How to Obtain Maximum Environmental Applicability from Natural Silicates
Abstract
:1. Introduction
2. Results and Discussion
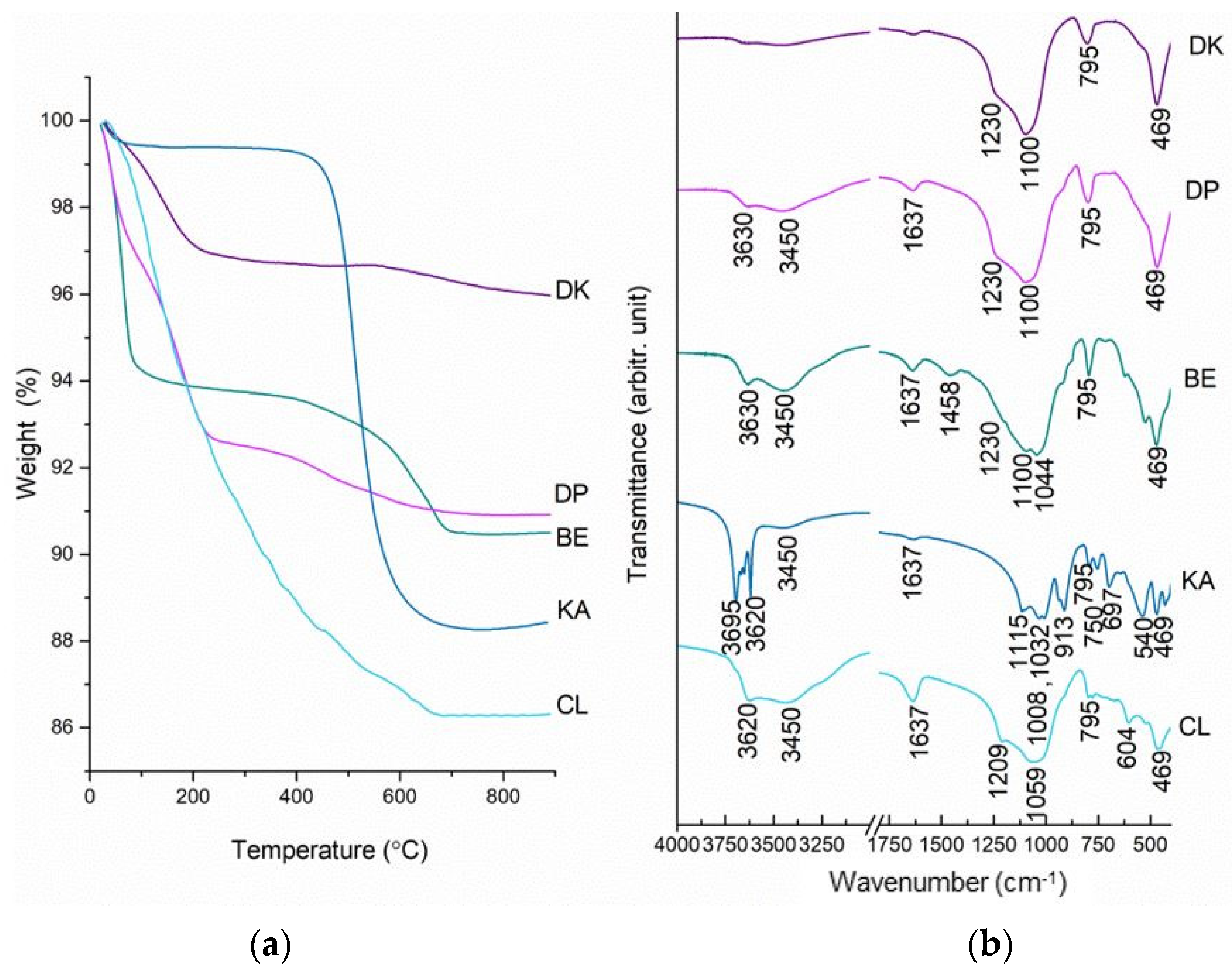
2.1. Thermal, Spectral and Structural Properties of Investigated Minerals
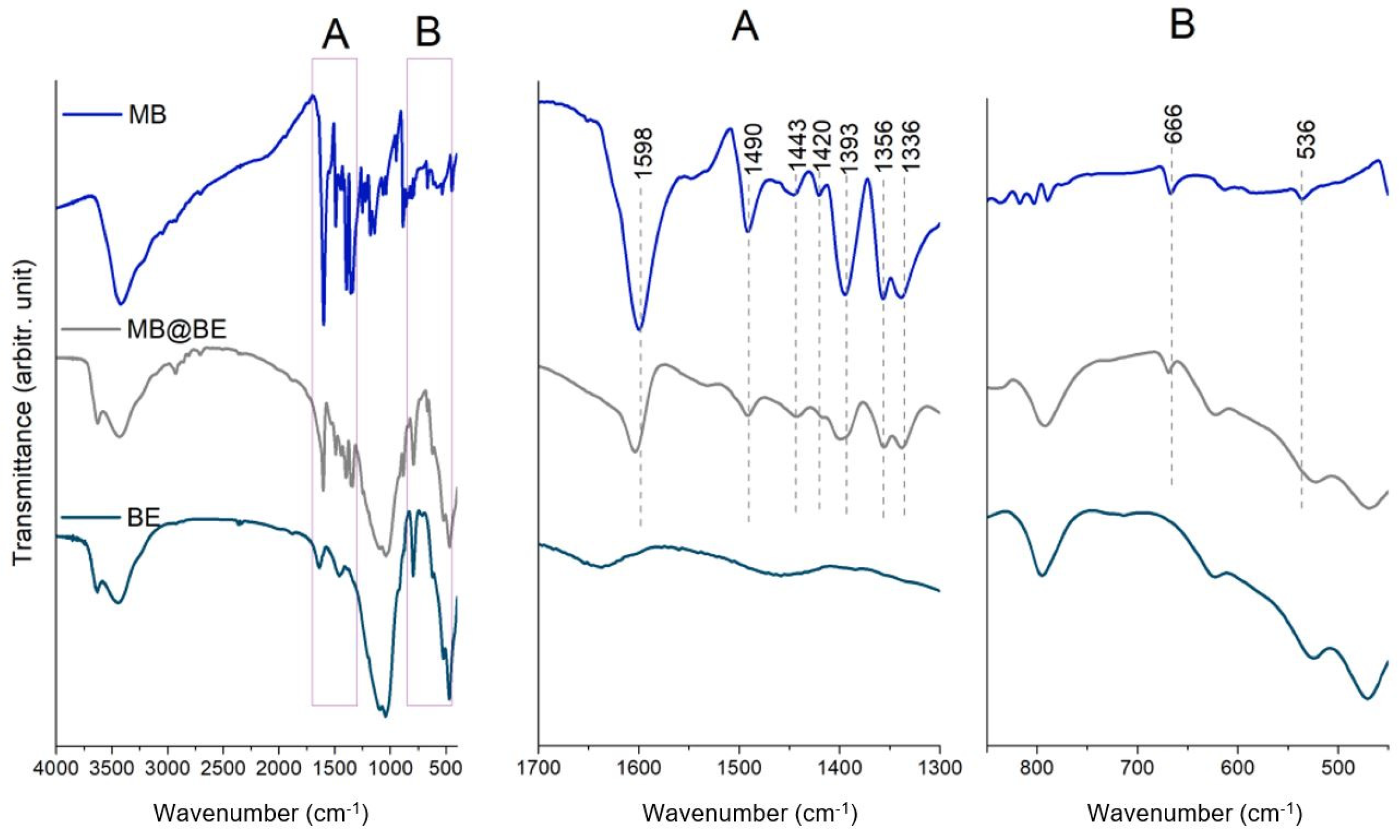
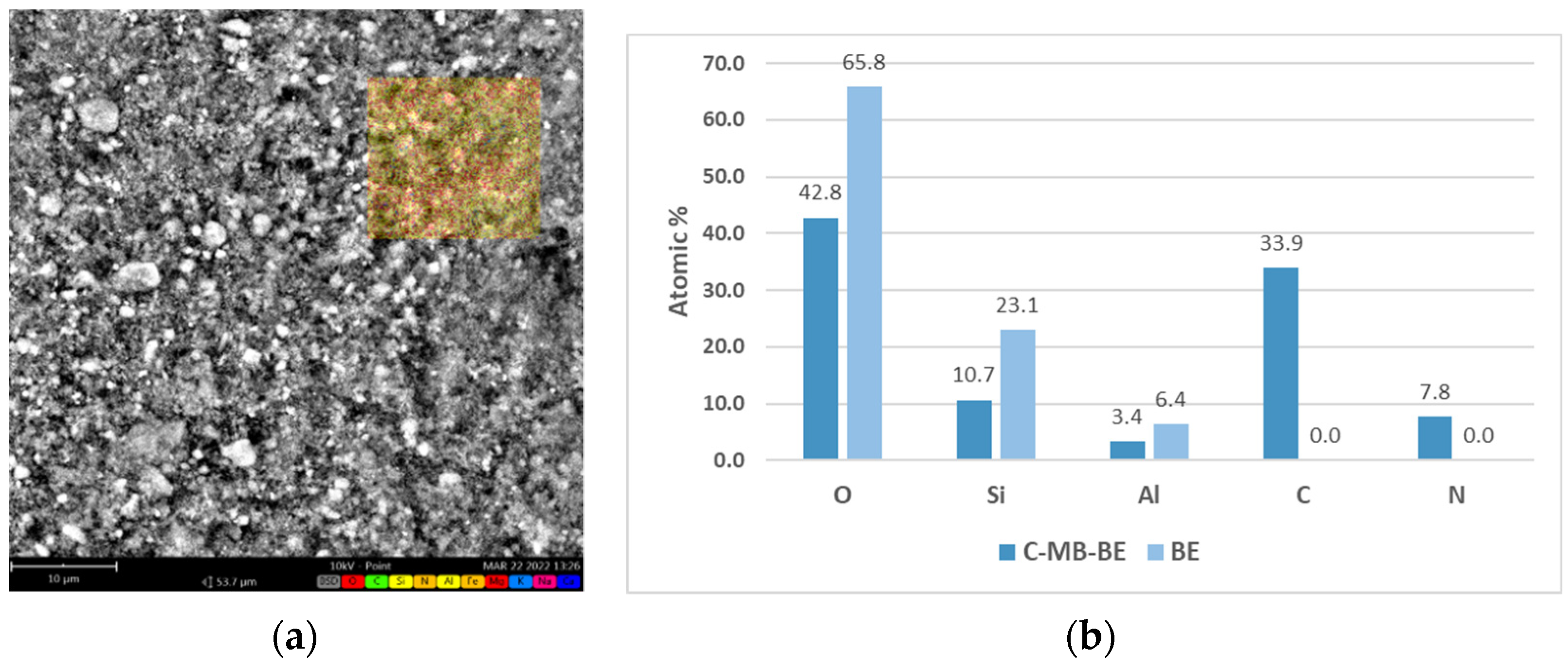
2.2. Methylene Blue Adsorption-Quantitative, Spectral and Elemental Analysis
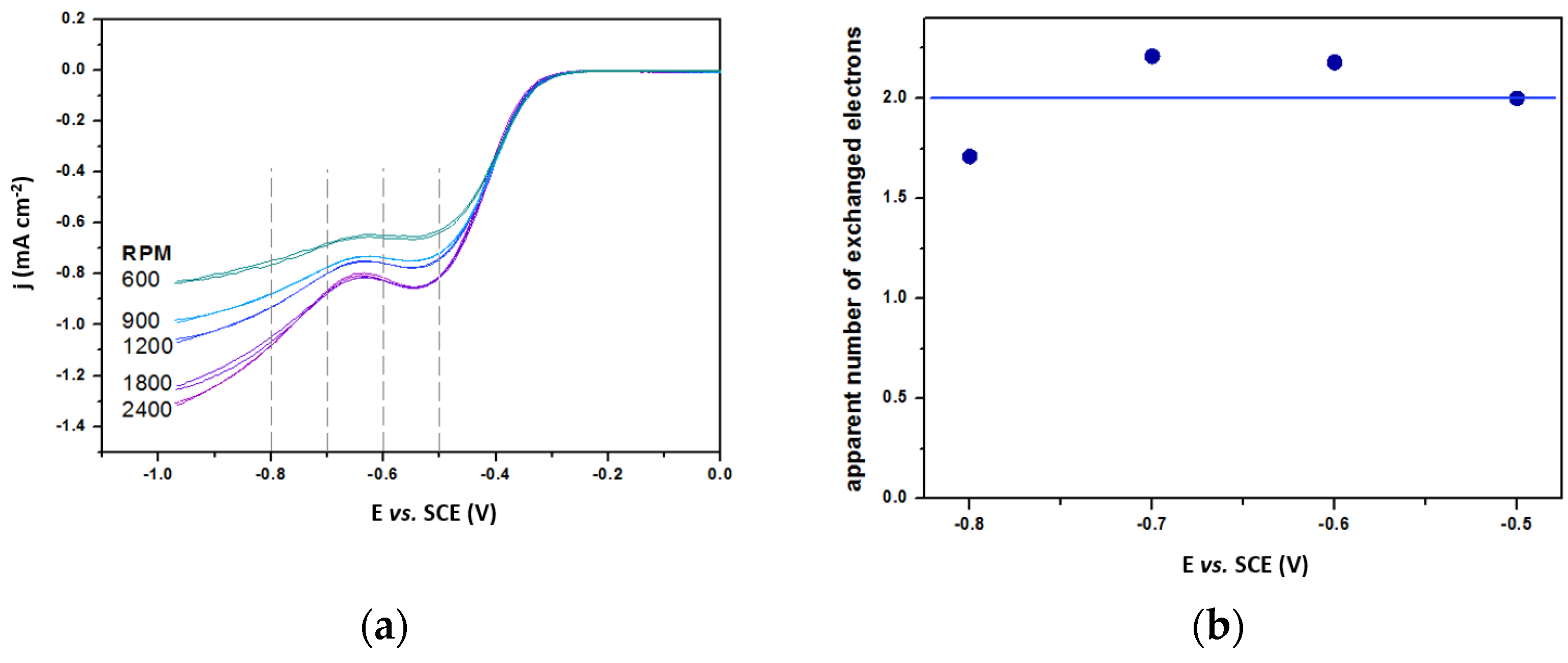
2.3. Electro-Applicability of Spent Adsorbent
3. Materials and Methods
3.1. Materials
3.2. Characterization of the Natural Silicates
3.3. Methylene Blue Adsorption, Adsorbents Treatment and Characterization
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ganguly, S.; Das, P.; Das, T.K.; Ghosh, S.; Das, S.; Bose, M.; Mondal, M.; Das, A.K.; Das, N.C. Acoustic Cavitation Assisted Destratified Clay Tactoid Reinforced in Situ Elastomer-Mimetic Semi-IPN Hydrogel for Catalytic and Bactericidal Application. Ultrason. Sonochemistry 2020, 60, 104797. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, N.C. Water Uptake Kinetics and Control Release of Agrochemical Fertilizers from Nanoclay-Assisted Semi-Interpenetrating Sodium Acrylate-Based Hydrogel. Polym.-Plast. Technol. Eng. 2017, 56, 744–761. [Google Scholar] [CrossRef]
- Krajišnik, D.; Milojević, M.; Malenović, A.A.; Daković, A.; Ibrić, S.; Savić, S.S.; Dondur, V.; Matijašević, S.; Radulović, A.; Daniels, R.; et al. Cationic Surfactants-Modified Natural Zeolites: Improvement of the Excipients Functionality. Drug Dev. Ind. Pharm. 2010, 36, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Cerri, G.; Farina, M.; Brundu, A.; Daković, A.; Giunchedi, P.; Gavini, E.; Rassu, G. Natural Zeolites for Pharmaceutical Formulations: Preparation and Evaluation of a Clinoptilolite-Based Material. Microporous Mesoporous Mater. 2016, 223, 58–67. [Google Scholar] [CrossRef]
- Krajišnik, D.; Daković, A.; Malenović, A.; Milojević-Rakić, M.; Dondur, V.; Radulović, Ž.; Milić, J.; Radulović, Z.; Milić, J. Investigation of Adsorption and Release of Diclofenac Sodium by Modified Zeolites Composites. Appl. Clay Sci. 2013, 83–84, 322–326. [Google Scholar] [CrossRef]
- Krajišnik, D.; Daković, A.; Milić, J.; Marković, M. Zeolites as Potential Drug Carriers. In Modified Clay and Zeolite Nanocomposite Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 27–55. [Google Scholar]
- Pagnacco, M.; Maksimović, J.; Mudrinić, T.; Banković, P.; Nedić-Vasiljević, B.; Milutinović-Nikolić, A. Oscillatory Briggs-Rauscher Reaction as “Fingerprint” for Bentonite Identification: The Fine-Tuning of Oscillatory Dynamics with Addition of Clay. ChemistrySelect 2020, 5, 8137–8141. [Google Scholar] [CrossRef]
- Jovic-Jovicic, N.; Bankovic, P.; Mojovic, Z.; Nedic-Vasiljevic, B.; Marinovic, S.; Mudrinic, T.; Milutinovic-Nikolic, A. Ecologically Friendly Chitosan-Montmorillonite Bio-Nanocomposite as Adsorbent for Textile Dyes from Aqueous Solutions. Sci. Sinter. 2017, 49, 419–429. [Google Scholar] [CrossRef]
- Janićijević, J.; Milić, J.; Čalija, B.; Micov, A.; Stepanović-Petrović, R.; Tomić, M.; Daković, A.; Dobričić, V.; Nedić Vasiljević, B.; Krajišnik, D. Potentiation of the Ibuprofen Antihyperalgesic Effect Using Inorganically Functionalized Diatomite. J. Mater. Chem. B 2018, 6, 5812–5822. [Google Scholar] [CrossRef] [PubMed]
- Janićijević, J.; Krajišnik, D.; Čalija, B.; Vasiljević, B.N.; Dobričić, V.; Daković, A.; Antonijević, M.D.; Milić, J. Modified Local Diatomite as Potential Functional Drug Carrier—A Model Study for Diclofenac Sodium. Int. J. Pharm. 2015, 496, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Milojević-Rakić, M.; Popadić, D.; Janošević Ležaić, A.; Jevremović, A.; Nedić Vasiljević, B.; Uskoković-Marković, S.; Bajuk-Bogdanović, D. MFI, BEA and FAU Zeolite Scavenging Role in Neonicotinoids and Radical Species Elimination. Environ. Sci. Processes Impacts 2022, 24, 265–276. [Google Scholar] [CrossRef]
- Barakan, S.; Aghazadeh, V. The Advantages of Clay Mineral Modification Methods for Enhancing Adsorption Efficiency in Wastewater Treatment: A Review. Environ. Sci. Pollut. Res. 2021, 28, 2572–2599. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Zhang, X.; Zhou, S. Adsorption of Methylene Blue in Water onto Activated Carbon by Surfactant Modification. Water 2020, 12, 587. [Google Scholar] [CrossRef] [Green Version]
- Rosly, N.Z.; Abdullah, A.H.; Ahmad Kamarudin, M.; Ashari, S.E.; Alang Ahmad, S.A. Adsorption of Methylene Blue Dye by Calix [6]Arene-Modified Lead Sulphide (Pbs): Optimisation Using Response Surface Methodology. Int. J. Environ. Res. Public Health 2021, 18, 397. [Google Scholar] [CrossRef] [PubMed]
- Rahmawati, F.; Ridassepri, A.F.; Chairunnisa; Wijayanta, A.T.; Nakabayashi, K.; Miyawaki, J.; Miyazaki, T. Carbon from Bagasse Activated with Water Vapor and Its Adsorption Performance for Methylene Blue. Appl. Sci. 2021, 11, 678. [Google Scholar] [CrossRef]
- Harikishore Kumar Reddy, D.; Vijayaraghavan, K.; Kim, J.A.; Yun, Y.-S. Valorisation of Post-Sorption Materials: Opportunities, Strategies, and Challenges. Adv. Colloid Interface Sci. 2017, 242, 35–58. [Google Scholar] [CrossRef]
- Tian, G.; Wang, W.; Zong, L.; Kang, Y.; Wang, A. From Spent Dye-Loaded Palygorskite to a Multifunctional Palygorskite/Carbon/Ag Nanocomposite. RSC Adv. 2016, 6, 41696–41706. [Google Scholar] [CrossRef]
- Feng, Y.; Dionysiou, D.D.; Wu, Y.; Zhou, H.; Xue, L.; He, S.; Yang, L. Adsorption of Dyestuff from Aqueous Solutions through Oxalic Acid-Modified Swede Rape Straw: Adsorption Process and Disposal Methodology of Depleted Bioadsorbents. Bioresour. Technol. 2013, 138, 191–197. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, W.; Kan, X.; Dong, L.; Jiang, Z.; Li, H.; Yang, H.; Cheng, R. Sorption of Methylene Blue by Carboxymethyl Cellulose and Reuse Process in a Secondary Sorption. Colloids Surf. A Physicochem. Eng. Asp. 2011, 380, 143–151. [Google Scholar] [CrossRef]
- Dimitriadis, S.; Nomikou, N.; McHale, A.P. Pt-Based Electro-Catalytic Materials Derived from Biosorption Processes and Their Exploitation in Fuel Cell Technology. Biotechnol. Lett. 2007, 29, 545–551. [Google Scholar] [CrossRef]
- Zhang, Y.; An, Y.; Wu, L.; Chen, H.; Li, Z.; Dou, H.; Murugadoss, V.; Fan, J.; Zhang, X.; Mai, X.; et al. Metal-Free Energy Storage Systems: Combining Batteries with Capacitors Based on a Methylene Blue Functionalized Graphene Cathode. J. Mater. Chem. A 2019, 7, 19668–19675. [Google Scholar] [CrossRef]
- Badeenezhad, A.; Azhdarpoor, A.; Bahrami, S.; Yousefinejad, S. Removal of Methylene Blue Dye from Aqueous Solutions by Natural Clinoptilolite and Clinoptilolite Modified by Iron Oxide Nanoparticles. Mol. Simul. 2019, 45, 564–571. [Google Scholar] [CrossRef]
- Hong, S.; Wen, C.; He, J.; Gan, F.; Ho, Y.-S. Adsorption Thermodynamics of Methylene Blue onto Bentonite. J. Hazard. Mater. 2009, 167, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Mouni, L.; Belkhiri, L.; Bollinger, J.-C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from Aqueous Solutions by Adsorption on Kaolin: Kinetic and Equilibrium Studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Khraisheh, M.A.M.; Ahmad, M.N.M.; Allen, S. Adsorption Behaviour of Methylene Blue onto Jordanian Diatomite: A Kinetic Study. J. Hazard. Mater. 2009, 165, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, X.; Ma, Z.; Wang, Q.; Yang, J. Synthesis of Nano-ZnO/Diatomite Composite and Research on Photoelectric Application. Catalysts 2021, 11, 1232. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Xue, N.; Wang, P.; Pei, M.; Guo, W. Ultra-Highly Efficient Removal of Methylene Blue Based on Graphene Oxide/TiO2/Bentonite Sponge. Materials 2020, 13, 824. [Google Scholar] [CrossRef] [Green Version]
- Kuntubek, A.; Kinayat, N.; Meiramkulova, K.; Poulopoulos, S.G.; Bear, J.C.; Inglezakis, V.J. Catalytic Oxidation of Methylene Blue by Use of Natural Zeolite-Based Silver and Magnetite Nanocomposites. Processes 2020, 8, 471. [Google Scholar] [CrossRef] [Green Version]
- Samarghandi, M.R.; Dargahi, A.; Shabanloo, A.; Nasab, H.Z.; Vaziri, Y.; Ansari, A. Electrochemical Degradation of Methylene Blue Dye Using a Graphite Doped PbO2 Anode: Optimization of Operational Parameters, Degradation Pathway and Improving the Biodegradability of Textile Wastewater. Arab. J. Chem. 2020, 13, 6847–6864. [Google Scholar] [CrossRef]
- Droguett, T.; Mora-Gómez, J.; García-Gabaldón, M.; Ortega, E.; Mestre, S.; Cifuentes, G.; Pérez-Herranz, V. Electrochemical Degradation of Reactive Black 5 Using Two-Different Reactor Configuration. Sci. Rep. 2020, 10, 4482. [Google Scholar] [CrossRef]
- Łuba, M.; Mikołajczyk, T.; Pierożyński, B.; Smoczyński, L.; Wojtacha, P.; Kuczyński, M. Electrochemical Degradation of Industrial Dyes in Wastewater through the Dissolution of Aluminum Sacrificial Anode of Cu/Al Macro-Corrosion Galvanic Cell. Molecules 2020, 25, 4108. [Google Scholar] [CrossRef]
- Irfan Khan, M.; Khan, H.U.; Azizli, K.; Sufian, S.; Man, Z.; Siyal, A.A.; Muhammad, N.; Faiz ur Rehman, M. The Pyrolysis Kinetics of the Conversion of Malaysian Kaolin to Metakaolin. Appl. Clay Sci. 2017, 146, 152–161. [Google Scholar] [CrossRef]
- Bourliva, A.; Michailidis, K.; Sikalidis, C.; Filippidis, A. Spectroscopic and Thermal Study of Bentonites from Milos Island, Greece. Bull. Geol. Soc. Greece 2013, 47, 2020. [Google Scholar] [CrossRef] [Green Version]
- Krajišnik, D.; Daković, A.; Milojević, M.; Malenović, A.; Kragović, M.; Bajuk-Bogdanović, D.; Dondur, V.; Milić, J. Properties of Diclofenac Sodium Sorption onto Natural Zeolite Modified with Cetylpyridinium Chloride. Colloids Surf B Biointerfaces 2011, 83, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Farmer, V.C. Transverse and Longitudinal Crystal Modes Associated with OH Stretching Vibrations in Single Crystals of Kaolinite and Dickite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 927–930. [Google Scholar] [CrossRef]
- Russell, J.D.; Fraser, A.R. Infrared Methods. In Clay Mineralogy: Spectroscopic and Chemical Determinative Methods; Springer: Dordrecht, The Netherlands, 1994; pp. 11–67. [Google Scholar]
- El Ouardi, Y.; Branger, C.; Toufik, H.; Laatikainen, K.; Ouammou, A.; Lenoble, V. An Insight of Enhanced Natural Material (Calcined Diatomite) Efficiency in Nickel and Silver Retention: Application to Natural Effluents. Environ. Technol. Innov. 2020, 18, 100768. [Google Scholar] [CrossRef]
- Zaitan, H.; Bianchi, D.; Achak, O.; Chafik, T. A Comparative Study of the Adsorption and Desorption of O-Xylene onto Bentonite Clay and Alumina. J. Hazard. Mater. 2008, 153, 852–859. [Google Scholar] [CrossRef]
- Jović-Jovičić, N.; Milutinović-Nikolić, A.; Banković, P.; Dojčinović, B.; Nedić, B.; Gržetić, I.; Jovanović, D. Synthesis, Characterization and Adsorptive Properties of Organobentonites. Acta Phys. Pol. A 2010, 117, 849–854. [Google Scholar] [CrossRef]
- Zhirong, L.; Azhar Uddin, M.; Zhanxue, S. FT-IR and XRD Analysis of Natural Na-Bentonite and Cu(II)-Loaded Na-Bentonite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1013–1016. [Google Scholar] [CrossRef]
- Madejová, J.; Gates, W.P.; Petit, S. IR Spectra of Clay Minerals. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2017; pp. 107–149. [Google Scholar]
- Faghihian, H.; Kabiri-Tadi, M. Removal of Zirconium from Aqueous Solution by Modified Clinoptilolite. J. Hazard. Mater. 2010, 178, 66–73. [Google Scholar] [CrossRef]
- Fernández-Pérez, A.; Valdés-Solís, T.; Marbán, G. Visible Light Spectroscopic Analysis of Methylene Blue in Water; the Resonance Virtual Equilibrium Hypothesis. Dye. Pigment. 2019, 161, 448–456. [Google Scholar] [CrossRef]
- Kahr, G.; Madsen, F.T. Determination of the Cation Exchange Capacity and the Surface Area of Bentonite, Illite and Kaolinite by Methylene Blue Adsorption. Appl. Clay Sci. 1995, 9, 327–336. [Google Scholar] [CrossRef]
- Yang, R.T. Adsorption: Fundamentals and Applications; Wiley-Interscience: Hoboken, NJ, USA, 2003; ISBN 0-471-29741-0. [Google Scholar]
- Rupar, J.; Bajuk-Bogdanović, D.; Milojević-Rakić, M.; Krstić, J.; Upadhyay, K.; Gavrilov, N.; Janošević Ležaić, A. Tailored Porosity Development in Carbons via Zn2+ Monodispersion: Fitting Supercapacitors. Microporous Mesoporous Mater. 2022, 335, 111790. [Google Scholar] [CrossRef]
- Gavrilov, N.; Pašti, I.A.; Mitrić, M.; Travas-Sejdić, J.; Ćirić-Marjanović, G.; Mentus, S.V. Electrocatalysis of Oxygen Reduction Reaction on Polyaniline-Derived Nitrogen-Doped Carbon Nanoparticle Surfaces in Alkaline Media. J. Power Sources 2012, 220, 306–316. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, R.; Wei, W.; Wei, W.; Zou, W.; Li, J.; Ni, B.-J.; Chen, H. Recycling Spent Water Treatment Adsorbents for Efficient Electrocatalytic Water Oxidation Reaction. Resour. Conserv. Recycl. 2022, 178, 106037. [Google Scholar] [CrossRef]
- Pérez-Díaz, P.J.; Medina-Ramírez, A.; Esquivel, I.R.G.; Ruiz, G.G.; Ruiz-Camacho, B. Effect of X Zeolite-Carbon Composite Ratio as Support of Pt Nanoparticles for MOR and ORR. Ionics 2021, 27, 1813–1828. [Google Scholar] [CrossRef]
- Katafias, A.; Lipińska, M.; Strutyński, K. Alkaline Hydrogen Peroxide as a Degradation Agent of Methylene Blue—Kinetic and Mechanistic Studies. React. Kinet. Mech. Catal. 2010, 101, 251–266. [Google Scholar] [CrossRef]
- Zhan, R.; Song, S.; Liu, Y.; Dong, S. Mechanisms of Methylene Blue Electrode Processes Studied by in Situ Electron Paramagnetic Resonance and Ultraviolet–Visible Spectroelectrochemistry. J. Chem. Soc. Faraday Trans. 1990, 86, 3125–3127. [Google Scholar] [CrossRef]
- Adamčíková, L.; Pavlíková, K.; Ševčík, P. The Decay of Methylene Blue in Alkaline Solution. React. Kinet. Catal. Lett. 2000, 69, 91–94. [Google Scholar] [CrossRef]
- Salamifar, S.E.; Mehrgardi, M.A.; Kazemi, S.H.; Mousavi, M.F. Cyclic Voltammetry and Scanning Electrochemical Microscopy Studies of Methylene Blue Immobilized on the Self-Assembled Monolayer of n-Dodecanethiol. Electrochim. Acta 2010, 56, 896–904. [Google Scholar] [CrossRef]
- Kelley, S.O.; Barton, J.K.; Jackson, N.M.; Hill, M.G. Electrochemistry of Methylene Blue Bound to a DNA-Modified Electrode. Bioconjugate Chem. 1997, 8, 31–37. [Google Scholar] [CrossRef]
- Begum, R.; Najeeb, J.; Sattar, A.; Naseem, K.; Irfan, A.; Al-Sehemi, A.G.; Farooqi, Z.H. Chemical Reduction of Methylene Blue in the Presence of Nanocatalysts: A Critical Review. Rev. Chem. Eng. 2020, 36, 749–770. [Google Scholar] [CrossRef]
- Pegu, R.; Majumdar, K.J.; Talukdar, D.J.; Pratihar, S. Oxalate Capped Iron Nanomaterial: From Methylene Blue Degradation to Bis(Indolyl)Methane Synthesis. RSC Adv. 2014, 4, 33446–33456. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Y.; Feng, Y.; Xie, X.; Li, X.; Yang, S. Different Adsorption-Degradation Behavior of Methylene Blue and Congo Red in Nanoceria/H2O2 System under Alkaline Conditions. Sci. Rep. 2019, 9, 4964. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popadić, D.; Gavrilov, N.; Ignjatović, L.; Krajišnik, D.; Mentus, S.; Milojević-Rakić, M.; Bajuk-Bogdanović, D. How to Obtain Maximum Environmental Applicability from Natural Silicates. Catalysts 2022, 12, 519. https://doi.org/10.3390/catal12050519
Popadić D, Gavrilov N, Ignjatović L, Krajišnik D, Mentus S, Milojević-Rakić M, Bajuk-Bogdanović D. How to Obtain Maximum Environmental Applicability from Natural Silicates. Catalysts. 2022; 12(5):519. https://doi.org/10.3390/catal12050519
Chicago/Turabian StylePopadić, Daliborka, Nemanja Gavrilov, Ljubiša Ignjatović, Danina Krajišnik, Slavko Mentus, Maja Milojević-Rakić, and Danica Bajuk-Bogdanović. 2022. "How to Obtain Maximum Environmental Applicability from Natural Silicates" Catalysts 12, no. 5: 519. https://doi.org/10.3390/catal12050519
APA StylePopadić, D., Gavrilov, N., Ignjatović, L., Krajišnik, D., Mentus, S., Milojević-Rakić, M., & Bajuk-Bogdanović, D. (2022). How to Obtain Maximum Environmental Applicability from Natural Silicates. Catalysts, 12(5), 519. https://doi.org/10.3390/catal12050519








