Mechanism Insight into Catalytic Performance of Ni12P5 over Ni2P toward the Catalytic Deoxygenation of Butyric Acid
Abstract
:1. Introduction
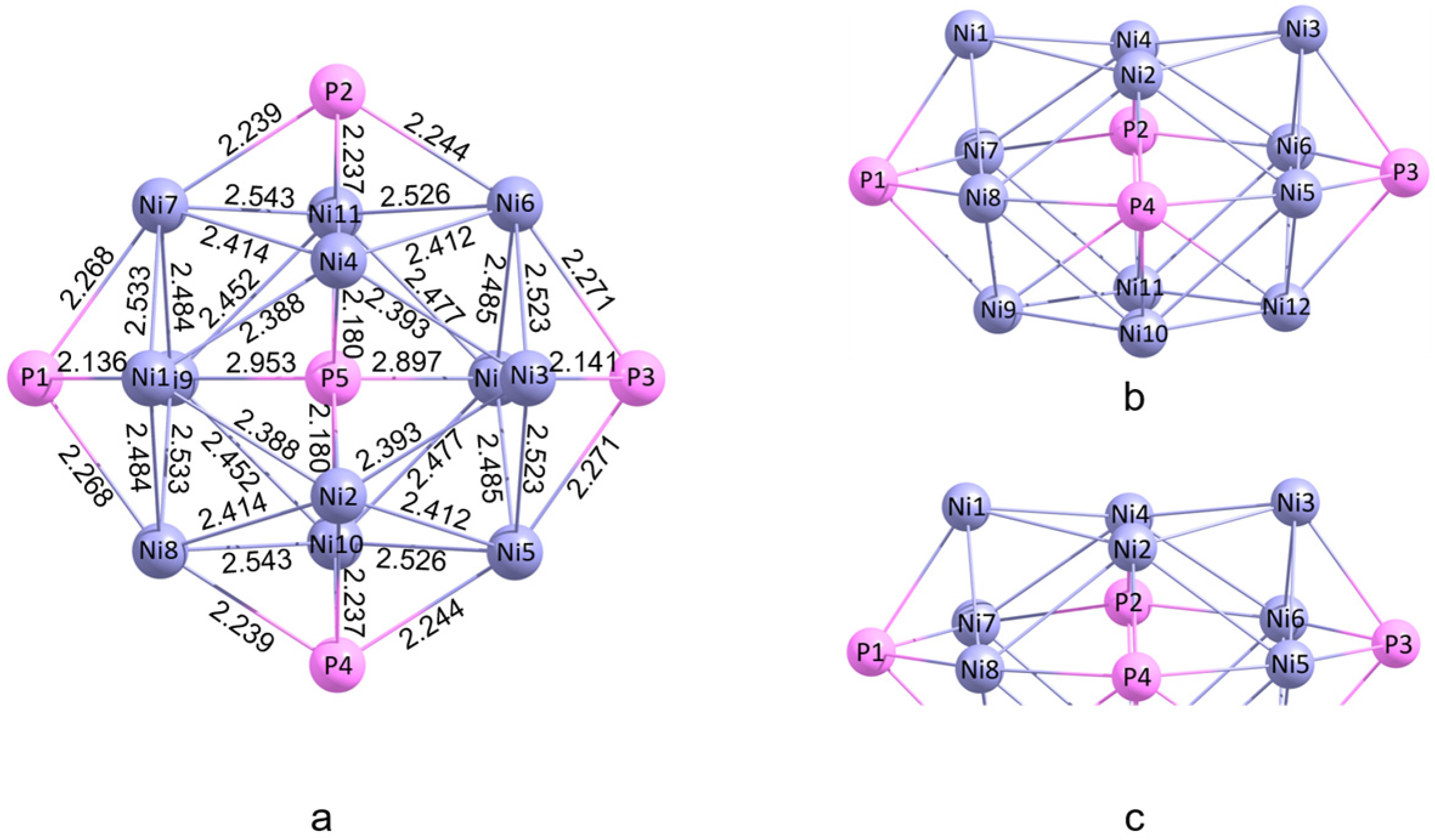
2. Computational Methods
3. Results and Discussion
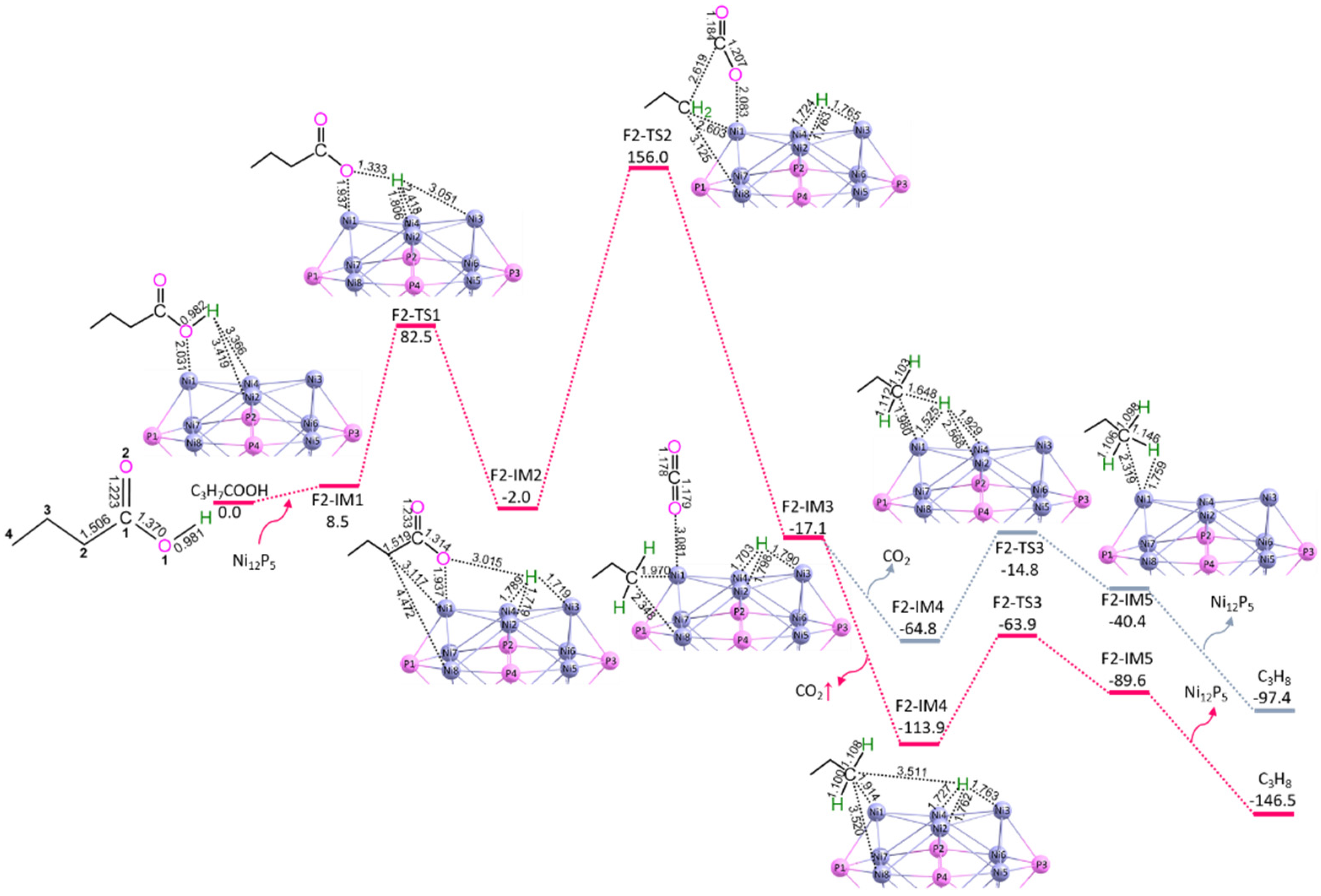
3.1. C3H7COOH → C3H8 + CO2
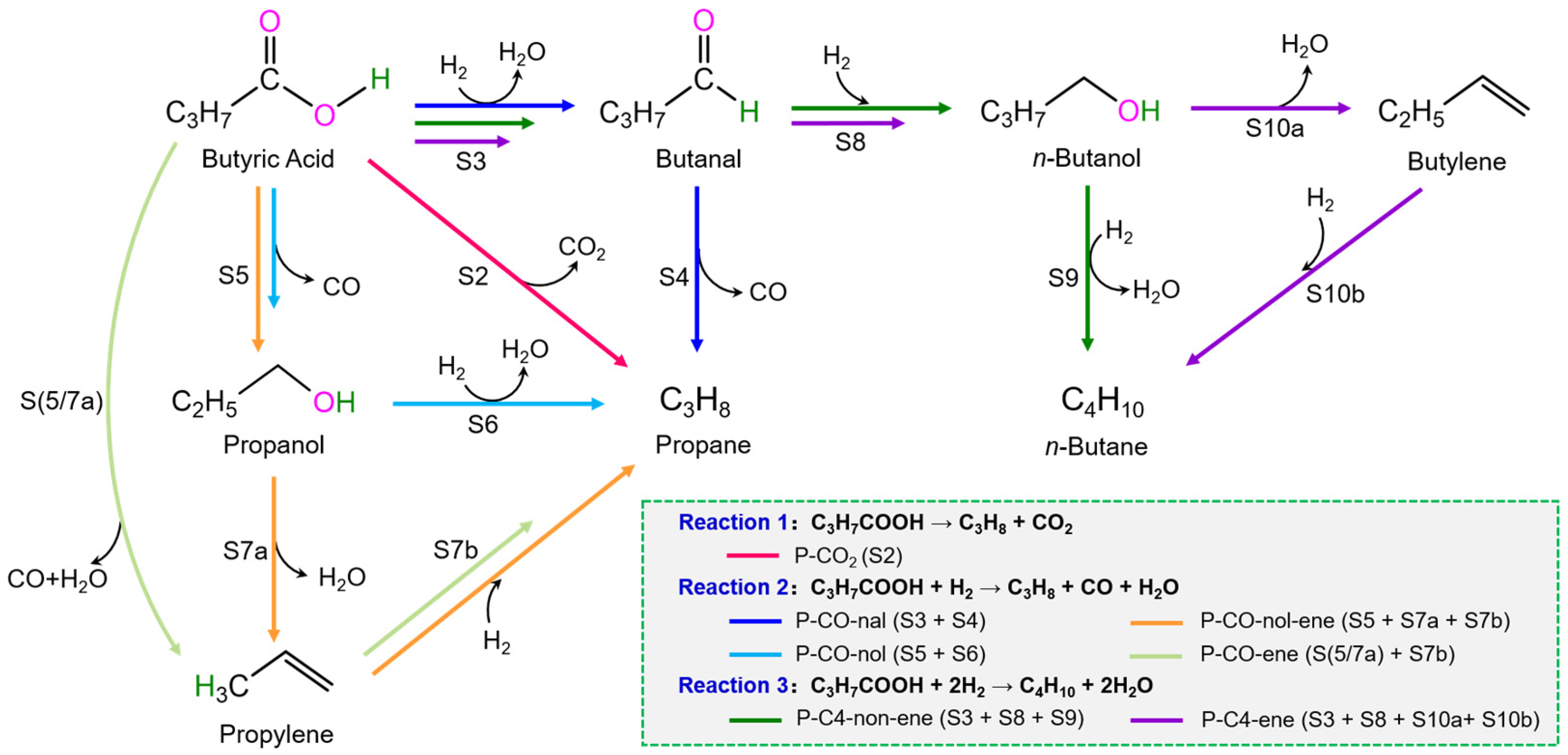
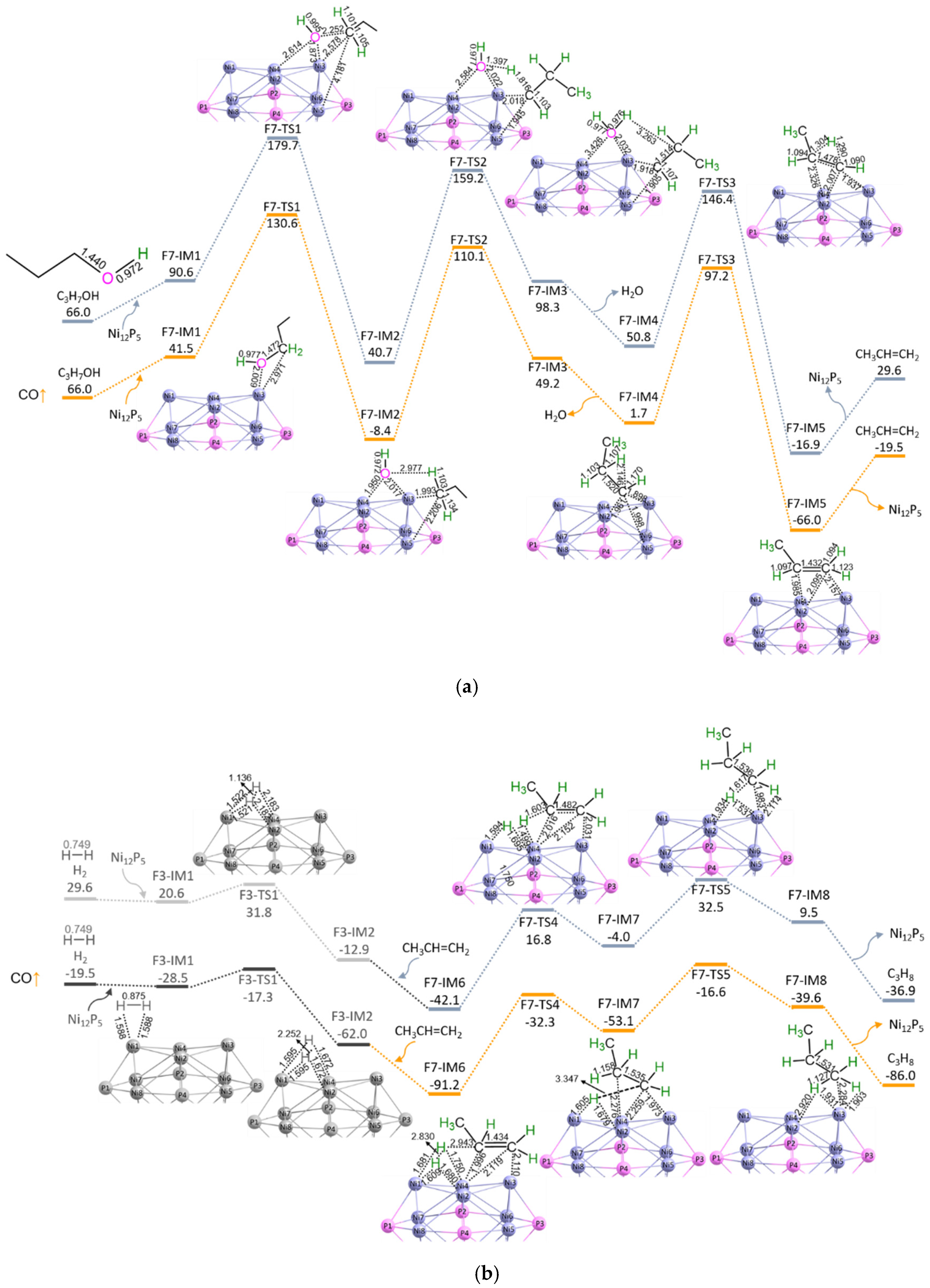
3.2. C3H7COOH + H2 → C3H8 + CO + H2O
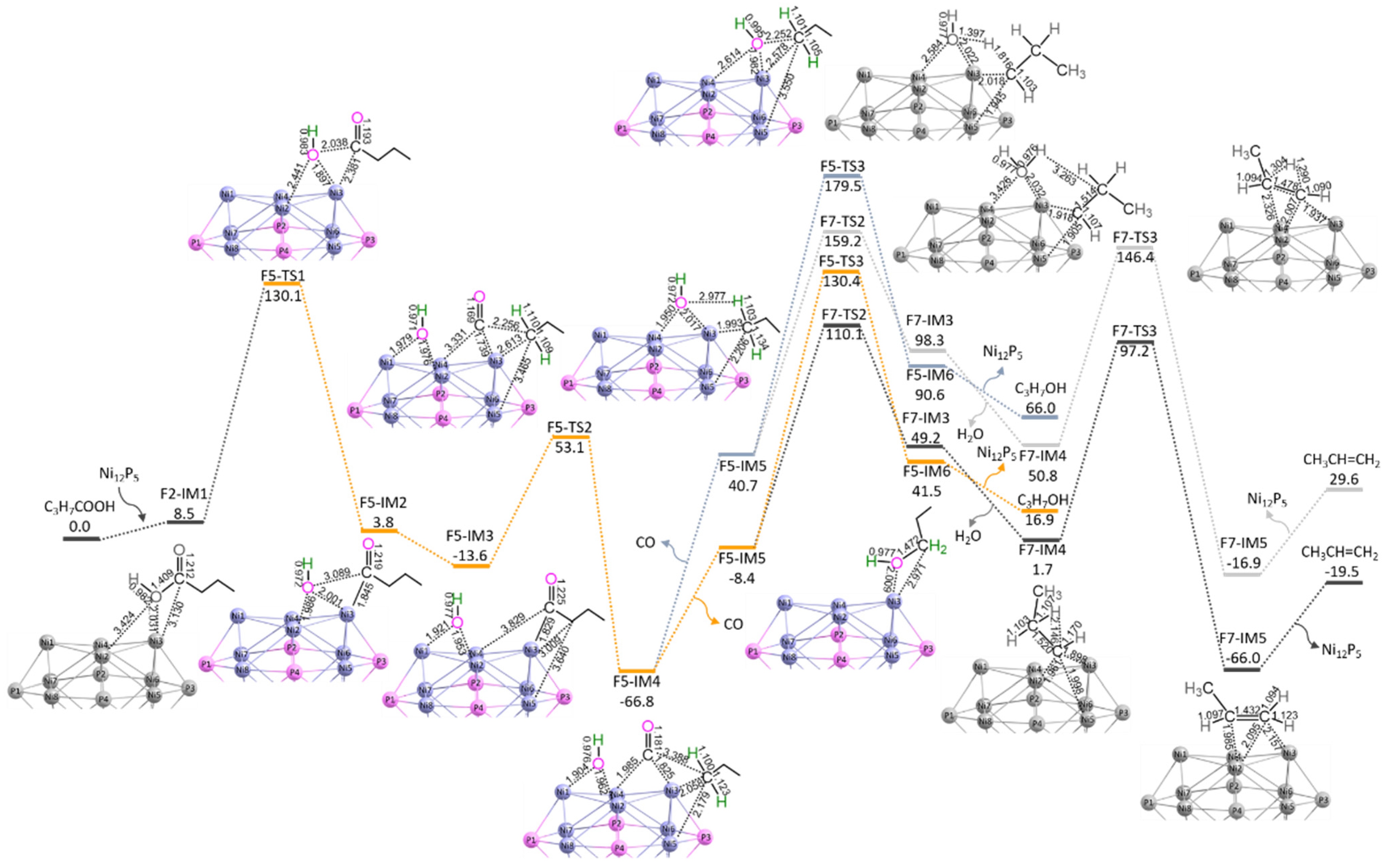
3.2.1. P-CO-nal (S3 + S4)
3.2.2. P-CO-nol (S5 + S6)
3.2.3. P-CO-nol-ene (S5 + S7a + S7b)
3.2.4. P-CO-ene (S5/7a + S7b)
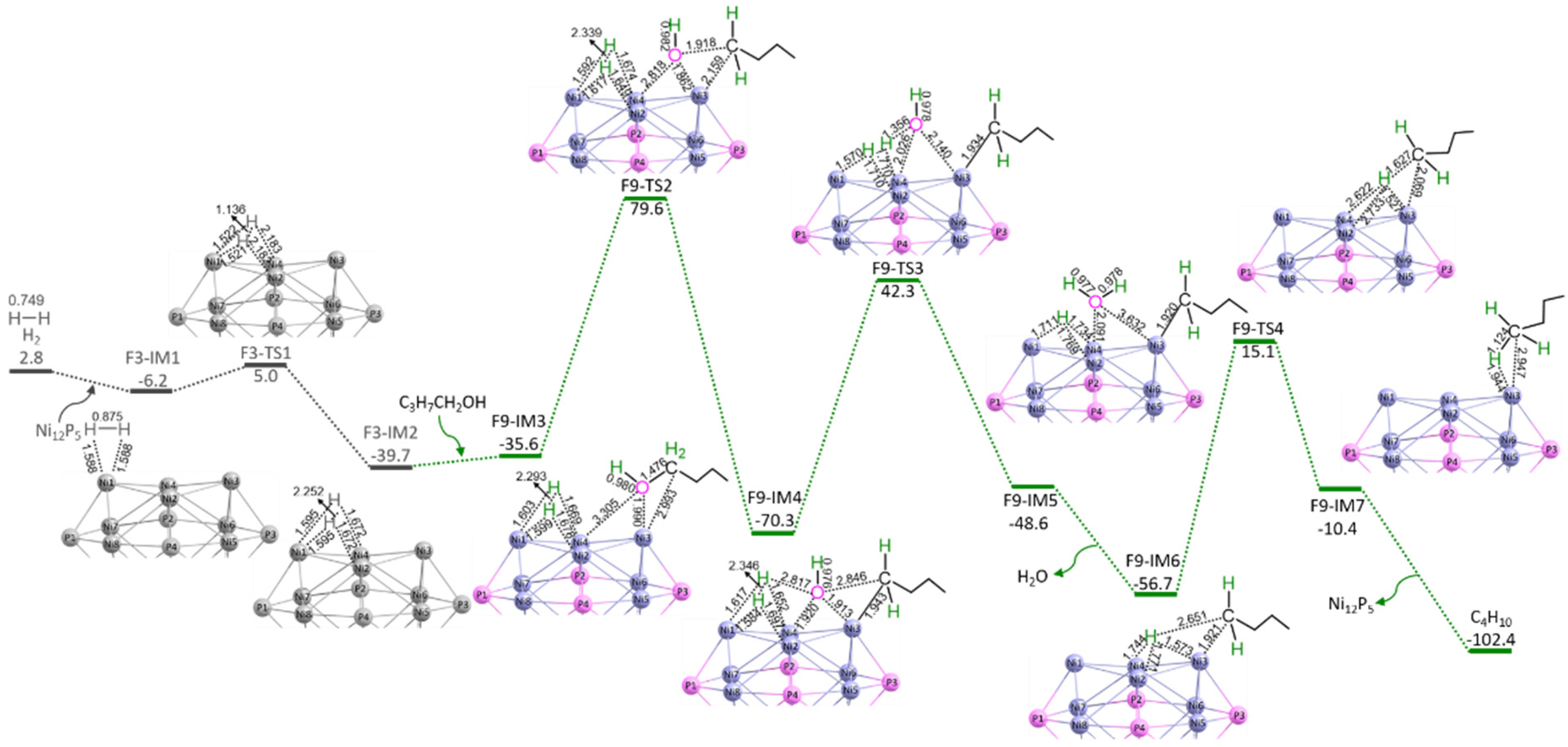
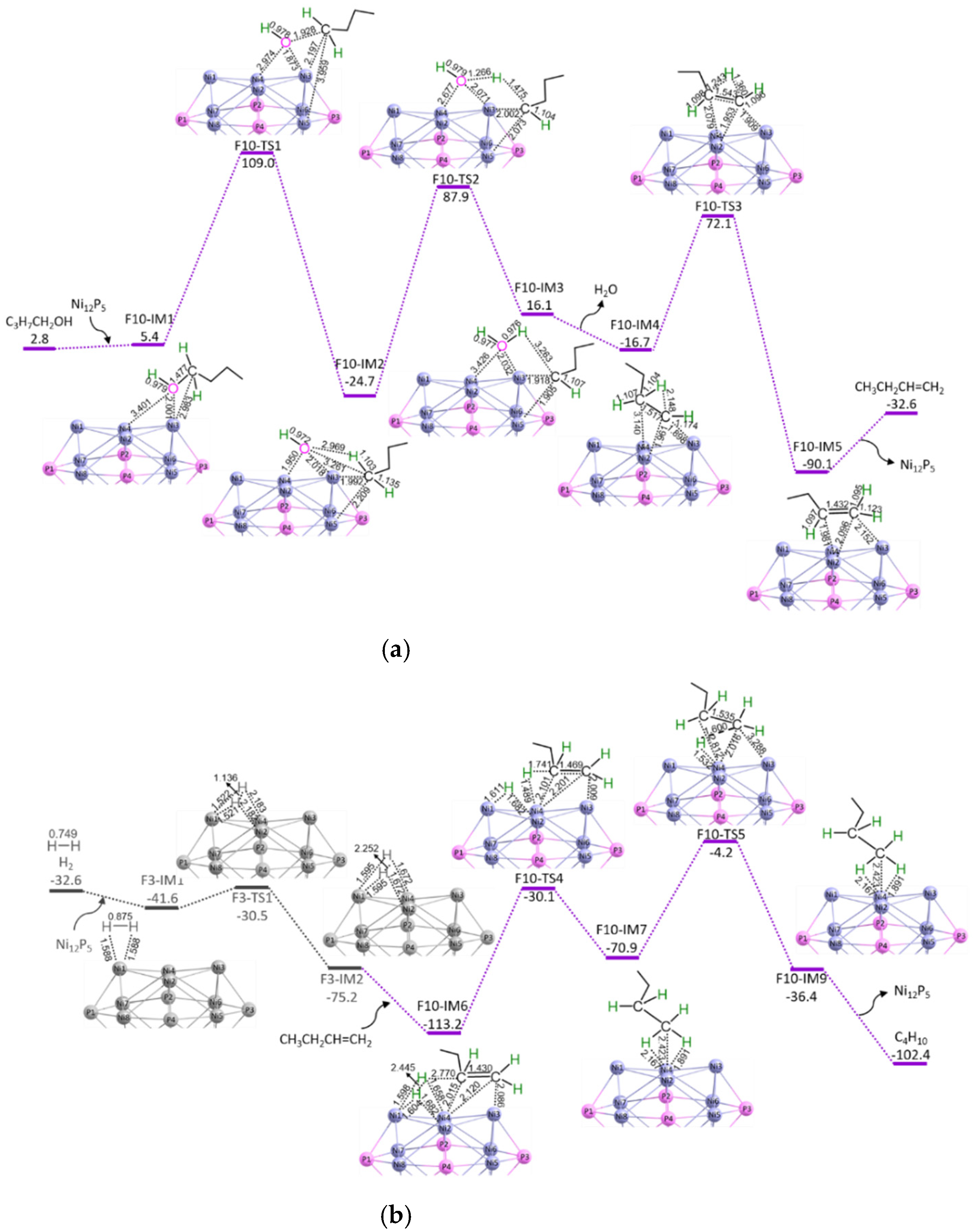
3.3. C3H7COOH + 2H2 → C4H10 + 2H2O
3.3.1. P-C4-non-ene (S3 + S8 + S9)
3.3.2. P-C4-ene (S3 + S8 + S10a + S10b)
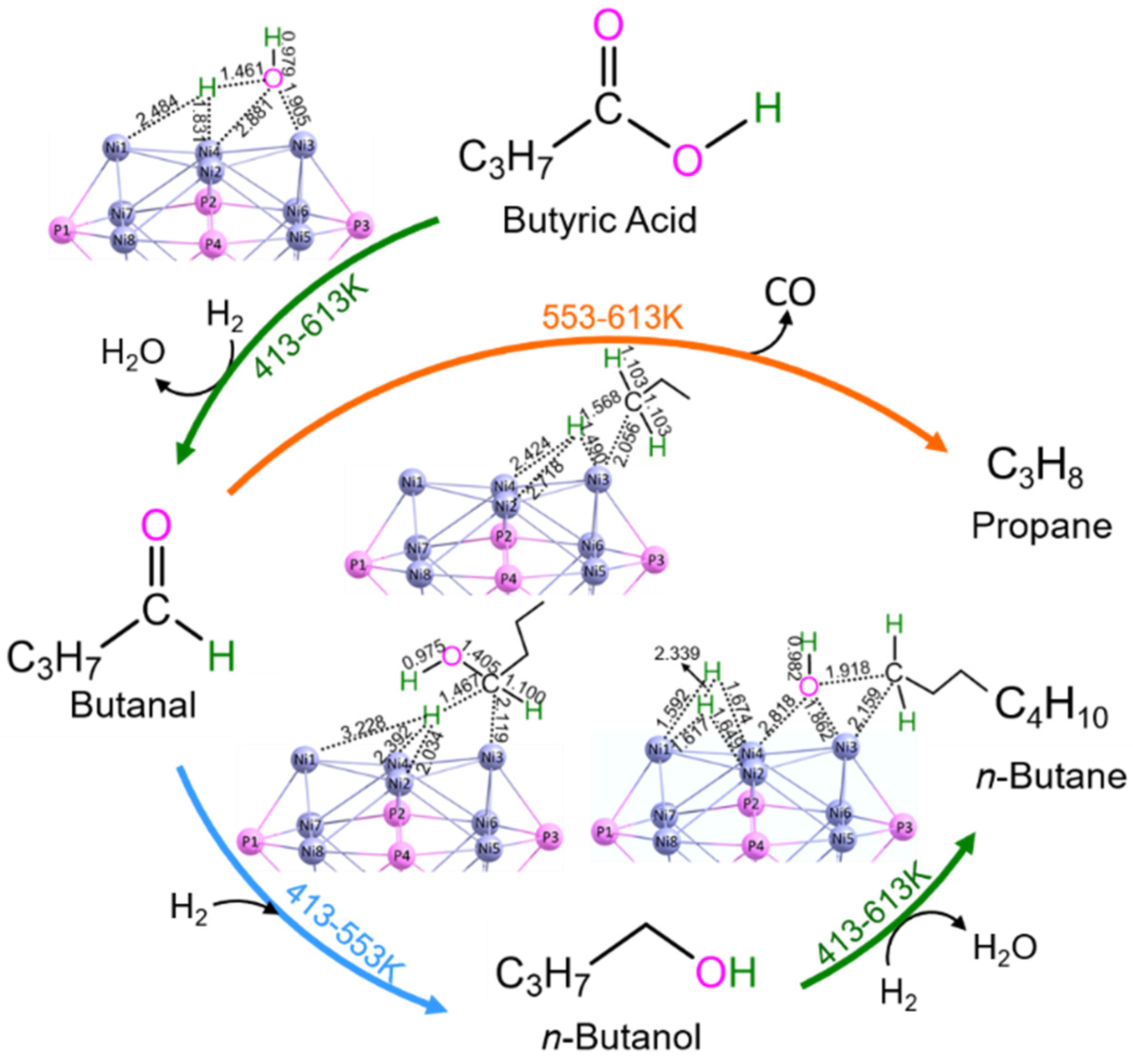
3.4. Catalytic Selectivity Dependent on Temperature
3.4.1. From Butyric Acid
3.4.2. From Butanal
3.4.3. From n-Butanol
3.5. Comparison of Ni12P5 Cluster with Ni12P6 Cluster in the Catalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ooi, X.Y.; Gao, W.; Ong, H.C.; Lee, H.V.; Juan, J.C.; Chen, W.H.; Lee, K.T. Overview on catalytic deoxygenation for biofuel synthesis using metal oxide supported catalysts. Renew. Sustain. Energy Rev. 2019, 112, 834–852. [Google Scholar] [CrossRef]
- Norouzi, N.; Talebi, S. An overview on the green petroleum production. Chem. Rev. Lett. 2020, 3, 38–52. [Google Scholar] [CrossRef]
- Mahdi, H.I.; Bazargan, A.; McKay, G.; Azelee, N.I.W.; Meili, L. Catalytic deoxygenation of palm oil and its residue in green diesel production: A current technological review. Chem. Eng. Res. Des. 2021, 174, 158–187. [Google Scholar] [CrossRef]
- Itthibenchapong, V.; Srifa, A.; Kaewmeesri, R.; Kidkhunthod, P.; Faungnawakij, K. Deoxygenation of palm kernel oil to jet fuel-like hydrocarbons using Ni-MoS2/γ-Al2O3 catalysts. Energy Convers. Manag. 2017, 134, 188–196. [Google Scholar] [CrossRef]
- Loe, R.; Santillan-Jimenez, E.; Morgan, T.; Sewell, L.; Ji, Y.Y.; Jones, S.; Isaacs, M.A.; Lee, A.F.; Crocker, M. Effect of Cu and Sn promotion on the catalytic deoxygenation of model and algal lipids to fuel-like hydrocarbons over supported Ni catalysts. Appl. Catal. B 2016, 191, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Xin, H.; Guo, K.; Li, D.; Yang, H.Q.; Hu, C.W. Production of high-grade diesel from palmitic acid over activated carbon-supported nickel phosphide catalysts. Appl. Catal. B 2016, 187, 375–385. [Google Scholar] [CrossRef]
- Wongnongwa, Y.; Jungsuttiwong, S.; Pimsuta, M.; Khemthong, P.; Kunaseth, M. Mechanistic and thermodynamic insights into the deoxygenation of palm oils using Ni2P catalyst: A combined experimental and theoretical study. Chem. Eng. J. 2020, 399, 125586. [Google Scholar] [CrossRef]
- Hermida, L.; Abdullah, A.Z.; Mohamed, A.R. Deoxygenation of fatty acid to produce diesel-like hydrocarbons: A review of process conditions, reaction kinetics and mechanism. Renew. Sustain. Energy Rev. 2015, 42, 1223–1233. [Google Scholar] [CrossRef]
- Hongloi, N.; Prapainainar, P.; Prapainainar, C. Review of green diesel production from fatty acid deoxygenation over Ni-based catalysts. Mol. Catal. 2022, 523, 111696. [Google Scholar] [CrossRef]
- Zhang, J.; Huo, X.C.; Li, Y.L.; Strathmann, T.J. Catalytic hydrothermal decarboxylation and cracking of fatty acids and lipids over Ru/C. ACS Sustain. Chem. Eng. 2019, 7, 14400–14410. [Google Scholar] [CrossRef]
- Liu, X.; Yang, M.; Deng, Z.H.; Dasgupta, A.; Guo, Y. Hydrothermal hydrodeoxygenation of palmitic acid over Pt/C catalyst: Mechanism and kinetic modeling. Chem. Eng. J. 2021, 407, 126332. [Google Scholar] [CrossRef]
- Yoosuk, B.; Sanggam, P.; Wiengket, S.; Prasassarakich, P. Hydrodeoxygenation of oleic acid and palmitic acid to hydrocarbon-like biofuel over unsupported Ni-Mo and Co-Mo sulfide catalysts. Renew. Energy 2019, 139, 1391–1399. [Google Scholar] [CrossRef]
- Ansari, K.B.; Gaikar, V.G. Investigating production of hydrocarbon rich bio-oil from grassy biomass using vacuum pyrolysis coupled with online deoxygenation of volatile products over metallic iron. Renew. Energy 2019, 130, 305–318. [Google Scholar] [CrossRef]
- Peroni, M.; Mancino, G.; Baráth, E.; Gutiérrez, O.Y.; Lercher, J.A. Bulk and γ-Al2O3-supported Ni2P and MoP for hydrodeoxygenation of palmitic acid. Appl. Catal. B 2016, 180, 301–311. [Google Scholar] [CrossRef]
- Toba, M.; Abe, Y.; Kuramochi, H.; Osako, M.; Mochizuki, T.; Yoshimura, Y. Hydrodeoxygenation of waste vegetable oil over sulfide catalysts. Catal. Today 2011, 164, 533–537. [Google Scholar] [CrossRef]
- Peroni, M.; Lee, I.; Huang, X.Y.; Baráth, E.; Gutiérrez, O.Y.; Lercher, J.A. Deoxygenation of palmitic acid on unsupported transition-metal phosphides. ACS Catal. 2017, 7, 6331–6341. [Google Scholar] [CrossRef]
- Liu, Y.H.; Yao, L.; Xin, H.; Wang, G.S.; Li, D.; Hu, C.W. The production of diesel-like hydrocarbons from palmitic acid over HZSM-22 supported nickel phosphide catalysts. Appl. Catal. B 2015, 174–175, 504–514. [Google Scholar] [CrossRef]
- Zhou, W.J.; Xin, H.; Yang, H.R.; Du, X.Z.; Yang, R.; Li, D.; Hu, C.W. The deoxygenation pathways of palmitic acid into hydrocarbons on silica-supported Ni12P5 and Ni2P catalysts. Catalysts 2018, 8, 153. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.; Wang, Z.M.; Liu, L.J.; Liu, T.H.; Li, D.; Yang, H.Q.; Hu, C.W. Theoretical insight into the deoxygenation molecular mechanism of butyric acid catalyzed by a Ni12P6 cluster. Catal. Sci. Technol. 2021, 11, 6425–6437. [Google Scholar] [CrossRef]
- Rundqvist, S.; Larsson, E. The Crystal Structure of Ni12P5. Acta Chem. Scand. 1959, 13, 551–560. [Google Scholar] [CrossRef]
- Delley, B. The conductor-like screening model for polymers and surfaces. Mol. Simul. 2006, 32, 117–123. [Google Scholar] [CrossRef]
- Klamt, A. The COSMO and COSMO-RS solvation models. WIREs Comput. Mol. Sci. 2018, 8, e1338. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Materials Studio, version 7.0; Accelrys Software Inc.: San Diego, CA, USA, 2013.
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Delley, B. Hardness conserving semilocal pseudopotentials. Phys. Rev. B 2002, 66, 155125. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Amatore, C.; Jutand, A. Mechanistic and kinetic studies of palladium catalytic systems. J. Organomet. Chem. 1999, 576, 254–278. [Google Scholar] [CrossRef]
- Kozuch, S.; Shalk, S. A Combined kinetic-quantum mechanical model for assessment of catalytic cycles: Application to cross-coupling and heck reactions. J. Am. Chem. Soc. 2006, 128, 3355–3365. [Google Scholar] [CrossRef]
- Kozuch, S.; Shalk, S. Kinetic-quantum chemical model for catalytic cycles: The haber-bosch process and the effect of reagent concentratio. J. Phys. Chem. A 2008, 112, 6032–6041. [Google Scholar] [CrossRef]
- Uhe, A.; Kozuch, S.; Shaik, S. Automatic analysis of computed catalytic cycles. J. Comput. Chem. 2011, 32, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Kozuch, S.; Shalk, S. How to Conceptualize Catalytic Cycles? The Energetic Span Model. Acc. Chem. Res. 2011, 44, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kozuch, S. Steady State Kinetics of Any Catalytic Network: Graph Theory, the Energy Span Model, the Analogy between Catalysis and Electrical Circuits, and the Meaning of “Mechanism”. ACS Catal. 2015, 5, 5242–5255. [Google Scholar] [CrossRef]
- Ren, L.K.; Yang, H.Q.; Hu, C.W. Theoretical study of the catalytic oxidation mechanism of 5-hydroxymethylfurfural to 2,5-diformylfuran by PMo-containing Keggin heteropolyacid. Catal. Sci. Technol. 2016, 6, 3776–3787. [Google Scholar] [CrossRef]
- Wigner, E. Calculation of the Rate of Elementary Association Reactions. J. Chem. Phys. 1937, 5, 720–725. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, S.; Li, D.; Liu, T.; Liu, L.; Yang, H.; Hu, C. Mechanism Insight into Catalytic Performance of Ni12P5 over Ni2P toward the Catalytic Deoxygenation of Butyric Acid. Catalysts 2022, 12, 569. https://doi.org/10.3390/catal12050569
Fu S, Li D, Liu T, Liu L, Yang H, Hu C. Mechanism Insight into Catalytic Performance of Ni12P5 over Ni2P toward the Catalytic Deoxygenation of Butyric Acid. Catalysts. 2022; 12(5):569. https://doi.org/10.3390/catal12050569
Chicago/Turabian StyleFu, Shuai, Dan Li, Tinghao Liu, Lijuan Liu, Huaqing Yang, and Changwei Hu. 2022. "Mechanism Insight into Catalytic Performance of Ni12P5 over Ni2P toward the Catalytic Deoxygenation of Butyric Acid" Catalysts 12, no. 5: 569. https://doi.org/10.3390/catal12050569
APA StyleFu, S., Li, D., Liu, T., Liu, L., Yang, H., & Hu, C. (2022). Mechanism Insight into Catalytic Performance of Ni12P5 over Ni2P toward the Catalytic Deoxygenation of Butyric Acid. Catalysts, 12(5), 569. https://doi.org/10.3390/catal12050569





