Pt/C-TiO2 as Oxygen Reduction Electrocatalysts against Sulfur Poisoning
Abstract
:1. Introduction
2. Results and Discussion
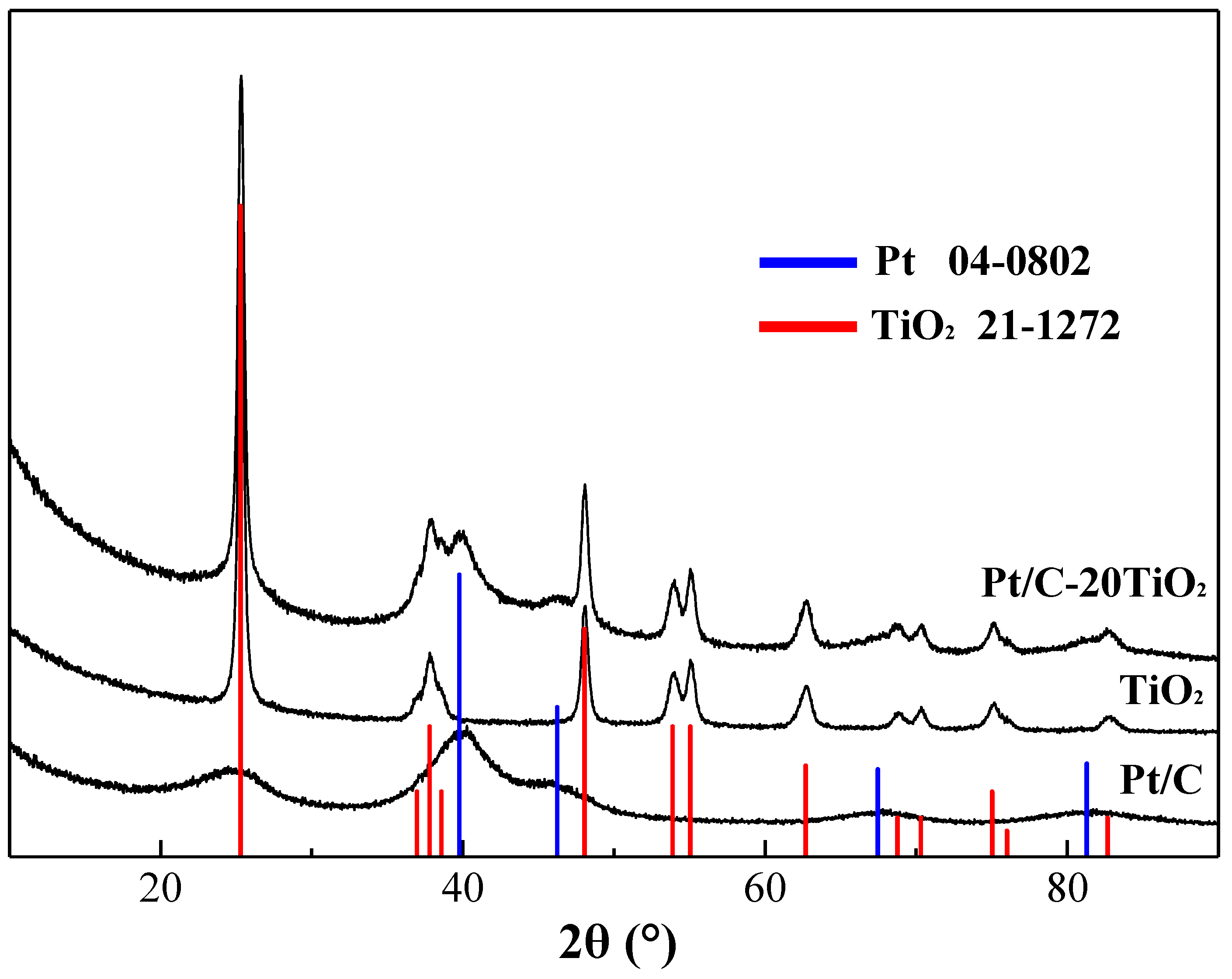
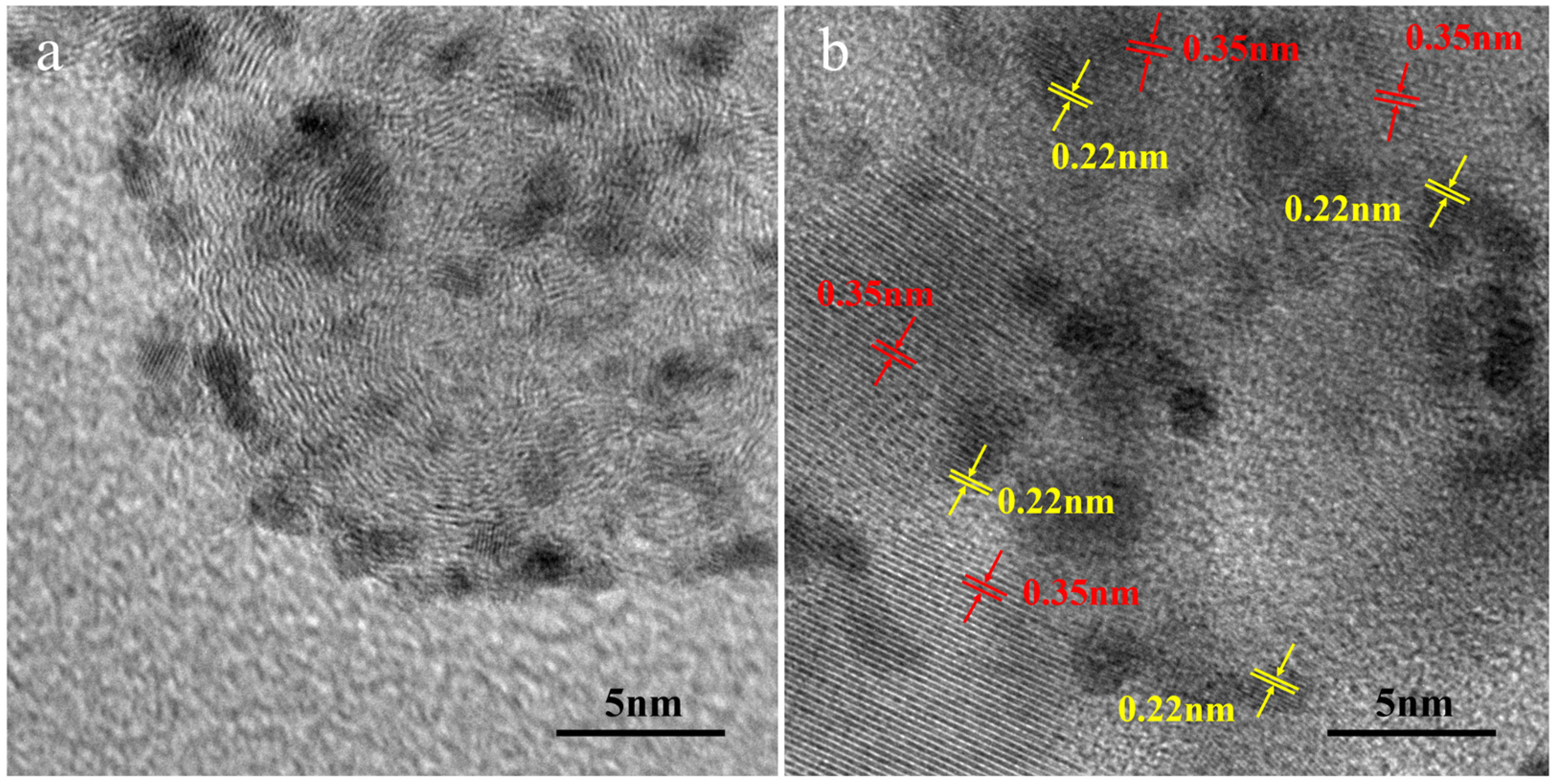
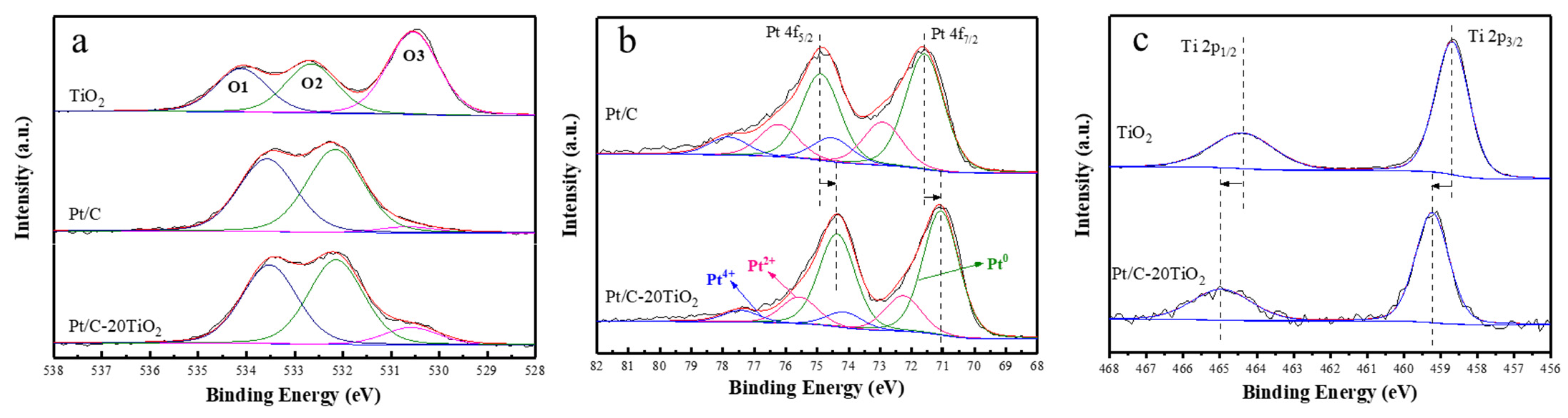
2.1. Characterization of Catalysts
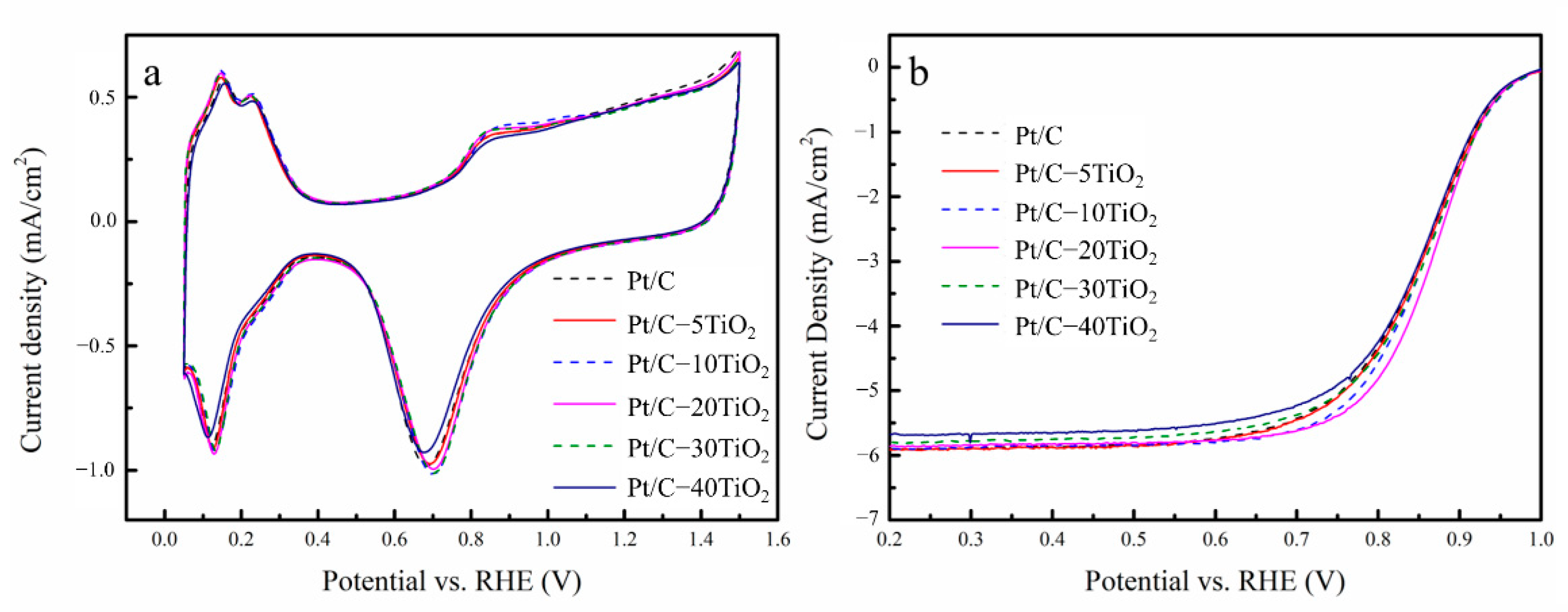
2.2. Electrocatalytic Performance
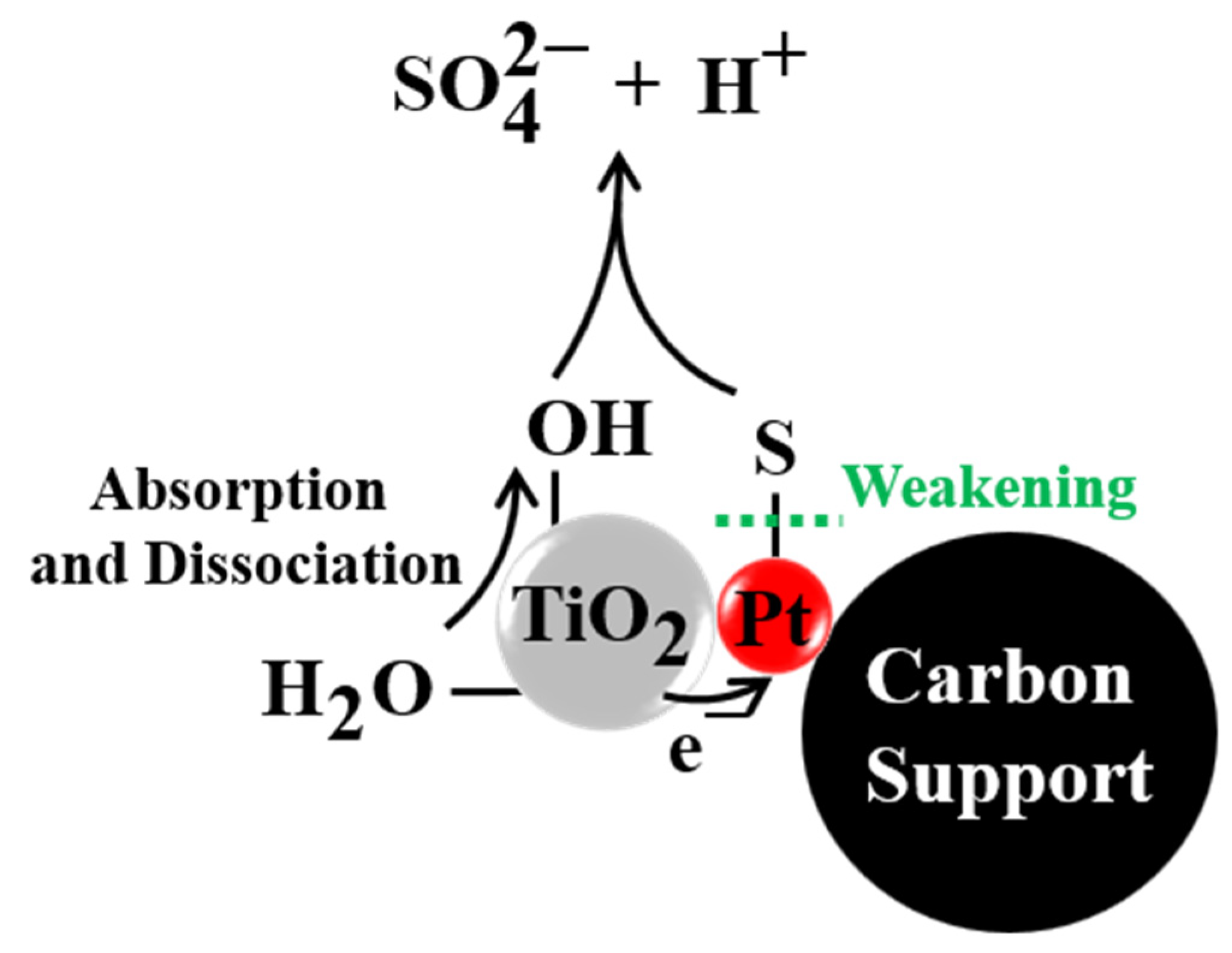
2.3. Mechanism of SO2 Tolerance
3. Materials and Methods
3.1. Chemicals and Materials Characterization
3.2. Synthesis of Catalysts
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, L.; Prabhakaran, V.; Xie, X.; Park, S.; Wang, Y.; Shao, Y. Low-PGM and PGM-free catalysts for proton exchange membrane fuel cells: Stability challenges and material solutions. Adv. Mater. 2021, 33, 1908232. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, B.; Hao, J.; Zhao, J.; Li, J.; Khakichi, A. Economical planning of fuel cell vehicle-to-grid integrated green buildings with a new hybrid optimization algorithm. Int. J. Hydrogen Energy 2022, 47, 8514–8531. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, T.; Wang, Z.; Xu, Y.; Li, X.; Wang, L.; Wang, H. Defect-Rich Porous Pd Metallene for Enhanced Alkaline Oxygen Reduction Electrocatalysis. Angew. Chem. Int. Ed. Engl. 2021, 133, 12134–12138. [Google Scholar] [CrossRef]
- Singh, P.; Gangadharan, P.K.; Khan, Z.; Kurungot, S.; Jaiswal, A. Cubic palladium nanorattles with solid octahedron gold core for catalysis and alkaline membrane fuel cell Applications. ChemCatChem 2019, 11, 4383–4392. [Google Scholar] [CrossRef]
- Malko, D.; Lopes, T.; Symianakis, E.; Kucernak, A. The intriguing poison tolerance of non-precious metal oxygen reduction reaction (ORR) catalysts. J. Mater. Chem. 2016, 4, 142–152. [Google Scholar] [CrossRef] [Green Version]
- Jing, F.; Hou, M.; Shi, W.; Fu, J.; Yu, H.; Ming, P.; Yi, B. The effect of ambient contamination on PEMFC performance. J. Power Sources 2007, 166, 172–176. [Google Scholar] [CrossRef]
- Garsany, Y.; Gould, B.D.; Baturina, O.A.; Swider-Lyons, K.E. Comparison of the Sulfur Poisoning of PBI and Nafion PEMFC Cathodes. Electrochem. Solid-State Lett. 2009, 12, B138. [Google Scholar] [CrossRef]
- Baturina, O.A.; Gould, B.D.; Korovina, A.; Garsany, Y.; Stroman, R.; Northrup, P.A. Products of SO2 adsorption on fuel cell electrocatalysts by combination of sulfur K-edge XANES and electrochemistry. Langmuir 2011, 27, 14930–14939. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Zhang, W.-Y.; Han, G.-K.; Zhou, Y.-W.; Li, L.-F.; Kang, C.; Kong, F.-P.; Gao, Y.-Z.; Du, C.-Y.; Wang, J.-J. Deactivation and regeneration of a benchmark Pt/C catalyst toward oxygen reduction reaction in the presence of poisonous SO2 and NO. Catal. Sci. Technol. 2022, accepted. [Google Scholar] [CrossRef]
- Awad, M.I.; Saleh, M.M.; Ohsaka, T. Impact of SO2 poisoning of platinum nanoparticles modified glassy carbon electrode on oxygen reduction. J. Power Sources 2011, 196, 3722–3728. [Google Scholar] [CrossRef]
- Reshetenko, T.; Laue, V.; Krewer, U.; Artyushkova, K. Study of degradation and spatial performance of low Pt-loaded proton exchange membrane fuel cells under exposure to sulfur dioxide in an oxidant stream. J. Power Sources 2020, 458, 228032. [Google Scholar] [CrossRef]
- Awad, M.I.; Saleh, M.M.; Ohsaka, T. Electroactivity regeneration of sulfur-poisoned platinum nanoparticle-modified glassy carbon electrode at low anodic potentials. J. Solid State Electrochem. 2015, 19, 1331–1340. [Google Scholar] [CrossRef]
- Borup, R.; Weber, A. Fuel Cell Performance and Durability Consortium. Available online: https://www.hydrogen.energy.gov/pdfs/review19/fc135_borup_2019_o.pdf (accessed on 30 April 2019).
- Mohtadi, R.; Lee, W.K.; Van Zee, J.W. Assessing durability of cathodes exposed to common air impurities. J. Power Sources 2004, 138, 216–225. [Google Scholar] [CrossRef]
- Garsany, Y.; Baturina, O.A.; Swider-Lyons, K.E. Impact of sulfur dioxide on the oxygen reduction reaction at Pt/Vulcan carbon electrocatalysts. J. Electrochem. Soc. 2007, 154, B670–B675. [Google Scholar] [CrossRef]
- Xie, F.; Shao, Z.-G.; Zhang, G.; Zhai, J.; Lu, W.; Qin, X.; Lin, W.; Yi, B. A quantitative research on S- and SO2-poisoning Pt/Vulcan carbon fuel cell catalyst. Electrochim. Acta 2012, 67, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.X.; Zhang, W.Y.; Han, G.K.; Zhou, Y.W.; Li, L.F.; Kong, F.P.; Gao, Y.Z.; Du, C.Y.; Wang, J.J.; Du, L.; et al. Deactivated Pt Electrocatalysts for the Oxygen Reduction Reaction: The Regeneration Mechanism and a Regenerative Protocol. ACS Catal. 2021, 11, 9293–9299. [Google Scholar] [CrossRef]
- Punyawudho, K.; Ma, S.; Van Zee, J.W.; Monnier, J.R. Effect of O2 on the adsorption of SO2 on carbon-supported Pt electrocatalysts. Langmuir 2011, 27, 7524–7530. [Google Scholar] [CrossRef]
- Von Deak, D.; Singh, D.; Biddinger, E.J.; King, J.C.; Bayram, B.; Miller, J.T.; Ozkan, U.S. Investigation of sulfur poisoning of CNx oxygen reduction catalysts for PEM fuel cells. J. Catal. 2012, 285, 145–151. [Google Scholar] [CrossRef]
- Baturina, O.A.; Gould, B.D.; Garsany, Y.; Swider-Lyons, K.E. Insights on the SO2 poisoning of Pt3Co/VC and Pt/VC fuel cell catalysts. Electrochim. Acta 2010, 55, 6676–6686. [Google Scholar] [CrossRef]
- Ramaker, D.E.; Gatewood, D.; Korovina, A.; Garsany, Y.; Swider-Lyons, K.E. Resolving Sulfur Oxidation and Removal from Pt and Pt3Co Electrocatalysts Using in Situ X-ray Absorption Spectroscopy. J. Phys. Chem. C 2010, 114, 11886–11897. [Google Scholar] [CrossRef]
- Xu, R.; Xu, F.; Pan, M.; Mu, S. Improving sulfur tolerance of noble metal catalysts by tungsten oxide-induced effects. RSC Adv. 2013, 3, 764–773. [Google Scholar] [CrossRef]
- Xu, F.; Cheng, K.; Yu, Y.; Mu, S. One-pot synthesis of Pt/CeO2/C catalyst for enhancing the SO2 electrooxidation. Electrochim. Acta 2017, 229, 253–260. [Google Scholar] [CrossRef]
- Xu, F.; Wang, D.; Sa, B.; Yu, Y.; Mu, S. One-pot synthesis of Pt/CeO2/C catalyst for improving the ORR activity and durability of PEMFC. Int. J. Hydrogen Energy 2017, 42, 13011–13019. [Google Scholar] [CrossRef]
- Liu, Y.; Du, L.; Kong, F.; Han, G.; Gao, Y.; Du, C.; Zuo, P.; Yin, G. Sulfur Dioxide-Tolerant Bimetallic PtRu Catalyst toward Oxygen Electroreduction. ACS Sustain. Chem. Eng. 2019, 8, 1295–1301. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Han, G.; Du, C.; Sun, Y.; Du, L.; An, M.; Yin, G.; Gao, Y.; Song, Y. Superior catalytic performance and CO tolerance of Ru@Pt/C-TiO2 electrocatalyst toward methanol oxidation reaction. Appl. Surf. Sci. 2019, 473, 943–950. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Liu, Y.; Feng, B.; Li, L.; Pan, H.; Kellogg, W.; Higgins, D.; Wu, G. Sn-doped TiO2 modified carbon to support Pt anode catalysts for direct methanol fuel cells. J. Power Sources 2015, 286, 354–361. [Google Scholar] [CrossRef]
- Ruiz-Camacho, B.; Martínez-González, J.; González-Huerta, R.; Tufiño-Velazquez, M. Kinetic study of oxygen reduction reaction and PEM fuel cell performance of Pt/TiO2-C electrocatalyst. Int. J. Hydrogen Energy 2014, 39, 16731–16739. [Google Scholar] [CrossRef]
- Yu, L.; Xi, J. TiO2 nanoparticles promoted Pt/C catalyst for ethanol electro-oxidation. Electrochim. Acta 2012, 67, 166–171. [Google Scholar] [CrossRef]
- Jiang, Z.-Z.; Wang, Z.-B.; Chu, Y.-Y.; Gu, D.-M.; Yin, G.-P. Ultrahigh stable carbon riveted Pt/TiO2–C catalyst prepared by in situ carbonized glucose for proton exchange membrane fuel cell. Energ. Environ. Sci. 2011, 4, 728–735. [Google Scholar] [CrossRef]
- Li, B.; Ding, Y.; Li, Q.; Guan, Z.; Zhang, M.; Yang, J. The photothermal effect enhance visible light-driven hydrogen evolution using urchin-like hollow RuO2/TiO2/Pt/C nanomaterial. J. Alloys Compd. 2022, 890, 161722. [Google Scholar] [CrossRef]
- Haidry, A.A.; Fatima, Q.; Mehmood, A.; Shahzad, A.; Ji, Y.; Saruhan, B. Adsorption Kinetics of NO2 Gas on Pt/Cr-TiO2/Pt-Based Sensors. Chemosensors 2021, 10, 11. [Google Scholar] [CrossRef]
- Vikrant, K.; Weon, S.; Kim, K.-H.; Sillanpää, M. Platinized titanium dioxide (Pt/TiO2) as a multi-functional catalyst for thermocatalysis, photocatalysis, and photothermal catalysis for removing air pollutants. Appl. Mater. Today 2021, 23, 100993. [Google Scholar] [CrossRef]
- Du, L.; Shao, Y.; Sun, J.; Yin, G.; Liu, J.; Wang, Y. Advanced catalyst supports for PEM fuel cell cathodes. Nano Energy 2016, 29, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Selvarani, G.; Maheswari, S.; Sridhar, P.; Pitchumani, S.; Shukla, A. Carbon-supported Pt–TiO2 as a methanol-tolerant oxygen-reduction catalyst for DMFCs. J. Electrochem. Soc. 2009, 156, B1354. [Google Scholar] [CrossRef]
- Lewera, A.; Timperman, L.; Roguska, A.; Alonso-Vante, N. Metal–support interactions between nanosized Pt and metal oxides (WO3 and TiO2) studied using X-ray photoelectron spectroscopy. J. Phys. Chem. C 2011, 115, 20153–20159. [Google Scholar] [CrossRef]
- Timperman, L.; Lewera, A.; Vogel, W.; Alonso-Vante, N. Nanostructured platinum becomes alloyed at oxide-composite substrate. Electrochem. Commun. 2010, 12, 1772–1775. [Google Scholar] [CrossRef]
- Camacho, B.R.; Morais, C.; Valenzuela, M.; Alonso-Vante, N. Enhancing oxygen reduction reaction activity and stability of platinum via oxide-carbon composites. Catal. Today 2013, 202, 36–43. [Google Scholar] [CrossRef]
- Jaksic, J.M.; Krstajic, N.V.; Vracar, L.M.; Neophytides, S.G.; Labou, D.; Falaras, P.; Jaksic, M.M. Spillover of primary oxides as a dynamic catalytic effect of interactive hypo-d-oxide supports. Electrochim. Acta 2007, 53, 349–361. [Google Scholar] [CrossRef]
- Jaksic, J.M.; Labou, D.; Papakonstantinou, G.D.; Siokou, A.; Jaksic, M.M. Novel spillover interrelating reversible electrocatalysts for oxygen and hydrogen electrode reactions. J. Phys. Chem. C 2010, 114, 18298–18312. [Google Scholar] [CrossRef]
- Lv, Q.; Yin, M.; Zhao, X.; Li, C.; Liu, C.; Xing, W. Promotion effect of TiO2 on catalytic activity and stability of Pt catalyst for electrooxidation of methanol. J. Power Sources 2012, 218, 93–99. [Google Scholar] [CrossRef]
- Gu, D.-M.; Chu, Y.-Y.; Wang, Z.-B.; Jiang, Z.-Z.; Yin, G.-P.; Liu, Y. Methanol oxidation on Pt/CeO2–C electrocatalyst prepared by microwave-assisted ethylene glycol process. Appl. Catal. B 2011, 102, 9–18. [Google Scholar] [CrossRef]

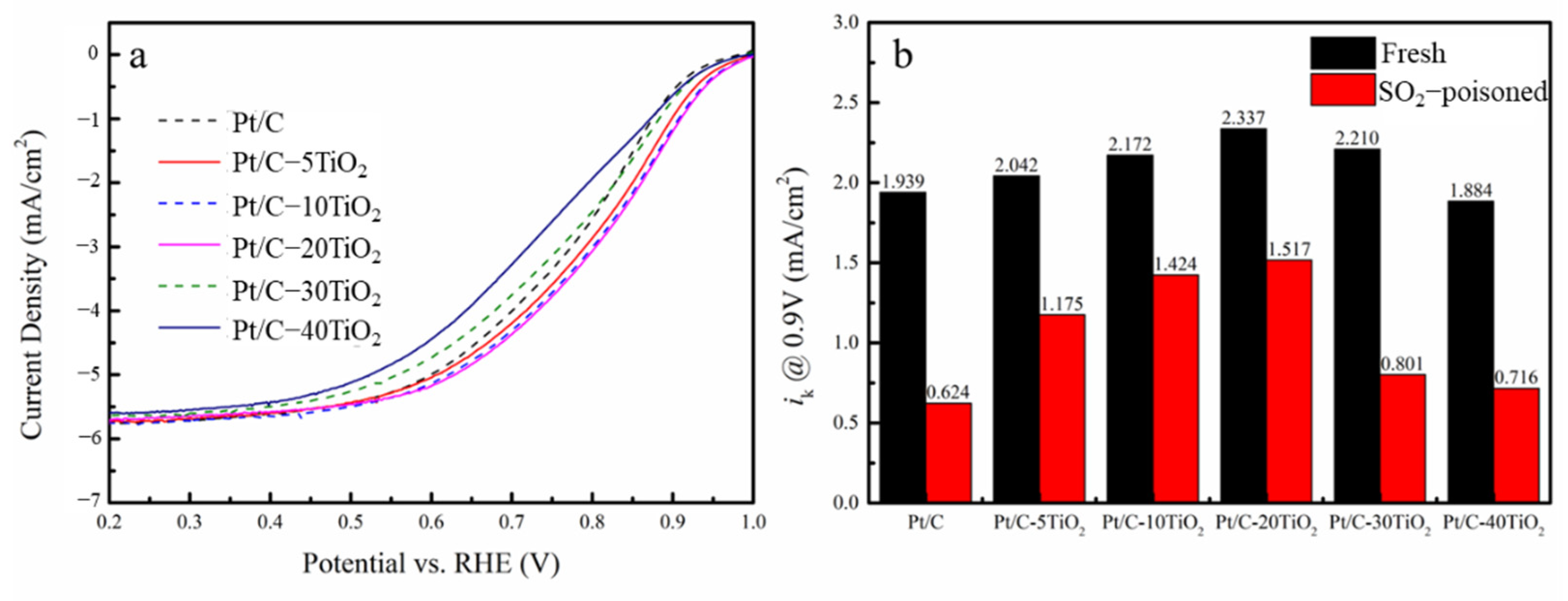
| Sample | Pristine | SO2 Poisoned | ||||||
|---|---|---|---|---|---|---|---|---|
| ECSA (m2 gPt−1) | ECSA (m2 gPt−1) | |||||||
| Pt/C | 97.07 | 0.853 | 1.939 | 27.06 | 0.782 | 0.624 | 71 | 67.8% |
| Pt/C-5TiO2 | 97.84 | 0.855 | 2.042 | 26.55 | 0.800 | 1.175 | 55 | 42.5% |
| Pt/C-10TiO2 | 102.04 | 0.858 | 2.172 | 28.32 | 0.809 | 1.424 | 49 | 34.4% |
| Pt/C-20TiO2 | 101.46 | 0.867 | 2.337 | 25.99 | 0.814 | 1.517 | 53 | 35.1% |
| Pt/C-30TiO2 | 99.11 | 0.86 | 2.210 | 29.82 | 0.775 | 0.801 | 85 | 63.7% |
| Pt/C-40TiO2 | 95.11 | 0.855 | 1.884 | 26.81 | 0.736 | 0.716 | 119 | 62.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ye, J.; Kong, F.; Du, C.; Zuo, P.; Du, L.; Yin, G. Pt/C-TiO2 as Oxygen Reduction Electrocatalysts against Sulfur Poisoning. Catalysts 2022, 12, 571. https://doi.org/10.3390/catal12050571
Liu Y, Ye J, Kong F, Du C, Zuo P, Du L, Yin G. Pt/C-TiO2 as Oxygen Reduction Electrocatalysts against Sulfur Poisoning. Catalysts. 2022; 12(5):571. https://doi.org/10.3390/catal12050571
Chicago/Turabian StyleLiu, Yuxin, Jing Ye, Fanpeng Kong, Chunyu Du, Pengjian Zuo, Lei Du, and Geping Yin. 2022. "Pt/C-TiO2 as Oxygen Reduction Electrocatalysts against Sulfur Poisoning" Catalysts 12, no. 5: 571. https://doi.org/10.3390/catal12050571
APA StyleLiu, Y., Ye, J., Kong, F., Du, C., Zuo, P., Du, L., & Yin, G. (2022). Pt/C-TiO2 as Oxygen Reduction Electrocatalysts against Sulfur Poisoning. Catalysts, 12(5), 571. https://doi.org/10.3390/catal12050571









