Inorganic Salt Catalysed Hydrothermal Carbonisation (HTC) of Cellulose
Abstract
:1. Introduction

2. Results and Discussion
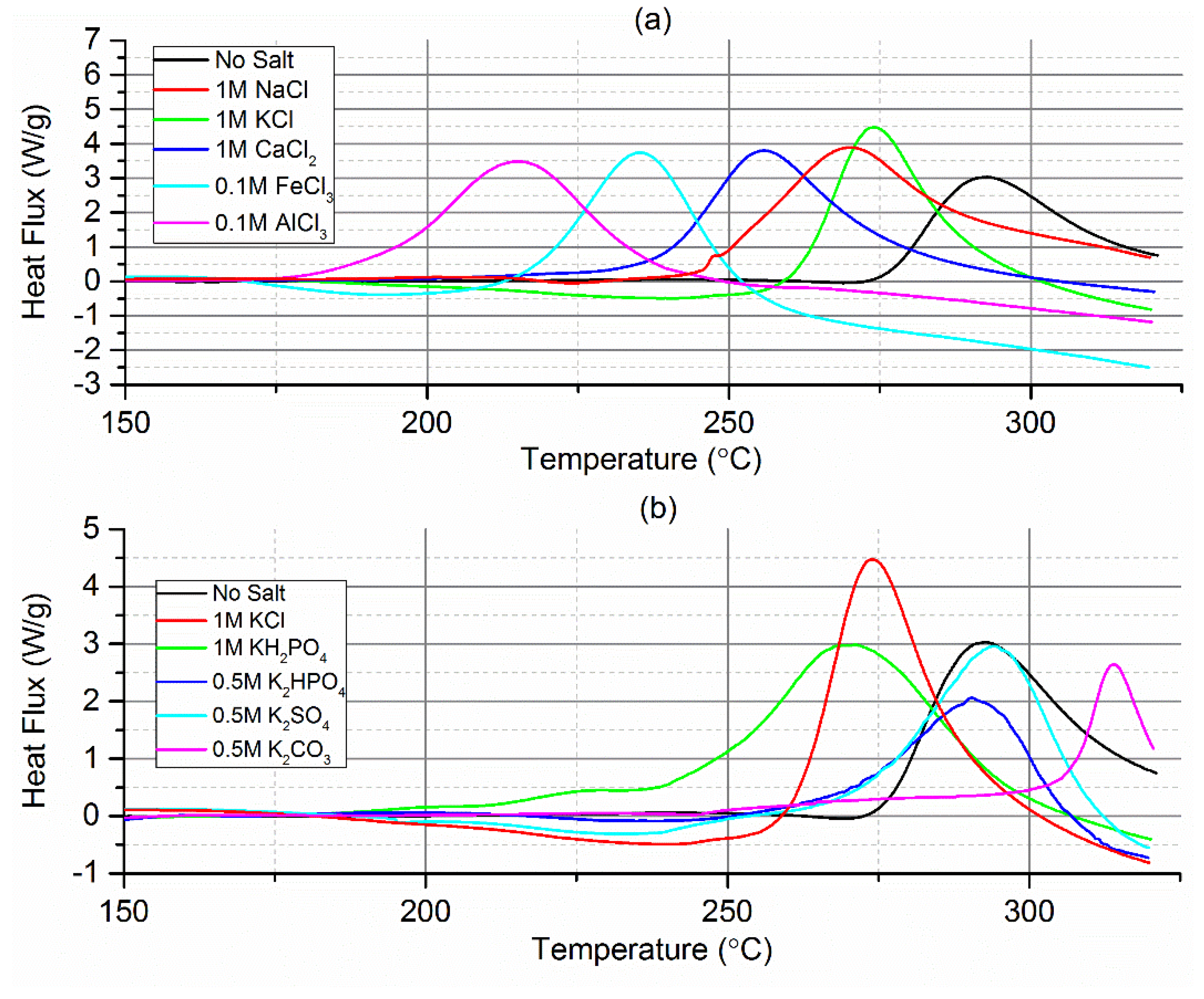
2.1. Differential Scanning Calorimetry (DSC)
2.2. Process Water Analysis
2.3. Hydrochar Analysis
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. HP-DSC
3.2.2. HTC Reactions
3.2.3. Proximate and Ultimate Analysis
3.2.4. Analysis of Process Waters by HPLC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mursito, A.T.; Hirajima, T.; Sasaki, K.; Kumagai, S. The effect of hydrothermal dewatering of Pontianak tropical peat on organics in wastewater and gaseous products. Fuel 2010, 89, 3934–3942. [Google Scholar] [CrossRef]
- Funke, A. Fate of Plant Available Nutrients during Hydrothermal Carbonization of Digestate. Chem. Ing. Tech. 2015, 87, 1713–1719. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, Y.; Wang, T.; Peng, C.; Li, S.; Wang, B.; Liu, X.; Li, C. Effect of temperature on the sulfur fate during hydrothermal carbonization of sewage sludge. Environ. Pollut. 2020, 260, 114067. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H. Cellulose and Pulp. In Forests and Forest Plants; Owens, J.N., Lund, H.G., Eds.; EOLSS Publications: Paris, France, 2009; Volume II, pp. 158–176. [Google Scholar]
- Jung, D.; Zimmermann, M.; Kruse, A. Hydrothermal Carbonization of Fructose: Growth Mechanism and Kinetic Model. ACS Sustain. Chem. Eng. 2018, 6, 13877–13887. [Google Scholar] [CrossRef]
- He, Q.; Yu, Y.; Wang, J.; Suo, X.; Liu, Y. Kinetic Study of the Hydrothermal Carbonization Reaction of Glucose and Its Product Structures. Ind. Eng. Chem. Res. 2021, 60, 4552–4561. [Google Scholar] [CrossRef]
- Kruse, A.; Zevaco, T.A. Properties of Hydrochar as Function of Feedstock, Reaction Conditions and Post-Treatment. Energies 2018, 11, 674. [Google Scholar] [CrossRef] [Green Version]
- Lucian, M.; Volpe, M.; Fiori, L. Hydrothermal Carbonization Kinetics of Lignocellulosic Agro-Wastes: Experimental Data and Modeling. Energies 2019, 12, 516. [Google Scholar] [CrossRef] [Green Version]
- Lucian, M.; Volpe, M.; Gao, L.; Piro, G.; Goldfarb, J.L.; Fiori, L. Impact of hydrothermal carbonization conditions on the formation of hydrochars and secondary chars from the organic fraction of municipal solid waste. Fuel 2018, 233, 257–268. [Google Scholar] [CrossRef]
- Qi, Y.; Song, B.; Qi, Y. The roles of formic acid and levulinic acid on the formation and growth of carbonaceous spheres by hydrothermal carbonization. RSC Adv. 2016, 6, 102428–102435. [Google Scholar] [CrossRef]
- Sasaki, M.; Goto, K.; Tajima, K.; Adschiri, T.; Arai, K. Rapid and selective retro-aldol condensation of glucose to glycolaldehyde in supercritical water. Green Chem. 2002, 4, 285–287. [Google Scholar] [CrossRef]
- Nicolae, S.A.; Au, H.; Modugno, P.; Luo, H.; Szego, A.E.; Qiao, M.; Li, L.; Yin, W.; Heeres, H.J.; Berge, N.; et al. Recent advances in hydrothermal carbonisation: From tailored carbon materials and biochemicals to applications and bioenergy. Green Chem. 2020, 22, 4747–4800. [Google Scholar] [CrossRef]
- Zhang, H.; Li, N.; Pan, X.; Wu, S.; Xie, J. Direct Transformation of Cellulose to Gluconic Acid in a Concentrated Iron(III) Chloride Solution under Mild Conditions. ACS Sustain. Chem. Eng. 2017, 5, 4066–4072. [Google Scholar] [CrossRef]
- Antonetti, C.; Licursi, D.; Fulignati, S.; Valentini, G.; Raspolli Galletti, A.M. New Frontiers in the Catalytic Synthesis of Levulinic Acid: From Sugars to Raw and Waste Biomass as Starting Feedstock. Catalysts 2016, 6, 196. [Google Scholar] [CrossRef]
- Assary, R.S.; Redfern, P.C.; Hammond, J.R.; Greeley, J.; Curtiss, L.A. Computational Studies of the Thermochemistry for Conversion of Glucose to Levulinic Acid. J. Phys. Chem. B 2010, 114, 9002–9009. [Google Scholar] [CrossRef]
- Tewari, Y.B.; Goldberg, R.N. Thermodynamics of hydrolysis of disaccharides. Cellobiose, gentiobiose, isomaltose, and maltose. J. Biol. Chem. 1989, 264, 3966–3971. [Google Scholar] [CrossRef]
- Dong, X.; Guo, S.; Wang, H.; Wang, Z.; Gao, X. Physicochemical characteristics and FTIR-derived structural parameters of hydrochar produced by hydrothermal carbonisation of pea pod (Pisum sativum Linn.) waste. Biomass Convers. Biorefinery 2019, 9, 531–540. [Google Scholar] [CrossRef]
- Düdder, H.; Wütscher, A.; Stoll, R.; Muhler, M. Synthesis and characterization of lignite-like fuels obtained by hydrothermal carbonization of cellulose. Fuel 2016, 171, 54–58. [Google Scholar] [CrossRef]
- Stirling, R.J.; Snape, C.E.; Meredith, W. The impact of hydrothermal carbonisation on the char reactivity of biomass. Fuel Processing Technol. 2018, 177, 152–158. [Google Scholar] [CrossRef]
- Gupta, D.; Mahajani, S.M.; Garg, A. Investigation on hydrochar and macromolecules recovery opportunities from food waste after hydrothermal carbonization. Sci. Total Environ. 2020, 749, 142294. [Google Scholar] [CrossRef]
- Paneque, M.; De la Rosa, J.M.; Kern, J.; Reza, M.T.; Knicker, H. Hydrothermal carbonization and pyrolysis of sewage sludges: What happen to carbon and nitrogen? J. Anal. Appl. Pyrolysis 2017, 128, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Idowu, I.; Li, L.; Flora, J.R.V.; Pellechia, P.J.; Darko, S.A.; Ro, K.S.; Berge, N.D. Hydrothermal carbonization of food waste for nutrient recovery and reuse. Waste Manag. 2017, 69, 480–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruse, A.; Koch, F.; Stelzl, K.; Wüst, D.; Zeller, M. Fate of Nitrogen during Hydrothermal Carbonization. Energy Fuels 2016, 30, 8037–8042. [Google Scholar] [CrossRef]
- Titirici, M.-M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, W.; Zhao, X.; Wang, D.P.; Liu, S.X. Carbon spheres obtained via citric acid catalysed hydrothermal carbonisation of cellulose. Mater. Res. Innov. 2013, 17, 546–551. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.I.; Ross, A.B.; Camargo-Valero, M.A. Mass and energy integration study of hydrothermal carbonization with anaerobic digestion of sewage sludge. Renew. Energy 2021, 167, 473–483. [Google Scholar] [CrossRef]
- Smith, A.M.; Whittaker, C.; Shield, I.; Ross, A.B. The potential for production of high quality bio-coal from early harvested Miscanthus by hydrothermal carbonisation. Fuel 2018, 220, 546–557. [Google Scholar] [CrossRef]
- Heilmann, S.M.; Davis, H.T.; Jader, L.R.; Lefebvre, P.A.; Sadowsky, M.J.; Schendel, F.J.; von Keitz, M.G.; Valentas, K.J. Hydrothermal carbonization of microalgae. Biomass Bioenergy 2010, 34, 875–882. [Google Scholar] [CrossRef]
- Heidari, M.; Salaudeen, S.; Dutta, A.; Acharya, B. Effects of Process Water Recycling and Particle Sizes on Hydrothermal Carbonization of Biomass. Energy Fuels 2018, 32, 11576–11586. [Google Scholar] [CrossRef]
- Stemann, J.; Putschew, A.; Ziegler, F. Hydrothermal carbonization: Process water characterization and effects of water recirculation. Bioresour. Technol. 2013, 143, 139–146. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Gu, C.; Han, Y.; Zan, S.; Wu, S. Effects of process water recirculation on solid and liquid products from hydrothermal carbonization of Laminaria. Bioresour. Technol. 2019, 292, 121996. [Google Scholar] [CrossRef]
- Uddin, M.H.; Reza, M.T.; Lynam, J.G.; Coronella, C.J. Effects of water recycling in hydrothermal carbonization of loblolly pine. Environ. Prog. Sustain. 2014, 33, 1309–1315. [Google Scholar] [CrossRef]
- Weiner, B.; Poerschmann, J.; Wedwitschka, H.; Koehler, R.; Kopinke, F.-D. Influence of Process Water Reuse on the Hydrothermal Carbonization of Paper. ACS Sustain. Chem. Eng. 2014, 2, 2165–2171. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Fierro, V.; Parmentier, J.; Vidal, L.; Gadonneix, P.; Celzard, A. Hydrothermal carbons produced from tannin by modification of the reaction medium: Addition of H+ and Ag+. Ind. Crop. Prod. 2015, 77, 364–374. [Google Scholar] [CrossRef]
- Rather, M.A.; Khan, N.S.; Gupta, R. Catalytic hydrothermal carbonization of invasive macrophyte Hornwort (Ceratophyllum demersum) for production of hydrochar: A potential biofuel. Int. J. Environ. Sci. Technol. 2017, 14, 1243–1252. [Google Scholar] [CrossRef]
- Ming, J.; Wu, Y.; Liang, G.; Park, J.-B.; Zhao, F.; Sun, Y.-K. Sodium salt effect on hydrothermal carbonization of biomass: A catalyst for carbon-based nanostructured materials for lithium-ion battery applications. Green Chem. 2013, 15, 2722–2726. [Google Scholar] [CrossRef]
- Lynam, J.G.; Coronella, C.J.; Yan, W.; Reza, M.T.; Vasquez, V.R. Acetic acid and lithium chloride effects on hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 2011, 102, 6192–6199. [Google Scholar] [CrossRef] [PubMed]
- Lynam, J.G.; Toufiq Reza, M.; Vasquez, V.R.; Coronella, C.J. Effect of salt addition on hydrothermal carbonization of lignocellulosic biomass. Fuel 2012, 99, 271–273. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Taran, O.P.; Bukhtiyarov, A.V.; Aymonier, C.; Prosvirin, I.P.; Parmon, V.N. Hydrothermal Solubilization–Hydrolysis–Dehydration of Cellulose to Glucose and 5-Hydroxymethylfurfural Over Solid Acid Carbon Catalysts. Top. Catal. 2018, 61, 1912–1927. [Google Scholar] [CrossRef]
- Remsing, R.C.; Swatloski, R.P.; Rogers, R.D.; Moyna, G. Mechanism of cellulose dissolution in the ionic liquid 1-n-butyl-3-methylimidazolium chloride: A 13C and 35/37Cl NMR relaxation study on model systems. Chem. Commun. 2006, 12, 1271–1273. [Google Scholar] [CrossRef]
- Sen, S.; Losey, B.P.; Gordon, E.E.; Argyropoulos, D.S.; Martin, J.D. Ionic Liquid Character of Zinc Chloride Hydrates Define Solvent Characteristics that Afford the Solubility of Cellulose. J. Phys. Chem. B 2016, 120, 1134–1141. [Google Scholar] [CrossRef]
- Yu, Y.; Lou, X.; Wu, H. Some Recent Advances in Hydrolysis of Biomass in Hot-Compressed Water and Its Comparisons with Other Hydrolysis Methods. Energy Fuels 2008, 22, 46–60. [Google Scholar] [CrossRef]
- Ahmadi Khoshooei, M.; Fazlollahi, F.; Maham, Y. A review on the application of differential scanning calorimetry (DSC) to petroleum products. J. Therm. Anal. Calorim. 2019, 138, 3455–3484. [Google Scholar] [CrossRef]
- Furushima, Y.; Nakada, M.; Takahashi, H.; Ishikiriyama, K. Study of melting and crystallization behavior of polyacrylonitrile using ultrafast differential scanning calorimetry. Polymer 2014, 55, 3075–3081. [Google Scholar] [CrossRef]
- Gupta, R.K.; Pant, B.; Agarwala, V.; Sinha, P.P. Differential scanning calorimetry and reaction kinetics studies of γ + α2 Ti aluminide. Mater. Chem. Phys. 2012, 137, 483–492. [Google Scholar] [CrossRef]
- Song, M.; Hourston, D.J. An application of modulated-temperature differential scanning calorimetry to the study of crystallisation kinetics in poly(ϵ-caprolactone)-poly(styrene-co-acrylonitrile) blends. Polymer 2000, 41, 8161–8165. [Google Scholar] [CrossRef]
- Ibbett, R.; Gaddipati, S.; Tucker, G. In-situ studies of hydrothermal reactions of lignocellulosic biomass using high-pressure differential scanning calorimetry. Biomass Bioenergy 2019, 121, 48–55. [Google Scholar] [CrossRef]
- Olsen, S.N.; Lumby, E.; McFarland, K.; Borch, K.; Westh, P. Kinetics of Enzymatic High-Solid Hydrolysis of Lignocellulosic Biomass Studied by Calorimetry. Appl. Biochem. Biotech. 2011, 163, 626–635. [Google Scholar] [CrossRef]
- Okano, T.; Qiao, K.; Bao, Q.; Tomida, D.; Hagiwara, H.; Yokoyama, C. Dehydration of fructose to 5-hydroxymethylfurfural (HMF) in an aqueous acetonitrile biphasic system in the presence of acidic ionic liquids. Appl. Catal. A-Gen. 2013, 451, 1–5. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, J.; Yagoub, A.E.A.; Ma, H.; Yu, X.; Hu, J.; Bao, X.; Liu, S. Conversion of glucose into 5-hydroxymethylfurfural in different solvents and catalysts: Reaction kinetics and mechanism. Egypt. J. Pet. 2017, 26, 477–487. [Google Scholar] [CrossRef] [Green Version]
- Assary, R.S.; Kim, T.; Low, J.J.; Greeley, J.; Curtiss, L.A. Glucose and fructose to platform chemicals: Understanding the thermodynamic landscapes of acid-catalysed reactions using high-level ab initio methods. Phys. Chem. Chem. Phys. 2012, 14, 16603–16611. [Google Scholar] [CrossRef]
- Setzer, W.N. A DFT Analysis of Thermal Decomposition Reactions Important to Natural Products. Nat. Prod. Commun. 2010, 5, 993–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knežević, D.; van Swaaij, W.P.M.; Kersten, S.R.A. Hydrothermal Conversion of Biomass: I, Glucose Conversion in Hot Compressed Water. Ind. Eng. Chem. Res. 2009, 48, 4731–4743. [Google Scholar] [CrossRef]
- Rebling, T.; von Frieling, P.; Buchholz, J.; Greve, T. Hydrothermal carbonization: Combination of heat of reaction measurements and theoretical estimations. J. Therm. Anal. Calorim. 2015, 119, 1941–1953. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Heat of reaction measurements for hydrothermal carbonization of biomass. Bioresour. Technol. 2011, 102, 7595–7598. [Google Scholar] [CrossRef]
- Ischia, G.; Cazzanelli, M.; Fiori, L.; Orlandi, M.; Miotello, A. Exothermicity of hydrothermal carbonization: Determination of heat profile and enthalpy of reaction via high-pressure differential scanning calorimetry. Fuel 2022, 310, 122312. [Google Scholar] [CrossRef]
- León, M.; Marcilla, A.F.; García, Á.N. Hydrothermal liquefaction (HTL) of animal by-products: Influence of operating conditions. Waste Manag. 2019, 99, 49–59. [Google Scholar] [CrossRef]
- Belkheiri, T.; Andersson, S.-I.; Mattsson, C.; Olausson, L.; Theliander, H.; Vamling, L. Hydrothermal liquefaction of kraft lignin in sub-critical water: The influence of the sodium and potassium fraction. Biomass Convers. Biorefinery 2018, 8, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Madsen, R.B.; Glasius, M. How Do Hydrothermal Liquefaction Conditions and Feedstock Type Influence Product Distribution and Elemental Composition? Ind. Eng. Chem. Res. 2019, 58, 17583–17600. [Google Scholar] [CrossRef]
- Zhu, Z.; Rosendahl, L.; Toor, S.S.; Chen, G. Optimizing the conditions for hydrothermal liquefaction of barley straw for bio-crude oil production using response surface methodology. Sci. Total Environ. 2018, 630, 560–569. [Google Scholar] [CrossRef]
- Hawthorne, F.C. A bond-topological approach to theoretical mineralogy: Crystal structure, chemical composition and chemical reactions. Phys. Chem. Miner. 2012, 39, 841–874. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, N.; Pan, X.; Wu, S.; Xie, J. Oxidative conversion of glucose to gluconic acid by iron(iii) chloride in water under mild conditions. Green Chem. 2016, 18, 2308–2312. [Google Scholar] [CrossRef]
- Brown, I.D. The Chemical Bond in Inorganic Chemistry: The Bond Valence Model, 1st ed.; Oxford University Press: New York, NY, USA, 2002; pp. 43–247. [Google Scholar]
- Kambo, H.S.; Minaret, J.; Dutta, A. Process Water from the Hydrothermal Carbonization of Biomass: A Waste or a Valuable Product? Waste Biomass Valorization 2018, 9, 1181–1189. [Google Scholar] [CrossRef]
- Makino, T.; Takano, H.; Kamiya, T.; Itou, T.; Sekiya, N.; Inahara, M.; Sakurai, Y. Restoration of cadmium-contaminated paddy soils by washing with ferric chloride: Cd extraction mechanism and bench-scale verification. Chemosphere 2008, 70, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-L.; Cai, Y.; Wu, Y.; Lu, Y.-X.; Yang, X.-L.; Yang, Y.-L. Enhancing the performance of a bioelectrochemically assisted osmotic membrane bioreactor based on reverse diffusion of organic and buffering draw solutes. Desalination 2020, 496, 114730. [Google Scholar] [CrossRef]
- Lu, X.; Flora, J.R.V.; Berge, N.D. Influence of process water quality on hydrothermal carbonization of cellulose. Bioresour. Technol. 2014, 154, 229–239. [Google Scholar] [CrossRef]
- Jung, D.; Duman, G.; Zimmermann, M.; Kruse, A.; Yanik, J. Hydrothermal carbonization of fructose—effect of salts and reactor stirring on the growth and formation of carbon spheres. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Persson, I. Ferric Chloride Complexes in Aqueous Solution: An EXAFS Study. J. Solut. Chem. 2018, 47, 797–805. [Google Scholar] [CrossRef] [Green Version]
- Akiya, N.; Savage, P.E. Roles of Water for Chemical Reactions in High-Temperature Water. Chem. Rev. 2002, 102, 2725–2750. [Google Scholar] [CrossRef]
- Lee, L.L. Molecular Thermodynamics of Electrolyte Solutions, 1st ed.; World Scientific: Singapore, 2008. [Google Scholar] [CrossRef]
- Paksung, N.; Pfersich, J.; Arauzo, P.J.; Jung, D.; Kruse, A. Structural Effects of Cellulose on Hydrolysis and Carbonization Behavior during Hydrothermal Treatment. ACS Omega 2020, 5, 12210–12223. [Google Scholar] [CrossRef]
- Garcés, D.; Faba, L.; Díaz, E.; Ordóñez, S. Aqueous-Phase Transformation of Glucose into Hydroxymethylfurfural and Levulinic Acid by Combining Homogeneous and Heterogeneous Catalysis. ChemSusChem 2019, 12, 924–934. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Lin, Z.; Niu, M.; Ren, S.; Wu, W. Conversion of Cellulose into Formic Acid by Iron(III)-Catalyzed Oxidation with O2 in Acidic Aqueous Solutions. ACS Omega 2018, 3, 14910–14917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kammoun, M.; Istasse, T.; Ayeb, H.; Rassaa, N.; Bettaieb, T.; Richel, A. Hydrothermal Dehydration of Monosaccharides Promoted by Seawater: Fundamentals on the Catalytic Role of Inorganic Salts. Front. Chem. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Kuzhiyil, N.; Dalluge, D.; Bai, X.; Kim, K.H.; Brown, R.C. Pyrolytic Sugars from Cellulosic Biomass. ChemSusChem 2012, 5, 2228–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marianou, A.A.; Michailof, C.C.; Ipsakis, D.; Triantafyllidis, K.; Lappas, A.A. Cellulose conversion into lactic acid over supported HPA catalysts. Green Chem. 2019, 21, 6161–6178. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, S.-K.; Jeong, G.-T. Efficient conversion of glucosamine to levulinic acid in a sulfamic acid-catalyzed hydrothermal reaction. RSC Adv. 2018, 8, 3198–3205. [Google Scholar] [CrossRef] [Green Version]
- Jung, D.; Körner, P.; Kruse, A. Kinetic study on the impact of acidity and acid concentration on the formation of 5-hydroxymethylfurfural (HMF), humins, and levulinic acid in the hydrothermal conversion of fructose. Biomass Convers. Biorefinery 2021, 11, 1155–1170. [Google Scholar] [CrossRef]
- Jiang, Z.; Yi, J.; Li, J.; He, T.; Hu, C. Promoting Effect of Sodium Chloride on the Solubilization and Depolymerization of Cellulose from Raw Biomass Materials in Water. ChemSusChem 2015, 8, 1901–1907. [Google Scholar] [CrossRef]
- Li, S.; Celzard, A.; Fierro, V.; Pasc, A. Salting Effect in the Hydrothermal Carbonisation of Bioresources. ChemistrySelect 2016, 1, 4161–4166. [Google Scholar] [CrossRef]
- Santos, V.S.; Moura, B.R.; Constantino, I.C.; Metzker, G.; Boscolo, M.; Cornélio, M.L.; Ferreira, O.P.; Mounier, J.L.S.; Hajjoul, H.; Bisinoti, M.C.; et al. Chelating properties of humic-like substances obtained from process water of hydrothermal carbonization. Environ. Technol. Innov. 2021, 23, 101688. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Cheng, K.; Antonietti, M. A hydrothermal process to turn waste biomass into artificial fulvic and humic acids for soil remediation. Sci. Total Environ. 2019, 686, 1140–1151. [Google Scholar] [CrossRef]
- Metrick, M.A.; MacDonald, G. Hofmeister ion effects on the solvation and thermal stability of model proteins lysozyme and myoglobin. Colloid Surf. A 2015, 469, 242–251. [Google Scholar] [CrossRef]
- Zongo, L.; Lange, H.; Crestini, C. A Study of the Effect of Kosmotropic and Chaotropic Ions on the Release Characteristics of Lignin Microcapsules under Stimuli-Responsive Conditions. ACS Omega 2019, 4, 6979–6993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, J.; Wang, J.; Graham, N.; Wilson, F. Coagulation of humic acid by aluminium sulphate in saline water conditions. Desalination 2002, 150, 1–14. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Han, G.; Liu, C.; Wang, X. Salt-Template Hydrothermal Carbonization for Pd NP-Loaded Porous Carbonaceous Material. Bioresources 2019, 14, 3630–3650. [Google Scholar] [CrossRef]
- Brown, A.E.; Finnerty, G.L.; Camargo-Valero, M.A.; Ross, A.B. Valorisation of macroalgae via the integration of hydrothermal carbonisation and anaerobic digestion. Bioresour. Technol. 2020, 312, 123539. [Google Scholar] [CrossRef]






| Salt | Temperature (°C) | Glucose | Fructose | Mannose | HMF | Levulinic Acid | Furfural | Formic Acid | Acetic Acid |
|---|---|---|---|---|---|---|---|---|---|
| None | 100 | - | - | - | - | - | - | - | - |
| 150 | - | - | - | - | - | - | - | - | |
| 200 | 240 | 120 | - | 610 | - | - | 670 | - | |
| 250 | - | - | - | - | 1520 | - | 460 | 1100 | |
| 1M KCl | 100 | - | - | - | - | - | - | - | - |
| 150 | - | - | - | - | - | - | - | - | |
| 200 | 10,550 | - | 500 | 3480 | - | - | 630 | - | |
| 250 | - | - | - | - | 8580 | - | 2220 | 950 | |
| 1M CaCl2 | 100 | - | - | - | - | - | - | - | - |
| 150 | 2780 | - | 4060 | 730 | - | - | - | - | |
| 200 | - | - | - | - | 13,180 | - | 7180 | 1210 | |
| 250 | - | - | - | - | 8790 | - | 1780 | 1100 | |
| 0.1M FeCl3 | 100 | 6260 | - | 7590 | - | - | - | - | - |
| 150 | 5890 | - | 2320 | - | - | - | 850 | - | |
| 200 | - | - | - | - | 25,700 | - | 11,250 | 770 | |
| 250 | - | - | - | - | 28,380 | - | - | 1170 | |
| 0.1M Al2(SO4)3 | 100 | - | - | - | - | - | - | - | - |
| 150 | 1790 | 870 | - | 1450 | - | 1160 | 580 | - | |
| 200 | - | - | - | - | 20,080 | - | 9640 | 1220 | |
| 250 | - | - | - | - | 18,060 | - | - | 1310 | |
| 0.1M FeCl3 + 1M KCl | 100 | 11,900 | - | 9650 | - | - | - | - | - |
| 150 | 24,430 | - | - | 1400 | 24,580 | 350 | 11,660 | 1770 | |
| 200 | - | - | - | - | 32,690 | - | 12,690 | 490 | |
| 250 | - | - | - | - | 31,380 | - | - | 1330 | |
| 0.1M FeCl3 + 5M KCl | 100 | 1280 | - | 4130 | - | - | - | - | - |
| 150 | 27,920 | - | 1360 | 1970 | 11,250 | 1960 | 4820 | 480 | |
| 200 | - | - | - | - | 31,020 | - | 13,060 | 830 | |
| 250 | - | - | - | - | 28,250 | - | - | 1180 | |
| 0.1M FeCl3 + 1M CaCl2 | 100 | 1790 | - | 5290 | - | - | - | - | - |
| 150 | 36,150 | 560 | 2230 | 1440 | 8870 | - | 3360 | 470 | |
| 200 | - | - | - | - | 30,000 | - | 10,460 | 500 | |
| 250 | - | - | - | - | 26,880 | - | - | 1320 | |
| 0.1M Al2(SO4)3 + 0.25M K2SO4 | 100 | 2940 | - | 8150 | - | - | - | - | - |
| 150 | 2960 | 400 | 2750 | - | - | - | - | - | |
| 200 | 9060 | - | - | 2610 | 8910 | - | 5190 | 770 | |
| 250 | - | - | - | - | 3280 | - | - | 770 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammerton, J.M.; Ross, A.B. Inorganic Salt Catalysed Hydrothermal Carbonisation (HTC) of Cellulose. Catalysts 2022, 12, 492. https://doi.org/10.3390/catal12050492
Hammerton JM, Ross AB. Inorganic Salt Catalysed Hydrothermal Carbonisation (HTC) of Cellulose. Catalysts. 2022; 12(5):492. https://doi.org/10.3390/catal12050492
Chicago/Turabian StyleHammerton, James M., and Andrew B. Ross. 2022. "Inorganic Salt Catalysed Hydrothermal Carbonisation (HTC) of Cellulose" Catalysts 12, no. 5: 492. https://doi.org/10.3390/catal12050492
APA StyleHammerton, J. M., & Ross, A. B. (2022). Inorganic Salt Catalysed Hydrothermal Carbonisation (HTC) of Cellulose. Catalysts, 12(5), 492. https://doi.org/10.3390/catal12050492






