Silica-Supported Copper (II) Oxide Cluster via Ball Milling Method for Catalytic Combustion of Ethyl Acetate
Abstract
:1. Introduction
2. Results
2.1. Appearances of the Prepared Catalysts
2.2. TG-DSC Results
2.3. ICP and N2 Physisorption Results
2.4. XRD Results
2.5. FT-IR Results
2.6. XPS Results
2.7. H2-TPR Results
2.8. HAADF-STEM Results
2.9. N2O Chemisorption Results
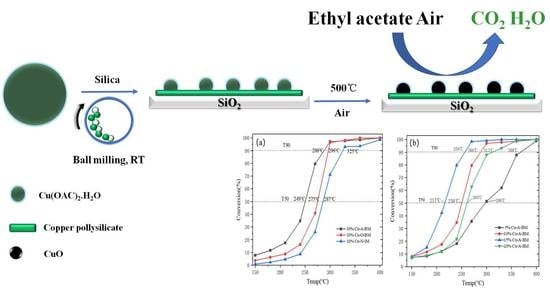
2.10. Catalytic Activity Results
3. Discussion
3.1. Structural Evolution of the W% Cu-A-BM Catalysts during Preparation
- Ball milling steps:
- (1)
- (2)
- Cu(OAC)2 · H2O + SiO2 → Cu(OAC)2 · H2O /SiO2
- Thermal pretreatment steps:
- (1)
- 50–200 °C
- (2)
- 200–400 °C
- (3)
- 400–500 °C+ O2 CuO/SiO2
3.2. Structure-Activity Relationship
4. Experimental
4.1. Catalyst Preparation
4.2. Catalyst Characterization
4.3. Catalytic Reaction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, H.; Yan, Y. Catalytic oxidation of ethyl acetate over CuO/ZSM-5 catalysts: Effect of preparation method. J. Taiwan Inst. Chem. Eng. 2018, 84, 162–172. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: A critical review. Appl. Catal. B: Environ. 2021, 281, 119447. [Google Scholar] [CrossRef]
- Dou, B.; Zhao, R.; Yan, N.; Zhao, C.; Hao, Q.; Hui, K.S.; Hui, K.N. A facilitated synthesis of hierarchically porous Cu–Ce–Zr catalyst using bacterial cellulose for VOCs oxidation. Mater. Chem. Phys. 2019, 237, 122181. [Google Scholar] [CrossRef]
- Kim, J.; Lee, B.-K. Enhanced photocatalytic decomposition of VOCs by visible-driven photocatalyst combined Cu-TiO2 and activated carbon fiber. Process Saf. Environ. Prot. 2018, 119, 164–171. [Google Scholar] [CrossRef]
- Munnik, P.; Wolters, M.; Gabrielsson, A.; Pollington, S.D.; Headdock, G.; Bitter, J.H.; de Jongh, P.E.; de Jong, K.P. Copper Nitrate Redispersion To Arrive at Highly Active Silica-Supported Copper Catalysts. J. Phys. Chem. C 2011, 115, 14698–14706. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Li, X.; Yin, Q.; Zhu, L.; Luo, Z. Highly active and selective Cu/SiO2 catalysts prepared by the urea hydrolysis method in dimethyl oxalate hydrogenation. Catal. Commun. 2011, 12, 1246–1250. [Google Scholar] [CrossRef]
- Chen, C.-C.; Lin, L.; Ye, R.-P.; Huang, L.; Zhu, L.-B.; Huang, Y.-Y.; Qin, Y.-Y.; Yao, Y.-G. Construction of Cu-Ce composite oxides by simultaneous ammonia evaporation method to enhance catalytic performance of Ce-Cu/SiO2 catalysts for dimethyl oxalate hydrogenation. Fuel 2021, 290, 120083. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, F.; Xue, J. Synthesis and structural characterization of silica dispersed copper nanomaterials with unusual thermal stability prepared by precipitation-gel method. J. Phys. Chem. C 2010, 114, 16104–16113. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, F.; Kang, H. Highly dispersed silica-supported copper nanoparticles prepared by precipitation-gel method: A simple but efficient and stable catalyst for glycerol hydrogenolysis. Chem. Mater. 2008, 20, 5090–5099. [Google Scholar] [CrossRef]
- Chen, L.; Guo, P.; Qiao, M.; Yan, S.; Li, H.; Shen, W.; Xu, H.; Fan, K. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2008, 257, 172–180. [Google Scholar] [CrossRef]
- He, X.; He, Q.; Deng, Y.; Peng, M.; Chen, H.; Zhang, Y.; Yao, S.; Zhang, M.; Xiao, D.; Ma, D.; et al. A versatile route to fabricate single atom catalysts with high chemoselectivity and regioselectivity in hydrogenation. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.S.; Lin, J.H.; You, J.H. Properties of Cu (thd) 2 as a precursor to prepare Cu/SiO2 catalyst using the atomic layer epitaxy technique. J. Am. Chem. Soc. 2006, 128, 15950–15951. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Chen, C.C.; Chen, C.T.; Kao, H.M. Synthesis of Cu nanoparticles in mesoporous silica SBA-15 functionalized with carboxylic acid groups. Chem. Commun. 2011, 47, 2288–2290. [Google Scholar] [CrossRef]
- Kohler, M.K.; Curry-Hyde, H.E.; Hughes, A.E. The structure of CuSiO2 catalysts prepared by the ion-exchange technique. J. Catal. 1987, 108, 323–333. [Google Scholar] [CrossRef]
- Zhang, K.; Chew, C.H.; Xu, G.Q. Synthesis and characterization of silica-copper oxide composite derived from microemulsion processing. Langmuir 1999, 15, 3056–3061. [Google Scholar] [CrossRef]
- Mo, L.; Saw, E.T.; Du, Y.; Borgna, A.; Ang, M.L.; Kathiraser, Y.; Li, Z.; Thitsartarn, W.; Lin, M.; Kawi, S. Highly dispersed supported metal catalysts prepared via in-situ self-assembled core-shell precursor route. Int. J. Hydrogen Energy 2015, 40, 13388–13398. [Google Scholar] [CrossRef]
- Mo, L.; Kawi, S. An in situ self-assembled core–shell precursor route to prepare ultrasmall copper nanoparticles on silica catalysts. J. Mater. Chem. A 2014, 2, 7837–7844. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friscic, T.; Grepioni, F.; Harris, K.D.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [Green Version]
- Kamolphop, U.; Taylor, S.F.R.; Breen, J.P.; Burch, R.; Delgado, J.J.; Chansai, S.; Hardacre, C.; Hengrasmee, S.; James, S.L. Low-Temperature Selective Catalytic Reduction (SCR) of NOx with n-Octane Using Solvent-Free Mechanochemically Prepared Ag/Al2O3 Catalysts. ACS Catal. 2011, 1, 1257–1262. [Google Scholar] [CrossRef]
- Yang, L.; Fan, C.; Luo, L.; Chen, Y.; Wu, Z.; Qin, Z.; Dong, M.; Fan, W.; Wang, J. Preparation of Pd/SiO2 Catalysts by a Simple Dry Ball-Milling Method for Lean Methane Oxidation and Probe of the State of Active Pd Species. Catalysts 2021, 11, 725. [Google Scholar] [CrossRef]
- He, X.; Deng, Y.; Zhang, Y.; He, Q.; Xiao, D.; Peng, M.; Zhao, Y.; Zhang, H.; Luo, R.; Gan, T.; et al. Mechanochemical Kilogram-Scale Synthesis of Noble Metal Single-Atom Catalysts. Cell Rep. Phys. Sci. 2020, 1, 100004. [Google Scholar] [CrossRef]
- Gan, T.; He, Q.; Zhang, H.; Xiao, H.; Liu, Y.; Zhang, Y.; He, X.; Ji, H. Unveiling the kilogram-scale gold single-atom catalysts via ball milling for preferential oxidation of CO in excess hydrogen. Chem. Eng. J. 2020, 389, 124490. [Google Scholar] [CrossRef]
- Amrute, A.P.; De Bellis, J.; Felderhoff, M.; Schüth, F. Mechanochemical synthesis of catalytic materials. Chem. Eur. J. 2021, 27, 6819–6847. [Google Scholar] [CrossRef]
- Zhang, K.; Hong, J.; Cao, G.; Zhan, D.; Tao, Y.; Cong, C. The kinetics of thermal dehydration of copper(II) acetate monohydrate in air. Therm. Acta 2005, 437, 145–149. [Google Scholar] [CrossRef]
- Lin, Z.; Han, D.; Li, S. Study on thermal decomposition of copper(II) acetate monohydrate in air. J. Therm. Anal. Calorim. 2011, 107, 471–475. [Google Scholar] [CrossRef]
- Habibi, M.H.; Karimi, B. Application of impregnation combustion method for fabrication of nanostructure CuO/ZnO composite oxide: XRD, FESEM, DRS and FTIR study. J. Ind. Eng. Chem. 2014, 20, 1566–1570. [Google Scholar] [CrossRef]
- Bette, S.; Costes, A.; Kremer, R.K.; Eggert, G.; Tang, C.C.; Dinnebier, R.E. On Verdigris, Part III: Crystal Structure, Magnetic and Spectral Properties of Anhydrous Copper(II) Acetate, a Paddle Wheel Chain. Z. Anorg.Und Allg. Chem. 2019, 645, 988–997. [Google Scholar] [CrossRef]
- Geng, L.; Li, G.; Zhang, X.; Wang, X.; Li, C.; Liu, Z.; Zhang, D.-S.; Zhang, Y.-Z.; Wang, G.; Han, H. Rational design of CuO/SiO2 nanocatalyst with anchor structure and hydrophilic surface for efficient hydrogenation of nitrophenol. J. Solid State Chem. 2021, 296, 121960. [Google Scholar] [CrossRef]
- Ren, Z.; Younis, M.N.; Zhao, H.; Li, C.; Yang, X.; Wang, E.; Wang, G. Silver modified Cu/SiO2 catalyst for the hydrogenation of methyl acetate to ethanol. Chin. J. Chem. Eng. 2020, 28, 1612–1622. [Google Scholar] [CrossRef]
- Chen, C.-C.; Lin, L.; Ye, R.-P.; Sun, M.-L.; Yang, J.-X.; Li, F.; Yao, Y.-G. Mannitol as a novel dopant for Cu/SiO2: A low-cost, environmental and highly stable catalyst for dimethyl oxalate hydrogenation without hydrogen prereduction. J. Catal. 2020, 389, 421–431. [Google Scholar] [CrossRef]
- Bian, Z.; Zhong, W.; Yu, Y.; Jiang, B.; Kawi, S. Cu/SiO2 derived from copper phyllosilicate for low-temperature water-gas shift reaction: Role of Cu+ sites. Int. J. Hydrogen Energy 2020, 45, 27078–27088. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Xu, Z.-N.; Peng, S.-Y.; Zhang, M.-J.; Lu, G.; Chen, Q.-S.; Chen, Y.; Guo, G.-C. High-Performance and Long-Lived Cu/SiO2 Nanocatalyst for CO2 Hydrogenation. ACS Catal. 2015, 5, 4255–4259. [Google Scholar] [CrossRef]
- He, M.; Luo, M.; Fang, P. Characterization of CuO Species and Thermal Solid-Solid Interaction in CuO/CeO2-Al2O3 Catalyst by In-Situ XRD, Raman Spectroscopy and TPR. J. Rare Earths 2020, 24, 188–192. [Google Scholar] [CrossRef]
- Marrero-Jerez, J.; Chinarro, E.; Moreno, B.; Peña-Martínez, J.; Núñez, P. CGO20–CuO composites synthesized by the combus-tion method and characterized by H2-TPR. Ceram. Int. 2015, 41, 10904–10909. [Google Scholar] [CrossRef]
- Van Der Grift, C.J.G.; Wielers, A.F.H.; Jogh, B. Effect of the reduction treatment on the structure and reactivity of sili-ca-supported copper particles. J. Catal. 1991, 131, 178–189. [Google Scholar] [CrossRef]











| Catalyst | Surafce Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | Cu Loading (wt%) | CuO Crystalline Size (XRD) (nm) |
|---|---|---|---|---|---|
| 5% Cu-A-BM | 298.3 | 0.43 | 22.9 | 5.1 | - |
| 10% Cu-A-BM | 251.6 | 0.36 | 17.2 | 9.9 | - |
| 15% Cu-A-BM | 188.7 | 0.27 | 18.0 | 13.3 | 7.3 |
| 20% Cu-A-BM | 132.6 | 0.31 | 24.8 | 18.4 | 14.5 |
| 10% Cu-O-BM | 244.3 | 0.35 | 19.2 | 9.8 | 16.2 |
| 10% Cu-N-IM | 139.4 | 0.29 | 33.2 | 10.1 | 22.9 |
| SiO2 | 390.1 | 0.97 | 33.2 | - | - |
| Pretreated Temperature/°C | CuO Crystalline Size | Cu2O Crystalline Size |
|---|---|---|
| 100 | 6.3 nm | - |
| 200 | 9.1 nm | - |
| 300 | 14.8 nm | 39.2 nm |
| 400 | 20.7 nm | 50.4 nm |
| 500 | 25.5 nm | - |
| Catalyst | Cu AR(%) a | CO AR(%) b | CP AR(%) c | CO/CP d |
|---|---|---|---|---|
| 5% Cu-A-BM | 1.33 | 0.59 | 0.74 | 0.79 |
| 10% Cu-A-BM | 1.71 | 0.99 | 0.72 | 1.38 |
| 15% Cu-A-BM | 2.68 | 1.54 | 1.14 | 1.35 |
| 20% Cu-A-BM | 1.26 | 0.69 | 0.57 | 1.21 |
| 10% Cu-O-BM | 0.71 | 0.57 | 0.14 | 4.04 |
| 10% Cu-N-IM | 0.49 | 0.49 | - | - |
| Catalyst | dChem (nm) a | D (%) b | S (m2 Cu/gCatal) c | dTEM (nm) d | dXRD (nm) e |
|---|---|---|---|---|---|
| 5% Cu-A-BM | 1.0 | 100.0 | 11.7 | - | - |
| 10% Cu-A-BM | 1.2 | 83.3 | 32.1 | - | - |
| 15% Cu-A-BM | 1.4 | 78.1 | 50.2 | 1.8 | 7.2 |
| 20% Cu-A-BM | 3.0 | 35.6 | 23.6 | - | 15.3 |
| 10% Cu-O-BM | 3.2 | 32.8 | 25.4 | - | 16.7 |
| 10% Cu-N-IM | 10.1 | 11.3 | 7.4 | - | 22.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.; Chen, H.; Ye, Y.; Zhang, H.; Xu, J.; Wang, L.; Mo, L. Silica-Supported Copper (II) Oxide Cluster via Ball Milling Method for Catalytic Combustion of Ethyl Acetate. Catalysts 2022, 12, 497. https://doi.org/10.3390/catal12050497
Ye Y, Chen H, Ye Y, Zhang H, Xu J, Wang L, Mo L. Silica-Supported Copper (II) Oxide Cluster via Ball Milling Method for Catalytic Combustion of Ethyl Acetate. Catalysts. 2022; 12(5):497. https://doi.org/10.3390/catal12050497
Chicago/Turabian StyleYe, Yuhang, Han Chen, Yuchuan Ye, Huiqiu Zhang, Jing Xu, Luhui Wang, and Liuye Mo. 2022. "Silica-Supported Copper (II) Oxide Cluster via Ball Milling Method for Catalytic Combustion of Ethyl Acetate" Catalysts 12, no. 5: 497. https://doi.org/10.3390/catal12050497
APA StyleYe, Y., Chen, H., Ye, Y., Zhang, H., Xu, J., Wang, L., & Mo, L. (2022). Silica-Supported Copper (II) Oxide Cluster via Ball Milling Method for Catalytic Combustion of Ethyl Acetate. Catalysts, 12(5), 497. https://doi.org/10.3390/catal12050497