Electro-Catalytic Properties of Palladium and Palladium Alloy Electro-Catalysts Supported on Carbon Nanofibers for Electro-Oxidation of Methanol and Ethanol in Alkaline Medium
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterisation of Materials
2.1.1. High Resolution-Transmission Electron Microscopy (HRTEM)
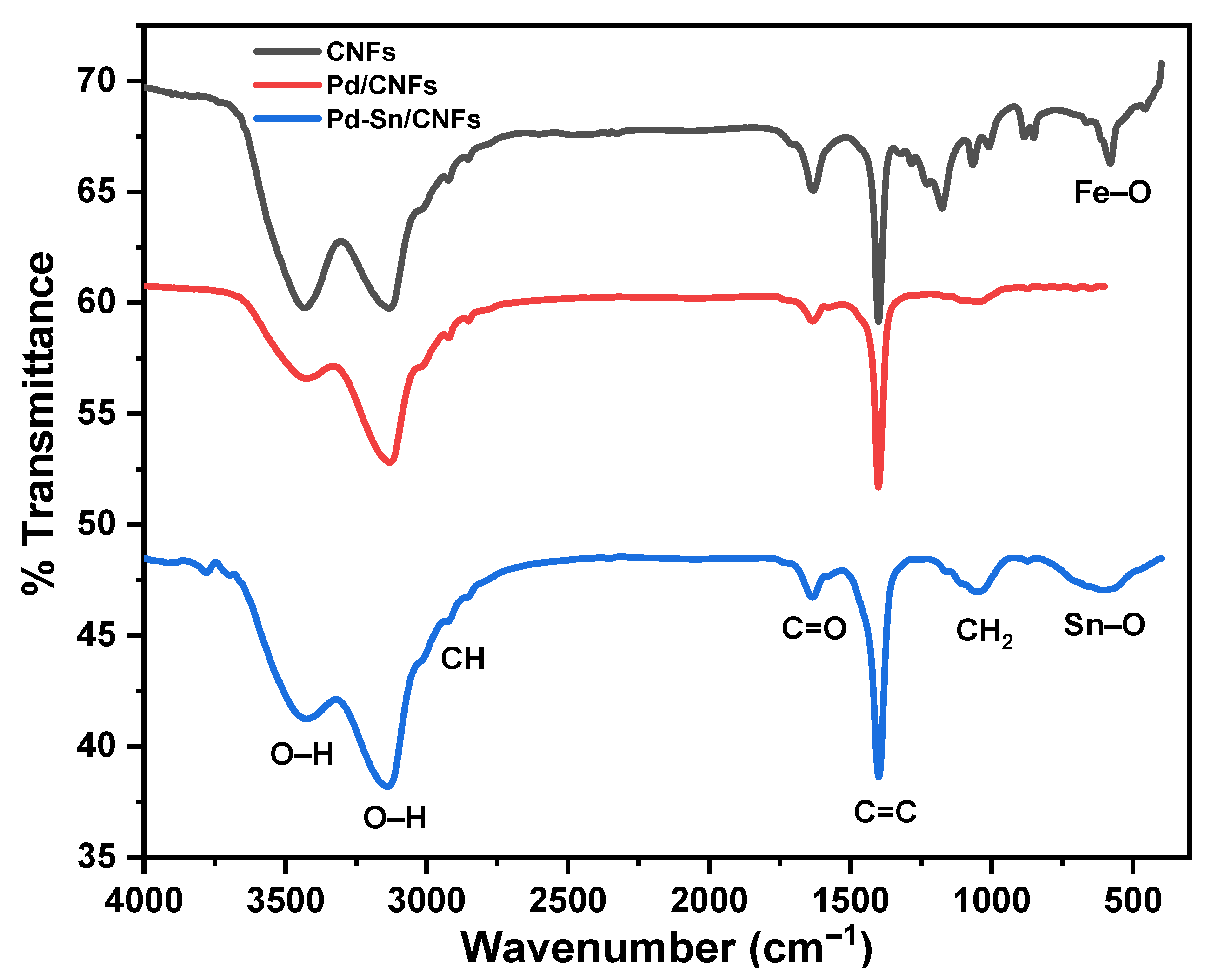
2.1.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.1.3. X-ray Diffraction (XRD)
2.1.4. X-ray Photoelectron Spectroscopy (XPS)
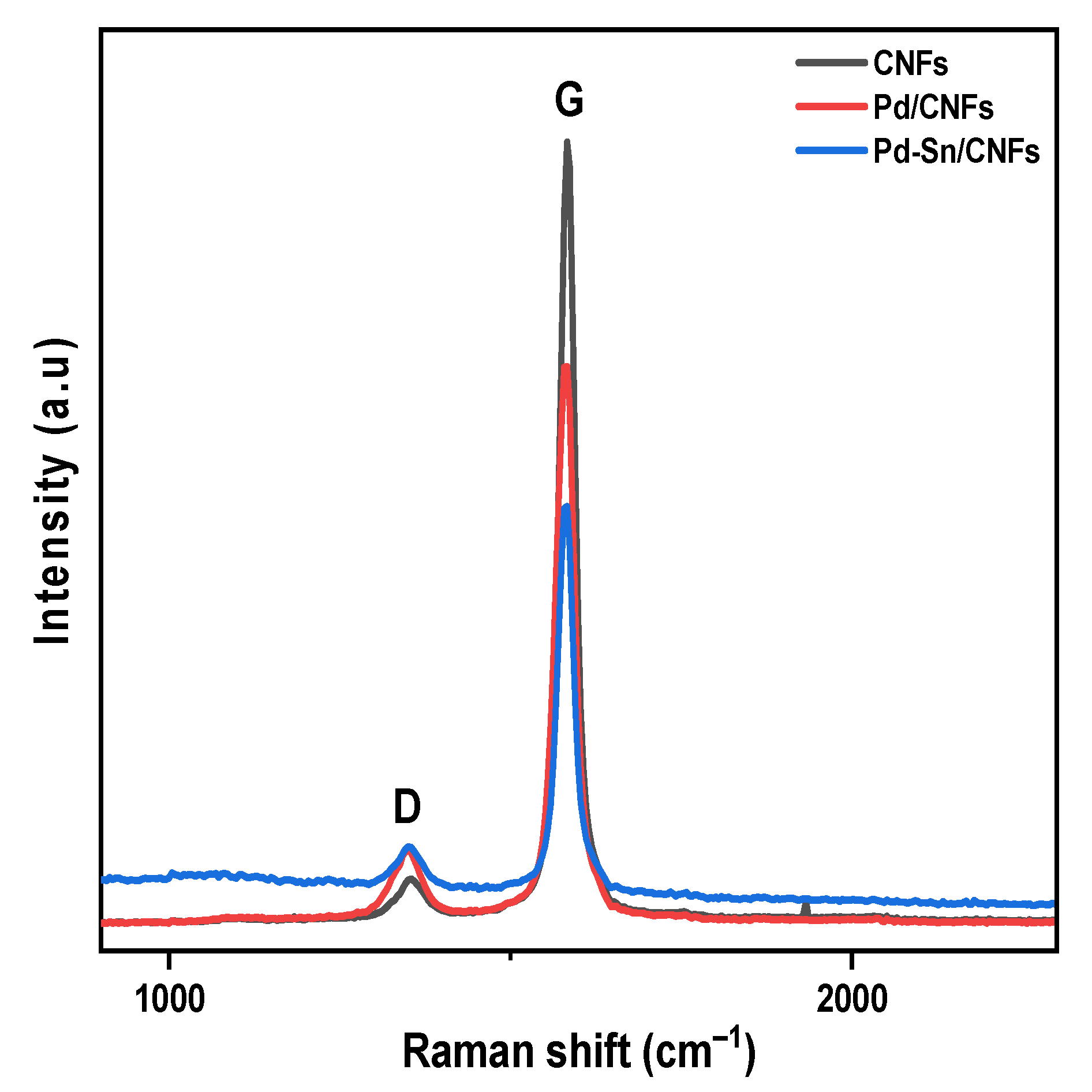
2.1.5. Raman Analysis
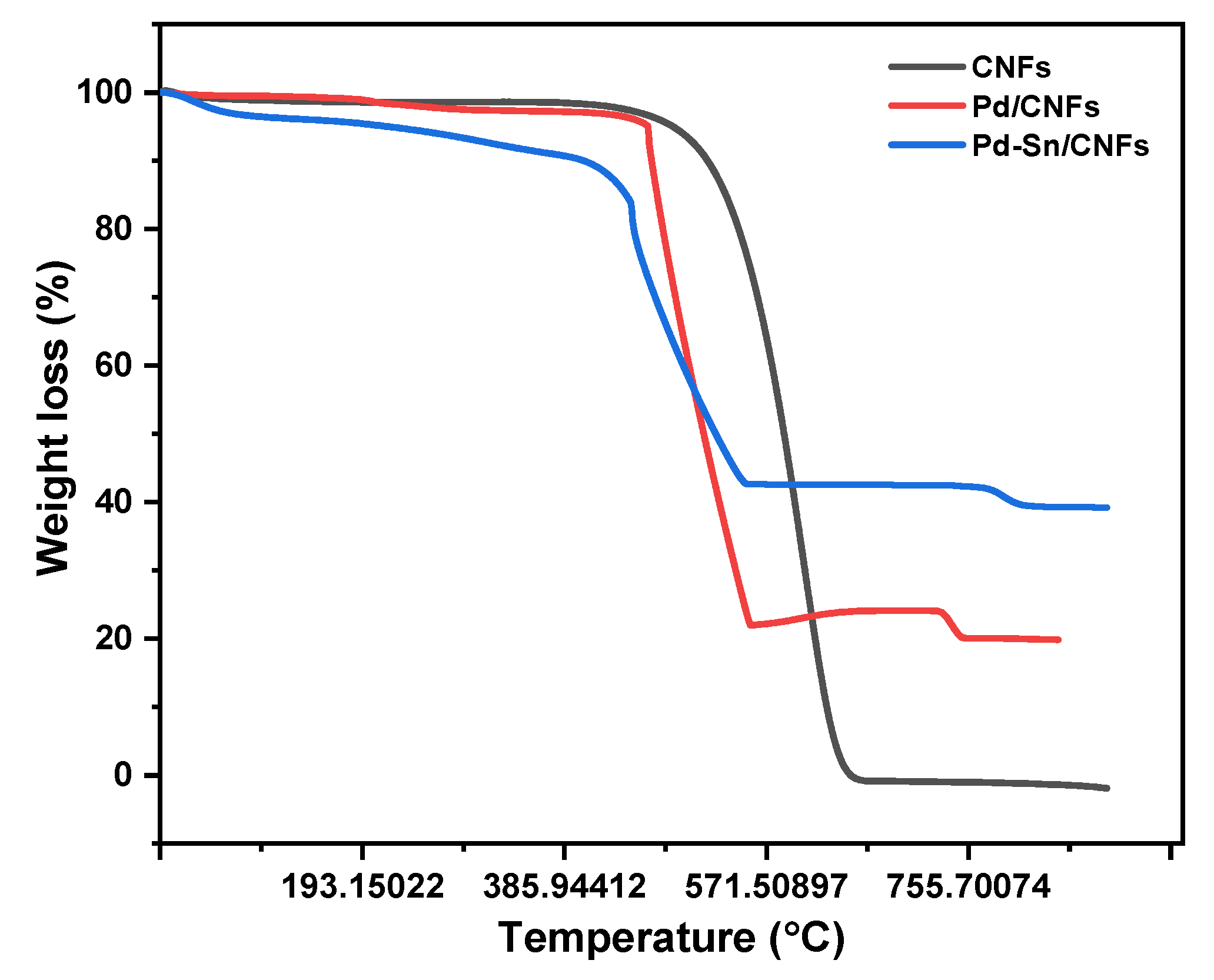
2.1.6. Thermogravimetric Analysis
2.1.7. Brunauer-Emmett-Teller (BET) Surface Area Analysis
2.2. Application of Electro-Catalysts in Alcohol Fuel Oxidation Reactions
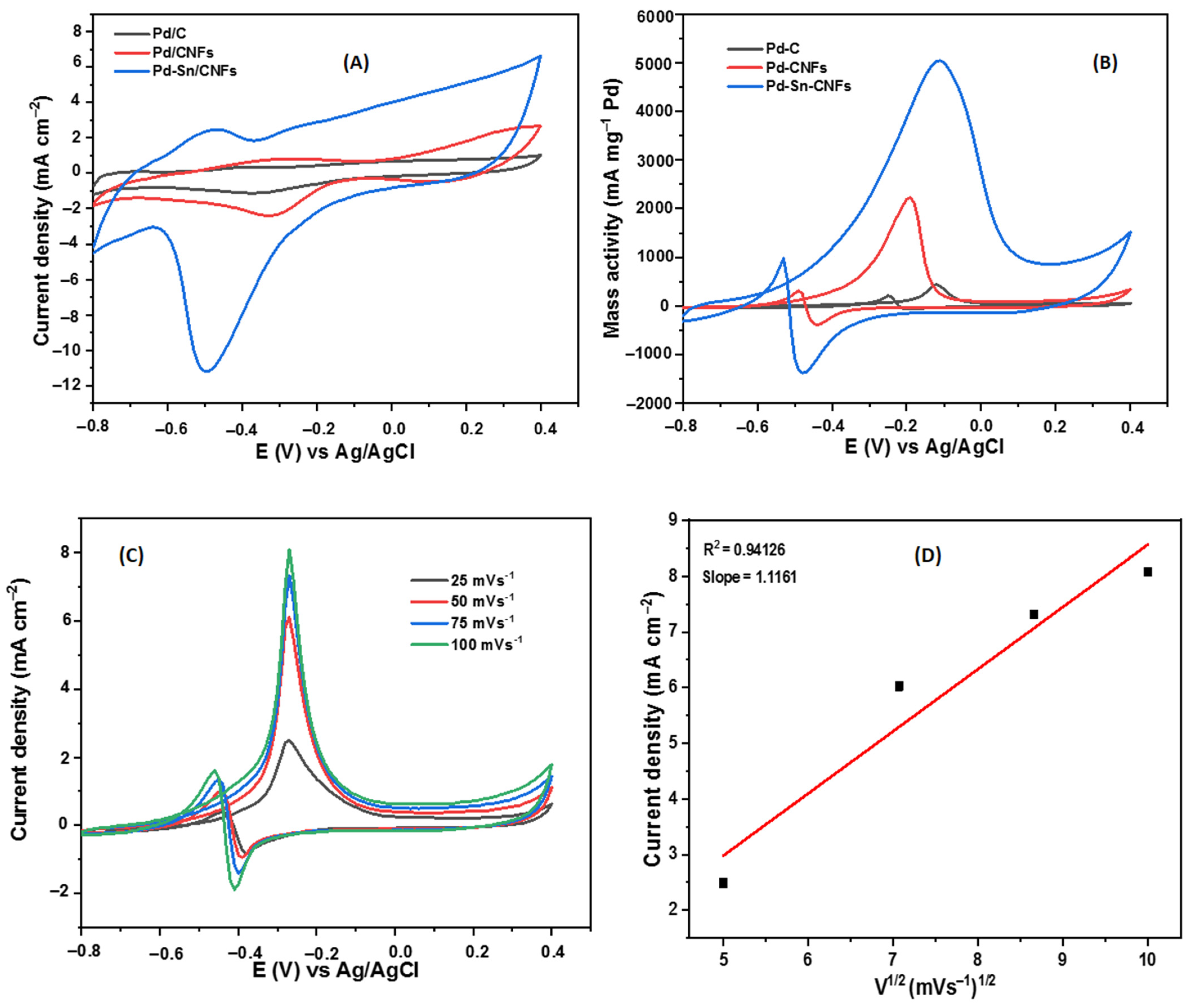
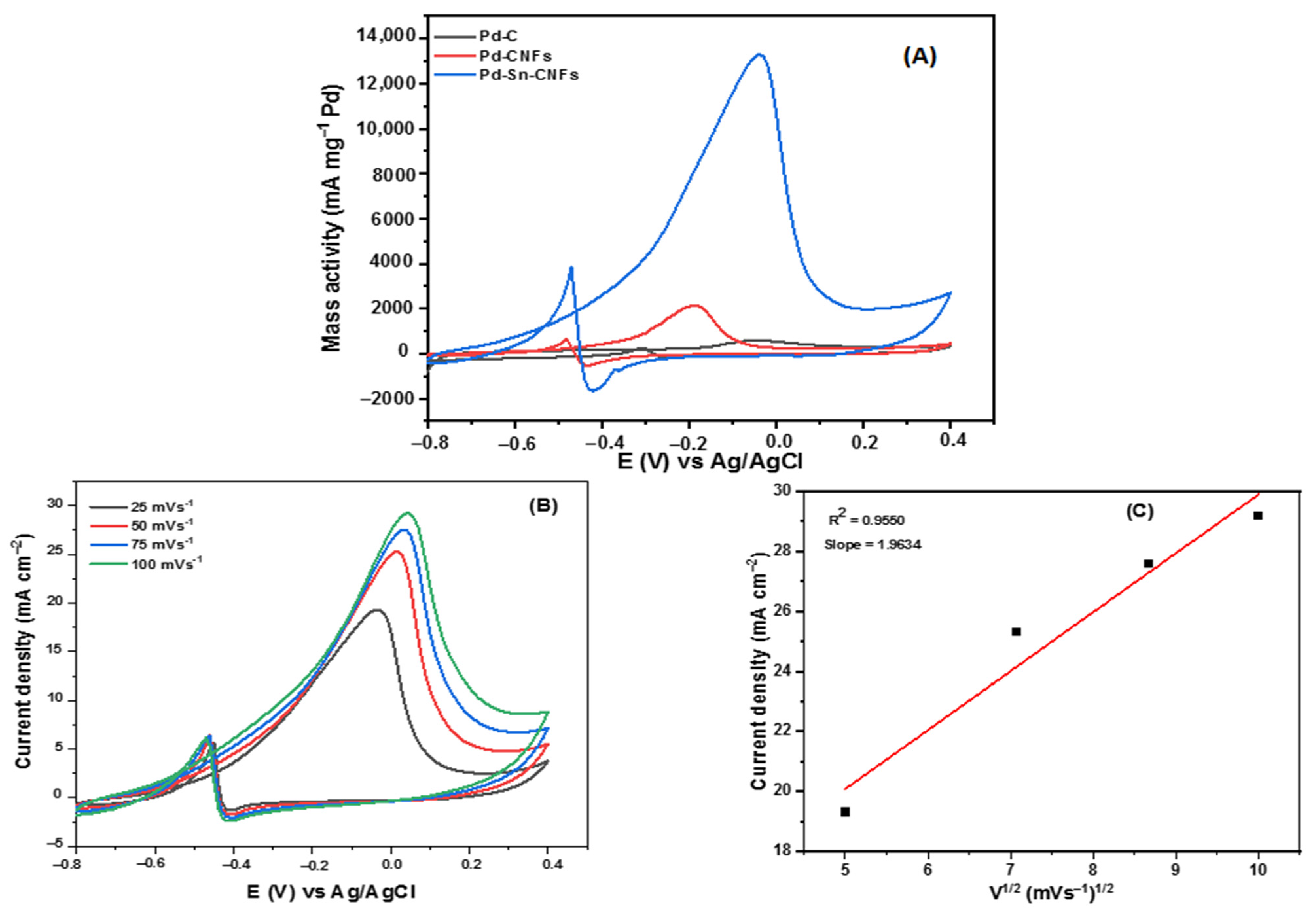
2.2.1. Methanol Electro-Oxidation
Cyclic Voltammetric Tests
Stability/Durability and Impedimetric Tests
2.2.2. Ethanol Electro-Oxidation
Cyclic Voltammetric Tests
2.2.3. Stability/Durability and Impedimetric Tests
3. Materials and Methods
3.1. Synthesis of Carbon Nanofibers (CNFs)
3.2. Functionalization of Carbon Nanofibers (CNFs)
3.3. Synthesis of Pd/CNFs and Pd-Sn/CNFs Electro-Catalysts
3.4. Sample Characterization
3.5. Electrochemical Characterisation
3.5.1. Electrode Preparation
3.5.2. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sikeyi, L.L.; Matthews, T.; Adekunle, A.S.; Maxakato, N.W. Electro-oxidation of Ethanol and Methanol on Pd/C, Pd/CNFs and Pd–Ru/CNFs Nanocatalysts in Alkaline Direct Alcohol Fuel Cell. Electroanalysis 2020, 32, 2681–2692. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.; Timmiati, S. Membranes for direct ethanol fuel cells: An overview. Appl. Energy 2016, 163, 334–342. [Google Scholar] [CrossRef]
- Wang, B.; Tao, L.; Cheng, Y.; Yang, F.; Jin, Y.; Zhou, C.; Yu, H.; Yang, Y. Electrocatalytic oxidation of small molecule alcohols over Pt, Pd, and Au catalysts: The effect of alcohol’s hydrogen bond donation ability and molecular structure properties. Catalysts 2019, 9, 387. [Google Scholar] [CrossRef] [Green Version]
- Gwebu, S.; Nomngongo, P.; Mashazi, P.; Nyokong, T.; Maxakato, N. Platinum nanoparticles supported on carbon nanodots as anode catalysts for direct alcohol fuel cells. Int. J. Electrochem. Sci. 2017, 12, 6365–6378. [Google Scholar] [CrossRef]
- An, L.; Zhao, T.; Shen, S.; Wu, Q.; Chen, R. Performance of a direct ethylene glycol fuel cell with an anion-exchange membrane. Int. J. Hydrogen Energy 2010, 35, 4329–4335. [Google Scholar] [CrossRef]
- Yu, E.H.; Wang, X.; Krewer, U.; Li, L.; Scott, K. Direct oxidation alkaline fuel cells: From materials to systems. Energy Environ. Sci. 2012, 5, 5668–5680. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Momma, T.; Osaka, T. Cell performance of Pd–Sn catalyst in passive direct methanol alkaline fuel cell using anion exchange membrane. J. Power Sources 2009, 189, 999–1002. [Google Scholar] [CrossRef]
- Jiang, L.; Hsu, A.; Chu, D.; Chen, R. Ethanol electro-oxidation on Pt/C and PtSn/C catalysts in alkaline and acid solutions. Int. J. Hydrogen Energy 2010, 35, 365–372. [Google Scholar] [CrossRef]
- Huang, H.; Wang, X. Recent progress on carbon-based support materials for electrocatalysts of direct methanol fuel cells. J. Mater. Chem. A 2014, 2, 6266–6291. [Google Scholar] [CrossRef]
- Hu, F.; Chen, C.; Wang, Z.; Wei, G.; Shen, P.K. Mechanistic study of ethanol oxidation on Pd–NiO/C electrocatalyst. Electrochim. Acta 2006, 52, 1087–1091. [Google Scholar] [CrossRef]
- Shen, S.; Zhao, T.; Xu, J.; Li, Y. Synthesis of PdNi catalysts for the oxidation of ethanol in alkaline direct ethanol fuel cells. J. Power Sources 2010, 195, 1001–1006. [Google Scholar] [CrossRef]
- Liu, J.; Ye, J.; Xu, C.; Tong, Y. Kinetics of ethanol electrooxidation at Pd electrodeposited on Ti. Electrochem. Commun. 2007, 9, 2334–2339. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, T.; Xu, J.; Liang, Z. Preparation and characterization of carbon-supported sub-monolayer palladium decorated gold nanoparticles for the electro-oxidation of ethanol in alkaline media. J. Power Sources 2009, 187, 80–84. [Google Scholar] [CrossRef]
- Qin, Y.-H.; Jia, Y.B.; Jiang, Y.; Niu, D.F.; Zhang, X.S.; Zhou, X.G.; Niu, L.; Yuan, W.K. Controllable synthesis of carbon nanofiber supported Pd catalyst for formic acid electrooxidation. Int. J. Hydrogen Energy 2012, 37, 7373–7377. [Google Scholar] [CrossRef]
- Faghihian, H.; Kooravand, M.; Atarodi, H. Synthesis of a novel carbon nanofiber structure for removal of lead. Korean J. Chem. Eng. 2013, 30, 357–363. [Google Scholar] [CrossRef]
- Maddah, B.; Soltaninezhad, M.; Adib, K.; Hasanzadeh, M. Activated carbon nanofiber produced from electrospun PAN nanofiber as a solid phase extraction sorbent for the preconcentration of organophosphorus pesticides. Sep. Sci. Technol. 2017, 52, 700–711. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Li, S.; Dong, Z.; Yang, H.; Guo, S.; Gou, G.; Ren, R.; Zhu, Z.; Jin, J.; Ma, J. Microenvironment Effects in Electrocatalysis: Ionic-Liquid-Like Coating on Carbon Nanotubes Enhances the Pd-Electrocatalytic Alcohol Oxidation. Chem.—Eur. J. 2013, 19, 2384–2391. [Google Scholar] [CrossRef]
- Du, W.; Mackenzie, K.E.; Milano, D.F.; Deskins, N.A.; Su, D.; Teng, X. Palladium–tin alloyed catalysts for the ethanol oxidation reaction in an alkaline medium. ACS Catal. 2012, 2, 287–297. [Google Scholar] [CrossRef]
- Suriani, A.B.; Dalila, A.R.; Mohamed, A.; Isa, I.M.; Kamari, A.; Hashim, N.; Soga, T.; Tanemura, M. Synthesis of carbon nanofibres from waste chicken fat for field electron emission applications. Mater. Res. Bull. 2015, 70, 524–529. [Google Scholar] [CrossRef]
- Ramos, A.; Cameán, I.; García, A.B. Graphitization thermal treatment of carbon nanofibers. Carbon 2013, 59, 2–32. [Google Scholar] [CrossRef]
- Cameán, I.; García, A.B.; Suelves, I.; Pinilla, J.L.; Lázaro, M.J.; Moliner, R. Graphitized carbon nanofibers for use as anodes in lithium-ion batteries: Importance of textural and structural properties. J. Power Sources 2012, 198, 303–307. [Google Scholar] [CrossRef]
- Souza, F.M.; Parreira, L.; Hammer, P.; Batista, B.; Santos, M. Niobium: A promising Pd co-electrocatalyst for ethanol electrooxidation reactions. J. Solid State Electrochem. 2018, 22, 1495–1506. [Google Scholar] [CrossRef] [Green Version]
- Vigier, F.; Coutanceau, C.; Hahn, F.; Belgsir, E.; Lamy, C. On the mechanism of ethanol electro-oxidation on Pt and PtSn catalysts: Electrochemical and in situ IR reflectance spectroscopy studies. J. Electroanal. Chem. 2004, 563, 81–89. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Chou, S.; Liu, H.; Wang, J. A 3D porous nitrogen-doped carbon-nanofiber-supported palladium composite as an efficient catalytic cathode for lithium–oxygen batteries. J. Mater. Chem. A 2017, 5, 1462–1471. [Google Scholar] [CrossRef]
- Li, G.; Pickup, P.G. Decoration of carbon-supported Pt catalysts with Sn to promote electro-oxidation of ethanol. J. Power Sources 2007, 173, 121–129. [Google Scholar] [CrossRef]
- Li, H.; Sun, G.; Cao, L.; Jiang, L.; Xin, Q. Comparison of different promotion effect of PtRu/C and PtSn/C electrocatalysts for ethanol electro-oxidation. Electrochim. Acta 2007, 52, 6622–6629. [Google Scholar] [CrossRef]
- Hu, G.; Nitze, F.; Sharifi, T.; Barzegar, H.R.; Wågberg, T. Self-assembled palladium nanocrystals on helical carbon nanofibers as enhanced electrocatalysts for electro-oxidation of small molecules. J. Mater. Chem. 2012, 22, 8541–8548. [Google Scholar] [CrossRef]
- Nitze, F.; Abou-Hamad, E.; Wågberg, T. Carbon nanotubes and helical carbon nanofibers grown by chemical vapour deposition on C60 fullerene supported Pd nanoparticles. Carbon 2011, 49, 1101–1107. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Ju, J.; Deng, N.; Wang, G.; Cheng, B.; Kang, W. ZnS nanoparticles anchored on porous carbon nanofibers as anode materials for lithium ion batteries. Electrochem. Commun. 2018, 96, 1–5. [Google Scholar] [CrossRef]
- Zou, G.; Zhang, D.; Dong, C.; Li, H.; Xiong, K.; Fei, L.; Qian, Y. Carbon nanofibers: Synthesis, characterization, and electrochemical properties. Carbon 2006, 44, 828–832. [Google Scholar] [CrossRef]
- Villarroel-Rocha, J.; Barrera, D.; Sapag, K. Introducing a self-consistent test and the corresponding modification in the Barrett, Joyner and Halenda method for pore-size determination. Microporous Mesoporous Mater. 2014, 200, 68–78. [Google Scholar] [CrossRef]
- Jeong, J.H.; Lee, Y.H.; Kim, B.-H. Relationship between microstructure and electrochemical properties of 2lignin-derived carbon nanofibers prepared by thermal treatment. Synth. Met. 2020, 260, 116287. [Google Scholar] [CrossRef]
- Oldham, K.B. Ultimate cyclic voltammetry: An analytical examination of the reversible case. J. Solid State Electrochem. 2013, 17, 2749–2756. [Google Scholar] [CrossRef]
- Fischer, D.A.; Vargas, I.T.; Pizarro, G.E.; Armijo, F.; Walczak, M. The effect of scan rate on the precision of determining corrosion current by Tafel extrapolation: A numerical study on the example of pure Cu in chloride containing medium. Electrochim. Acta 2019, 313, 457–467. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, L.; Xue, H.; Wang, Z.; Hu, H. Methanol electrocatalytic oxidation on highly dispersed platinum–ruthenium/graphene catalysts prepared in supercritical carbon dioxide–methanol solution. RSC Adv. 2012, 2, 9651–9659. [Google Scholar] [CrossRef]
- Bachiller-Baeza, B.; Peña-Bahamonde, J.; Castillejos-López, E.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Improved performance of carbon nanofiber-supported palladium particles in the selective 1,3-butadiene hydrogenation: Influence of carbon nanostructure, support functionalization treatment and metal precursor. Catal. Today 2015, 249, 63–71. [Google Scholar] [CrossRef]
- Selepe, C.T.; Gwebu, S.S.; Matthews, T.; Mashola, T.A.; Sikeyi, L.L.; Zikhali, M.; Maxakato, N.W. Effect of Sn Doping on Pd Electro-Catalysts for Enhanced Electro-Catalytic Activity towards Methanol and Ethanol Electro-Oxidation in Direct Alcohol Fuel Cells. Nanomaterials 2021, 11, 2725. [Google Scholar] [CrossRef]
- Raseruthe, K.E.; Matthews, T.; Gwebu, S.S.; Pillay, K.; Maxakato, N.W. Investigating the effect of carbon support on palladium-based catalyst towards electro-oxidation of ethylene glycol. Mater. Res. Express 2021, 8, 015017. [Google Scholar] [CrossRef]
- Karuppasamy, L.; Lee, G.-J.; Anandan, S.; Wu, J.J. Synthesis of shape-controlled Pd nanocrystals on carbon nanospheres and electrocatalytic oxidation performance for ethanol and ethylene glycol. Appl. Surf. Sci. 2020, 519, 146266. [Google Scholar] [CrossRef]
- Yan, Z.; He, G.; Shen, P.K.; Luo, Z.; Xie, J.; Chen, M. MoC–graphite composite as a Pt electrocatalyst support for highly active methanol oxidation and oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 4014–4022. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Chen, H.; Hu, X.; Ma, S. Fabrication of highly sensitive and stable hydroxylamine electrochemical sensor based on gold nanoparticles and metal–metalloporphyrin framework modified electrode. ACS Appl. Mater. Interfaces 2016, 8, 18173–18181. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Zuo, J.; Pang, H.; Tan, L.; Chen, T.; Ma, H. A dopamine electrochemical sensor based on Pd-Pt alloy nanoparticles decorated polyoxometalate and multiwalled carbon nanotubes. J. Electroanal. Chem. 2018, 827, 103–111. [Google Scholar] [CrossRef]
- Soundararajan, D.; Park, J.; Kim, K.; Ko, J. Pt–Ni alloy nanoparticles supported on CNF as catalyst for direct ethanol fuel cells. Curr. Appl. Phys. 2012, 12, 854–859. [Google Scholar] [CrossRef]
- Sharma, S. Recent developments in electrocatalysts and hybrid electrocatalyst support systems for polymer electrolyte fuel cells. In Electrocatalysts for Low Temperature Fuel Cells: Fundamentals and Recent Trends; Wiley-VCH: Weinheim, Germany, 2017; pp. 197–239. [Google Scholar]
- Cao, L.; Sun, G.; Li, H.; Xin, Q. Carbon-supported IrSn catalysts for direct ethanol fuel cell. Fuel Cells Bull. 2007, 2007, 12–16. [Google Scholar] [CrossRef]
- Ning, L.; Liu, X.; Deng, M.; Huang, Z.; Zhu, A.; Zhang, Q.; Liu, Q. Palladium-based nanocatalysts anchored on CNT with high activity and durability for ethanol electro-oxidation. Electrochim. Acta 2019, 297, 206–214. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Mahmoodi, R.; Daneshvari-Esfahlan, V. Ni@ Pd core-shell nanostructure supported on multi-walled carbon nanotubes as efficient anode nanocatalysts for direct methanol fuel cells with membrane electrode assembly prepared by catalyst coated membrane method. Energy 2018, 161, 1074–1084. [Google Scholar] [CrossRef]
- Zhang, J.; She, Y. Mechanism of methanol decomposition on the Pd/WC (0001) surface unveiled by first-principles calculations. Front. Chem. Sci. Eng. 2020, 14, 1052–1064. [Google Scholar] [CrossRef]
- Fayomi, O.; Daniyan, A.; Umoru, L.; Popoola, A. Data on the effect of current density relationship on the super-alloy composite coating by electrolytic route. Data Brief 2018, 18, 776–780. [Google Scholar] [CrossRef]
- Choudhury, A.; Chandra, H.; Arora, A. Application of solid oxide fuel cell technology for power generation—A review. Renew. Sustain. Energy Rev. 2013, 20, 430–442. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, T.-Y.; Yan, X.-M.; Xu, X.W.; Zhang, Q.D.; Zhao, B.L.; El Hamouti, I.; Hao, C.; He, G.-H. Benzimidazolium functionalized polysulfone-based anion exchange membranes with improved alkaline stability. Chin. J. Polym. Sci. 2018, 36, 129–138. [Google Scholar] [CrossRef]
- Sikeyi, L.; Adekunle, A.; Maxakato, N. Electro-catalytic activity of carbon nanofibers supported palladium nanoparticles for direct alcohol fuel cells in alkaline medium. Electrocatalysis 2019, 10, 420–428. [Google Scholar] [CrossRef]






| Electrocatalysts | Pd in Pd/CNFs | Pd in Pd-Sn/CNFs |
|---|---|---|
| Average crystal size | 3.91 nm | 1.90 nm |
| Lattice parameter | 0.4716 | 0.8876 |
| Electrocatalyst | Pd2+ (eV) | Pd0+ (eV) | Pd2+ (%) | Pd0+ |
|---|---|---|---|---|
| Pd/CNFs | 337.36 | 335.79 | 21 | 79 |
| Pd-Sn/CNFs | 337.62 | 334.74 | 42 | 58 |
| Sample | O1 (eV) | O2 (eV) | O3 (eV) | O4 (eV) |
|---|---|---|---|---|
| CNFs | 531.4 | 532.79 | 534.18 | 536.02 |
| Pd/CNFs | 531.3 | 532.71 | 534.22 | 536.07 |
| Pd-Sn/CNFs | 531.35 | 532.75 | 534.20 | 536.09 |
| Sample | C1 (eV) | C2 (eV) | C3 (eV) | C4 (eV) | C5 (eV) |
|---|---|---|---|---|---|
| CNFs | 284.64 | 285.78 | 286.80 | 289.48 | 292.13 |
| Pd/CNFs | 284.74 | 285.60 | 286.90 | 289.69 | 292.10 |
| Pd-Sn/CNFs | 284.78 | 285.64 | 286.88 | 289.52 | 292.11 |
| Nanomaterial | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| CNFs support | 19.6369 | 1.69 | 25.64 |
| Pd/CNFs electrocatalyst | 18.1912 | 1.82 | 27.55 |
| Pd-Sn/CNFs electrocatalyst | 17.4183 | 0.25 | 42.31 |
| Electro-Catalyst | Rs (Ohm) | Rct (Ohm) |
|---|---|---|
| Pd/C | 14.08 | 67.96 |
| Pd/CNFs | 6.28 | 51.43 |
| Pd-Sn/CNFs | 0.43 | 26.89 |
| Electro-Catalyst | Rs (Ohm) | Rct (Ohm) |
|---|---|---|
| Pd/C | 6.30 | 50.06 |
| Pd/CNFs | 1.11 | 27.68 |
| Pd-Sn/CNFs | 0.81 | 14.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selepe, C.T.; Gwebu, S.S.; Matthews, T.; Mashola, T.A.; Sikeyi, L.L.; Zikhali, M.; Mbokazi, S.P.; Makhunga, T.S.; Adegoke, K.A.; Maxakato, N.W. Electro-Catalytic Properties of Palladium and Palladium Alloy Electro-Catalysts Supported on Carbon Nanofibers for Electro-Oxidation of Methanol and Ethanol in Alkaline Medium. Catalysts 2022, 12, 608. https://doi.org/10.3390/catal12060608
Selepe CT, Gwebu SS, Matthews T, Mashola TA, Sikeyi LL, Zikhali M, Mbokazi SP, Makhunga TS, Adegoke KA, Maxakato NW. Electro-Catalytic Properties of Palladium and Palladium Alloy Electro-Catalysts Supported on Carbon Nanofibers for Electro-Oxidation of Methanol and Ethanol in Alkaline Medium. Catalysts. 2022; 12(6):608. https://doi.org/10.3390/catal12060608
Chicago/Turabian StyleSelepe, Cyril Tlou, Sandile Surprise Gwebu, Thabo Matthews, Tebogo Abigail Mashola, Ludwe Luther Sikeyi, Memory Zikhali, Siyabonga Patrick Mbokazi, Thobeka Sipho Makhunga, Kayode Adesina Adegoke, and Nobanathi Wendy Maxakato. 2022. "Electro-Catalytic Properties of Palladium and Palladium Alloy Electro-Catalysts Supported on Carbon Nanofibers for Electro-Oxidation of Methanol and Ethanol in Alkaline Medium" Catalysts 12, no. 6: 608. https://doi.org/10.3390/catal12060608
APA StyleSelepe, C. T., Gwebu, S. S., Matthews, T., Mashola, T. A., Sikeyi, L. L., Zikhali, M., Mbokazi, S. P., Makhunga, T. S., Adegoke, K. A., & Maxakato, N. W. (2022). Electro-Catalytic Properties of Palladium and Palladium Alloy Electro-Catalysts Supported on Carbon Nanofibers for Electro-Oxidation of Methanol and Ethanol in Alkaline Medium. Catalysts, 12(6), 608. https://doi.org/10.3390/catal12060608







