Novel Enzymatic Method for Imine Synthesis via the Oxidation of Primary Amines Using D-Amino Acid Oxidase from Porcine Kidney
Abstract
:1. Introduction
2. Results
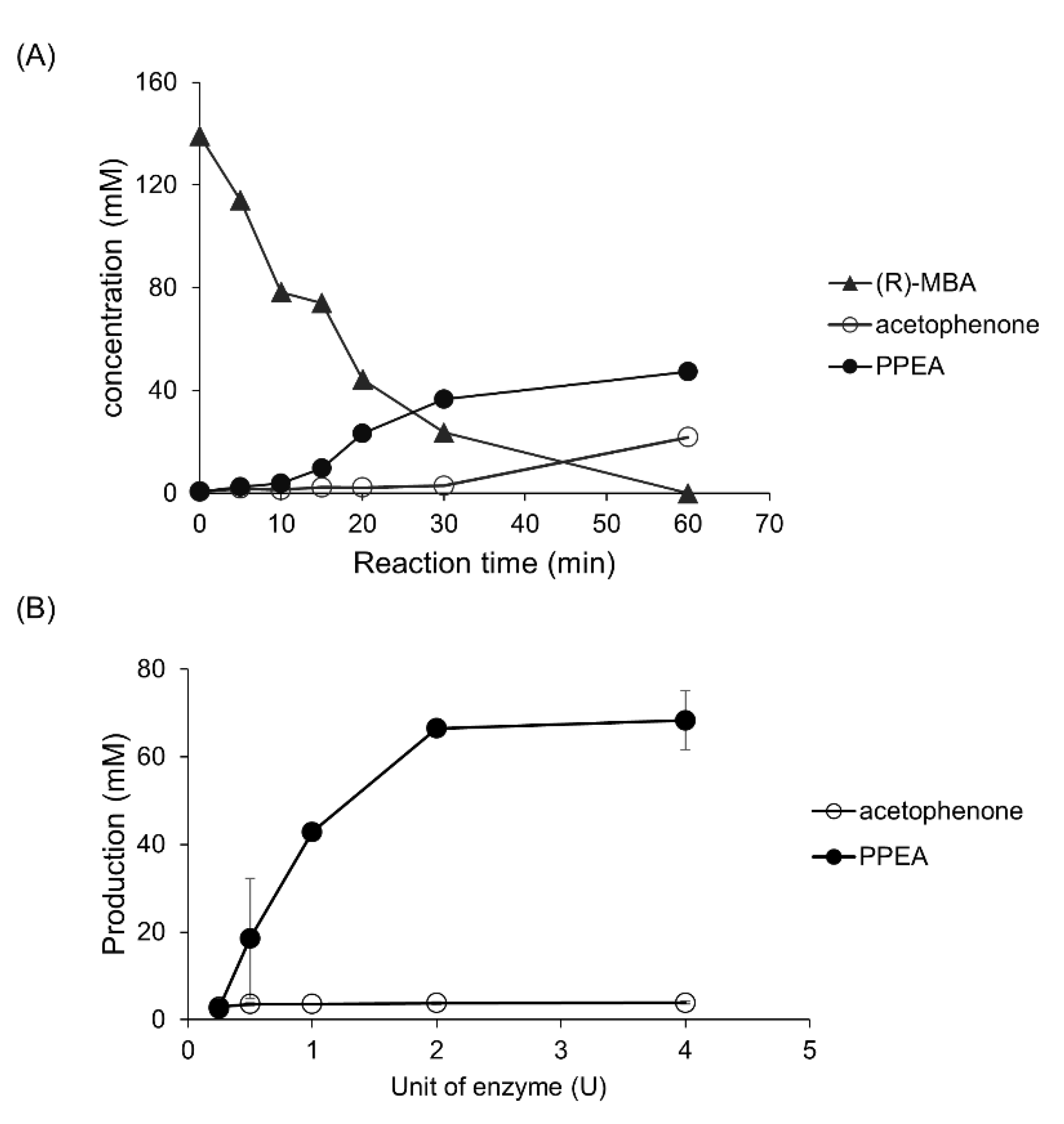
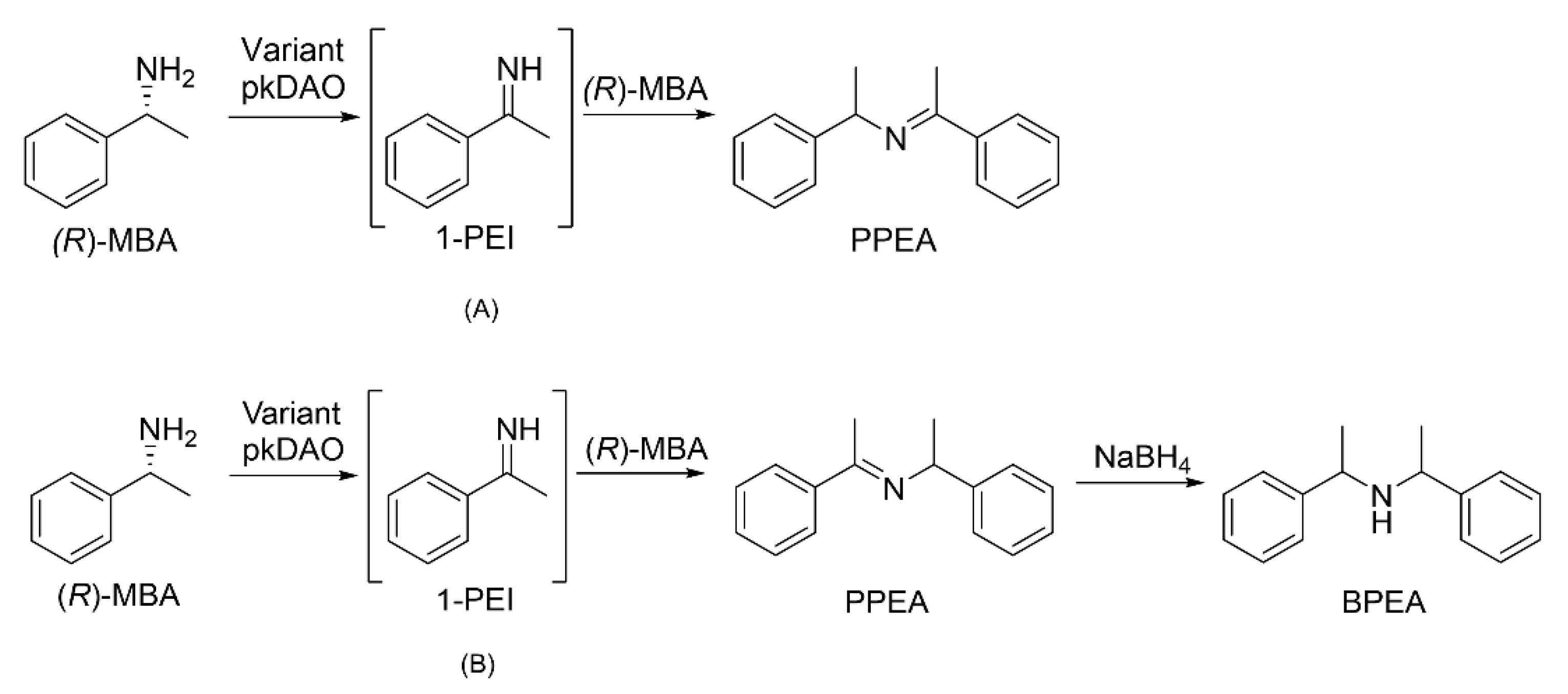
2.1. Production and Identification of PPEA from (R)-MBA
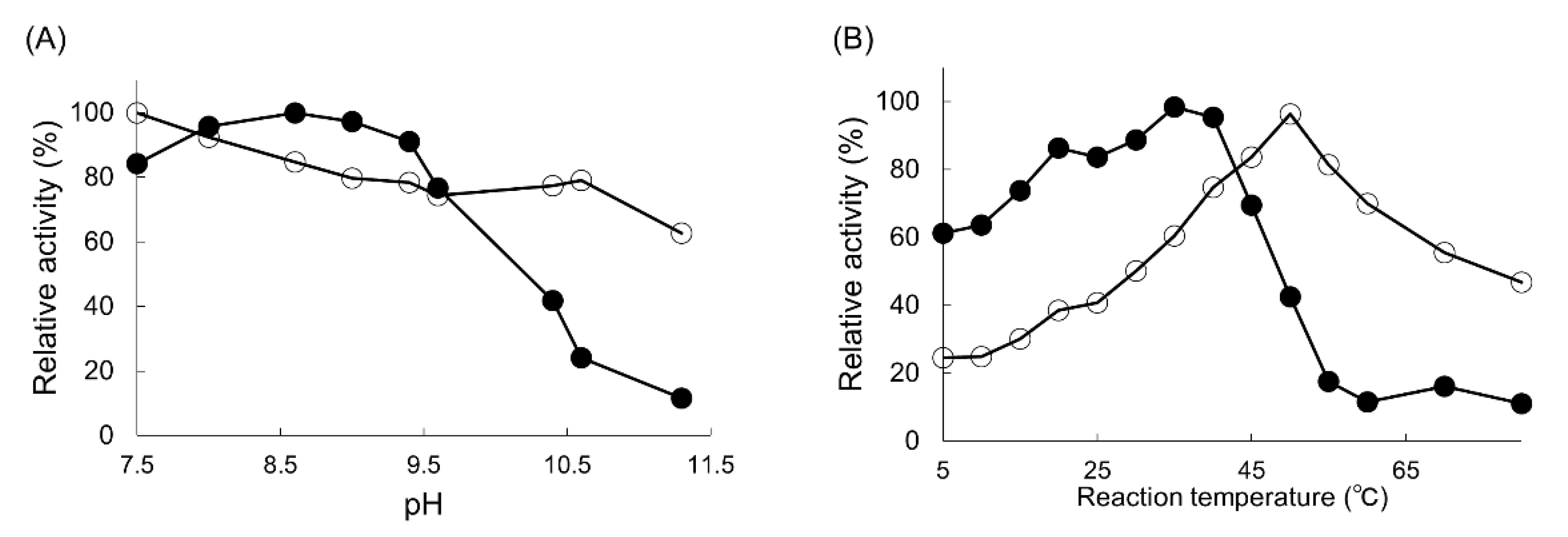
2.2. Optimization of the Reaction for PPEA Synthesis
2.3. Production of PPEA from (R)-MBA
2.4. Production of BPEA from (R)-MBA via PPEA
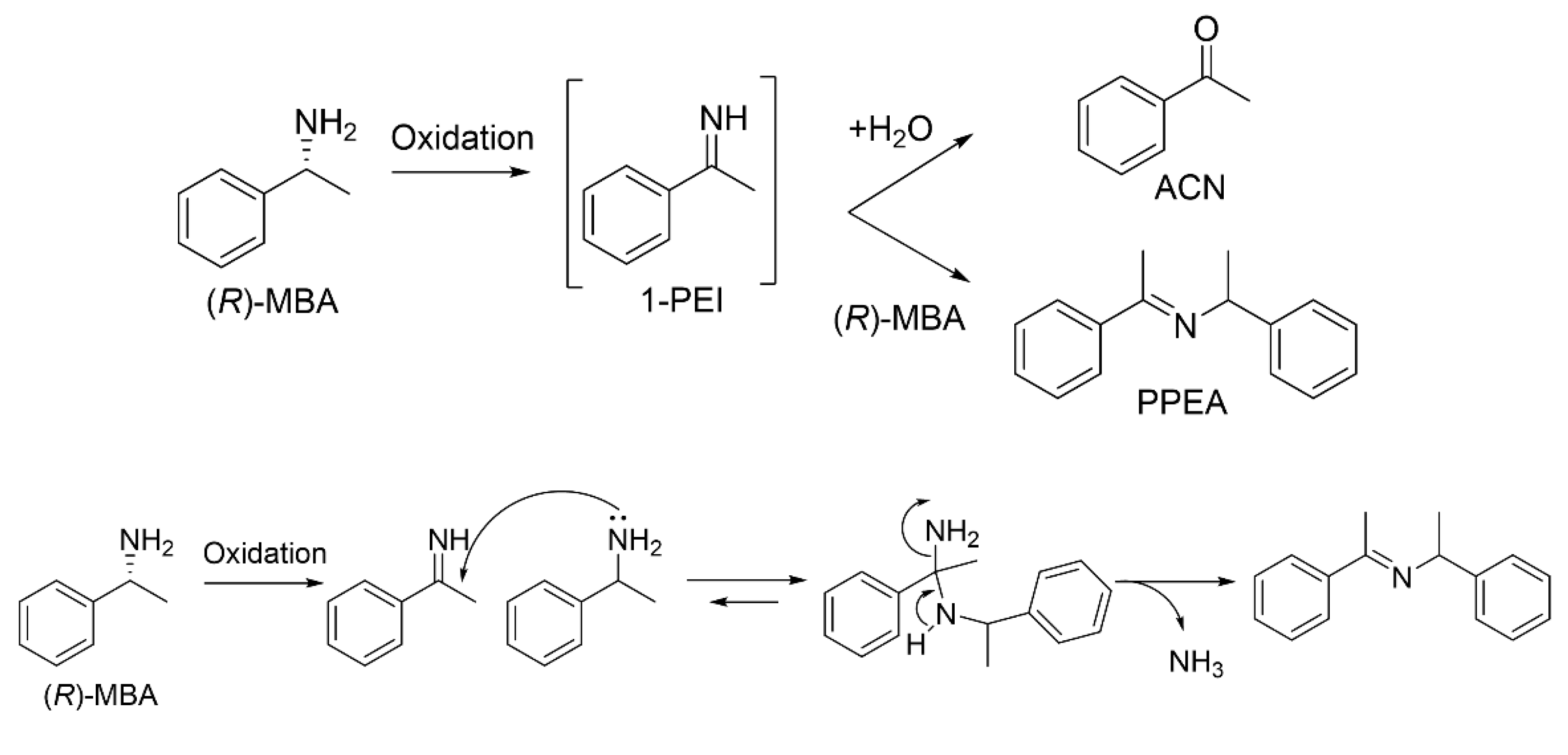
2.5. Reaction Mechanism of Imine Formation from Primary Amine with the Variant pkDAO
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Analysis of the Enzymatic Reaction by High-Performance Liquid Chromatography (HPLC)
4.3. Analysis of the Products in the Enzymatic Reaction Using GC/MS
4.4. Identification of the Reaction Products from (R)-MBA via GC/MS Analysis
4.5. Synthesis of 15N-Labeled n-Hexylamine
4.6. Kinetic Analysis of 1-Phenyl-N-(1-Phenylethylidene)Ethanamine (PPEA) Synthesis
4.7. Production of PPEA from (R)-MBA
4.8. Enzymatic Synthesis of N-Hexyl-1-Phenylethan-1-Imine from (R)-MBA and 15N-n-Hexylamine
4.9. Production of Bis(1-Phenylethyl)Amine (BPEA) from (R)-MBA and Identification of BPEA
4.10. Production and Identification of N-(1-Phenylethyl)Hexane-1-Amine from (R)-MBA and Hexylamine Using Enzymatic Reaction
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bloch, R. Additions of organometallic reagents to CN bonds: Reactivity and selectivity. Chem. Rev. 1998, 98, 1407–1438. [Google Scholar] [CrossRef] [PubMed]
- Neumann, R.; Levin, M. Selective aerobic oxidative dehydrogenation of alcohols and amines catalyzed by a supported molybdenum-vanadium heteropoly anion salt Na5PMo2V2O40. J. Org. Chem. 1991, 56, 5707–5710. [Google Scholar] [CrossRef]
- Shiri, P. Novel hybrid molecules based on triazole-β-lactam as potential biological agents. Mini Rev. Med. Chem. 2021, 21, 536–553. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Hamamoto, M.; Nishiyama, Y.; Ishii, Y. Oxidation of benzylic derivatives with dioxygen catalyzed by mixed addenda metallophosphate containing vanadium and molybdenum. Chem. Lett. 1993, 22, 1699–1702. [Google Scholar] [CrossRef]
- Éll, A.H.; Samec, J.S.; Brasse, C.; Bäckvall, J.-E. Dehydrogenation of aromatic amines to imines via ruthenium-catalyzed hydrogen transfer. Chem. Commun. 2002, 10, 1144–1145. [Google Scholar] [CrossRef]
- Nicolaou, K.; Mathison, C.J.; Montagnon, T. New reactions of IBX: Oxidation of nitrogen- and sulfur-containing substrates to afford useful synthetic intermediates. Angew. Chem. Int. Ed. 2003, 42, 4077–4082. [Google Scholar] [CrossRef]
- Gnanaprakasam, B.; Zhang, J.; Milstein, D. Direct synthesis of imines from alcohols and amines with liberation of H2. Angew. Chem. Int. Ed. 2010, 49, 1468–1471. [Google Scholar] [CrossRef]
- Donthiri, R.R.; Patil, R.D.; Adimurthy, S. NaOH-catalyzed imine synthesis: Aerobic oxidative coupling of alcohols and amines. Eur. J. Org. Chem. 2012, 2012, 4457–4460. [Google Scholar] [CrossRef]
- Ushakov, D.B.; Gilmore, K.; Kopetzki, D.; McQuade, D.T.; Seeberger, P.H. Continuous-flow oxidative cyanation of primary and secondary amines using singlet oxygen. Angew. Chem. Int. Ed. 2014, 53, 557–561. [Google Scholar] [CrossRef]
- Marui, K.; Nomoto, A.; Ueshima, M.; Ogawa, A. Eco-friendly copper sulfate-catalyzed oxidation of amines to imines by hydrogen peroxide in water. Tetrahedron Lett. 2015, 56, 1200–1202. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Zhang, L.; Lv, J.; Luo, S.; Cheng, J.-P. Bioinspired organocatalytic aerobic C–H oxidation of amines with an ortho-quinone catalyst. Org. Lett. 2015, 17, 1469–1472. [Google Scholar] [CrossRef] [PubMed]
- Ghislieri, D.; Green, A.P.; Pontini, M.; Willies, S.C.; Rowles, I.; Frank, A.; Grogan, G.; Turner, N.J. Engineering an enantioselective amine oxidase for the synthesis of pharmaceutical building blocks and alkaloid natural products. J. Am. Chem. Soc. 2013, 135, 10863–10869. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Li, B.; Qin, Y.; Dong, Y.; Ren, J.; Li, G. Recent progress in directed evolution of stereoselective monoamine oxidases. Bioresour. Bioprocess. 2019, 6, 37. [Google Scholar] [CrossRef]
- Heath, R.S.; Pontini, M.; Bechi, B.; Turner, N.J. Development of an R-selective amine oxidase with broad substrate specificity and high enantioselectivity. ChemCatChem 2014, 6, 996–1002. [Google Scholar] [CrossRef]
- Tani, Y.; Miyake, R.; Yukami, R.; Dekishima, Y.; China, H.; Saito, S.; Kawabata, H.; Mihara, H. Functional expression of L-lysine α-oxidase from Scomber japonicus in Escherichia coli for one-pot synthesis of L-pipecolic acid from DL-lysine. Appl. Microbiol. Biotechnol. 2015, 99, 5045–5054. [Google Scholar] [CrossRef]
- Kawahara, N.; Yasukawa, K.; Asano, Y. New enzymatic methods for the synthesis of primary α-aminonitriles and unnatural α-amino acids by oxidative cyanation of primary amines with D-amino acid oxidase from porcine kidney. Green Chem. 2017, 19, 418–424. [Google Scholar] [CrossRef]
- Yasukawa, K.; Nakano, S.; Asano, Y. Tailoring D-amino acid oxidase from the pig kidney to R-stereoselective amine oxidase and its use in the deracemization of α-methylbenzylamine. Angew. Chem. Int. Ed. 2014, 53, 4428–4431. [Google Scholar] [CrossRef]
- Yasukawa, K.; Motojima, F.; Ono, A.; Asano, Y. Expansion of the substrate specificity of porcine kidney D-amino acid oxidase for S-stereoselective oxidation of 4-Cl-benzhydrylamine. ChemCatChem 2018, 10, 3500–3505. [Google Scholar] [CrossRef] [Green Version]
- Nakano, S.; Yasukawa, K.; Tokiwa, T.; Ishikawa, T.; Ishitsubo, E.; Matsuo, N.; Ito, S.; Tokiwa, H.; Asano, Y. Origin of stereoselectivity and substrate/ligand recognition in an FAD-dependent R-selective amine oxidase. J. Phys. Chem. B 2016, 120, 10736–10743. [Google Scholar] [CrossRef]
- Asano, Y. Screening and development of enzymes for determination and transformation of amino acids, Biosci. Biotechnol. Biochem. 2019, 83, 1402–1416. [Google Scholar] [CrossRef]
- Asano, Y.; Yasukawa, K. Identification and development of amino acid oxidases. Curr. Opin. Chem. Biol. 2019, 49, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, K.; Kawahara, N.; Motojima, F.; Nakano, S.; Asano, Y. Porcine kidney D-amino acid oxidase-derived R-amine oxidases with new substrate specificities. The Enzymes 2020, 47, 117–136. [Google Scholar] [PubMed]
- Patil, R.D.; Adimurthy, S. Catalytic Methods for Imine Synthesis. Asian J. Org. Chem. 2013, 2, 726–744. [Google Scholar] [CrossRef]
- Abdel-Magid, A.F.; Carson, K.G.; Harris, B.D.; Maryanoff, C.A.; Shah, R.D. Reductive amination of aldehydes and ketones with sodium triacetoxyborohydride. studies on direct and indirect reductive amination procedures. J. Org. Chem. 1996, 61, 3849–3862. [Google Scholar] [CrossRef]
- Mirza-Aghayana, M.; Tavanaa, M.M.; Rahimifarda, M.; Boukherroub, R. Palladium on activated carbon catalyzed reductive amination of aldehydes and ketones by triethylsilane. Appl. Organomet. Chem. 2014, 28, 113–115. [Google Scholar] [CrossRef]
- Scheller, P.N.; Lenz, M.; Hammer, S.C.; Hauer, B.; Nestl, B.M. Imine reductase-catalyzed intermolecular reductive amination of aldehydes and ketones. ChemCatChem 2015, 7, 3239–3242. [Google Scholar] [CrossRef]
- Guzmán-Mejía, R.; Reyes-Rangel, G.; Juaristi, E. Preparation of chiral derivatives of β-Ala containing the α-phenylethyl group: Useful starting materials for the asymmetric synthesis of β-amino acids. Nat. Protoc. 2007, 2, 2759–2766. [Google Scholar] [CrossRef]
- Enthaler, S. Practical one-oot synthesis of secondary amines by zinc-catalyzed reductive amination. Catal. Lett. 2011, 141, 55–61. [Google Scholar] [CrossRef]
- Giovenzana, G.B.; Imperio, D.; Penoni, A.; Palmisano, G. Reductive amination with zinc powder in aqueous media. Beilstein J. Org. Chem. 2011, 7, 1095–1099. [Google Scholar] [CrossRef]
- Largeron, M. Protocols for the catalytic oxidation of primary amines to imines. Eur. J. Org. Chem. 2013, 24, 5225–5235. [Google Scholar] [CrossRef]
- Nugent, T.C.; Williams, R.V.; Dragan, A.; Méndez, A.A.; Iosub, A.V. An investigation of the observed, but counter-intuitive, stereoselectivity noted during chiral amine synthesis via N-chiral-ketimines. Beilstein J. Org. Chem. 2013, 9, 2103–2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuwahara, Y.; Shimizu, N.; Tanabe, T. Release of hydrogen cyanide via a post-secretion Schotten-Baumann reaction in defensive fluids of polydesmoid millipedes. J. Chem. Ecol. 2011, 37, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Tehara, N.; Uesugi, Y.; Jung, S.; Imai, N. Convenient peptide synthesis without protection of C-terminals. Chem. Lett. 2012, 41, 42–43. [Google Scholar] [CrossRef]
- Xu, B.; Tambar, U.K. Remote allylation of unactivated C (sp3)–H bonds triggered by photogenerated amidyl radicals. ACS Catal. 2019, 9, 4627–4631. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawahara, N.; Palasin, K.; Asano, Y. Novel Enzymatic Method for Imine Synthesis via the Oxidation of Primary Amines Using D-Amino Acid Oxidase from Porcine Kidney. Catalysts 2022, 12, 511. https://doi.org/10.3390/catal12050511
Kawahara N, Palasin K, Asano Y. Novel Enzymatic Method for Imine Synthesis via the Oxidation of Primary Amines Using D-Amino Acid Oxidase from Porcine Kidney. Catalysts. 2022; 12(5):511. https://doi.org/10.3390/catal12050511
Chicago/Turabian StyleKawahara, Nobuhiro, Kunwadee Palasin, and Yasuhisa Asano. 2022. "Novel Enzymatic Method for Imine Synthesis via the Oxidation of Primary Amines Using D-Amino Acid Oxidase from Porcine Kidney" Catalysts 12, no. 5: 511. https://doi.org/10.3390/catal12050511
APA StyleKawahara, N., Palasin, K., & Asano, Y. (2022). Novel Enzymatic Method for Imine Synthesis via the Oxidation of Primary Amines Using D-Amino Acid Oxidase from Porcine Kidney. Catalysts, 12(5), 511. https://doi.org/10.3390/catal12050511







