Polymeric Nanocapsule Enhances the Peroxidase-like Activity of Fe3O4 Nanozyme for Removing Organic Dyes
Abstract
:1. Introduction
2. Results
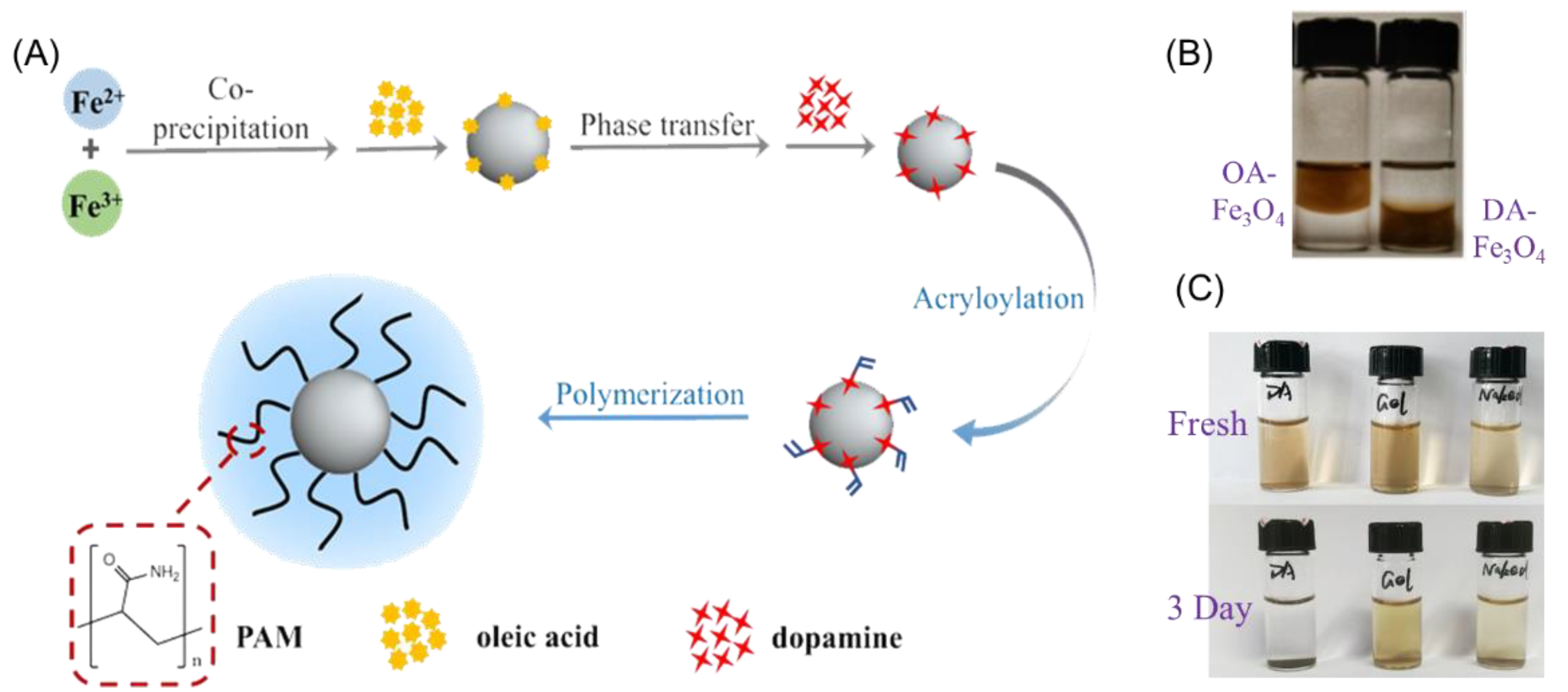
2.1. Design and Synthesis of Fe3O4@Gel
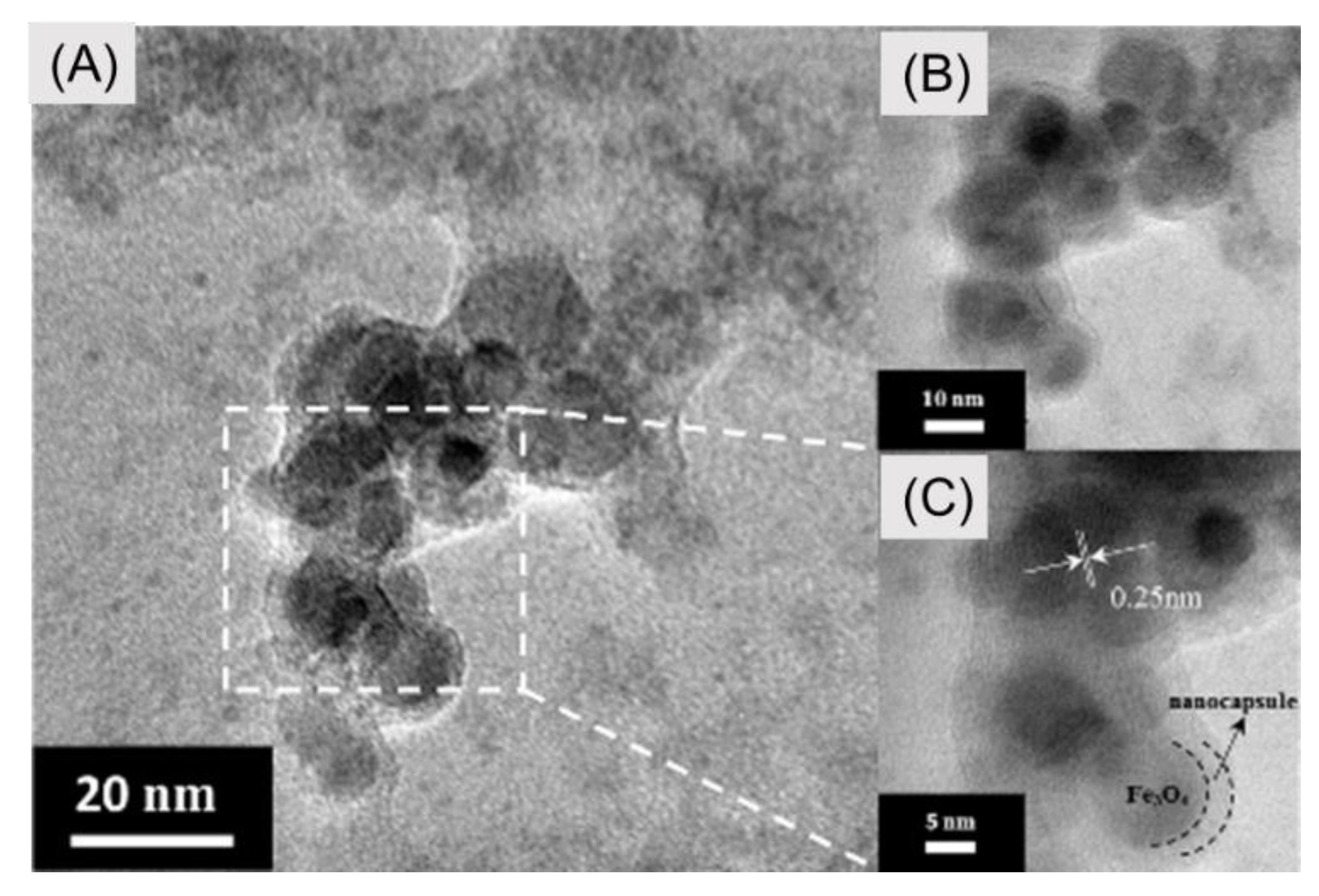
2.2. Physical Characterization of Samples
2.3. Peroxidase-Mimicking Activity of Fe3O4@Gel
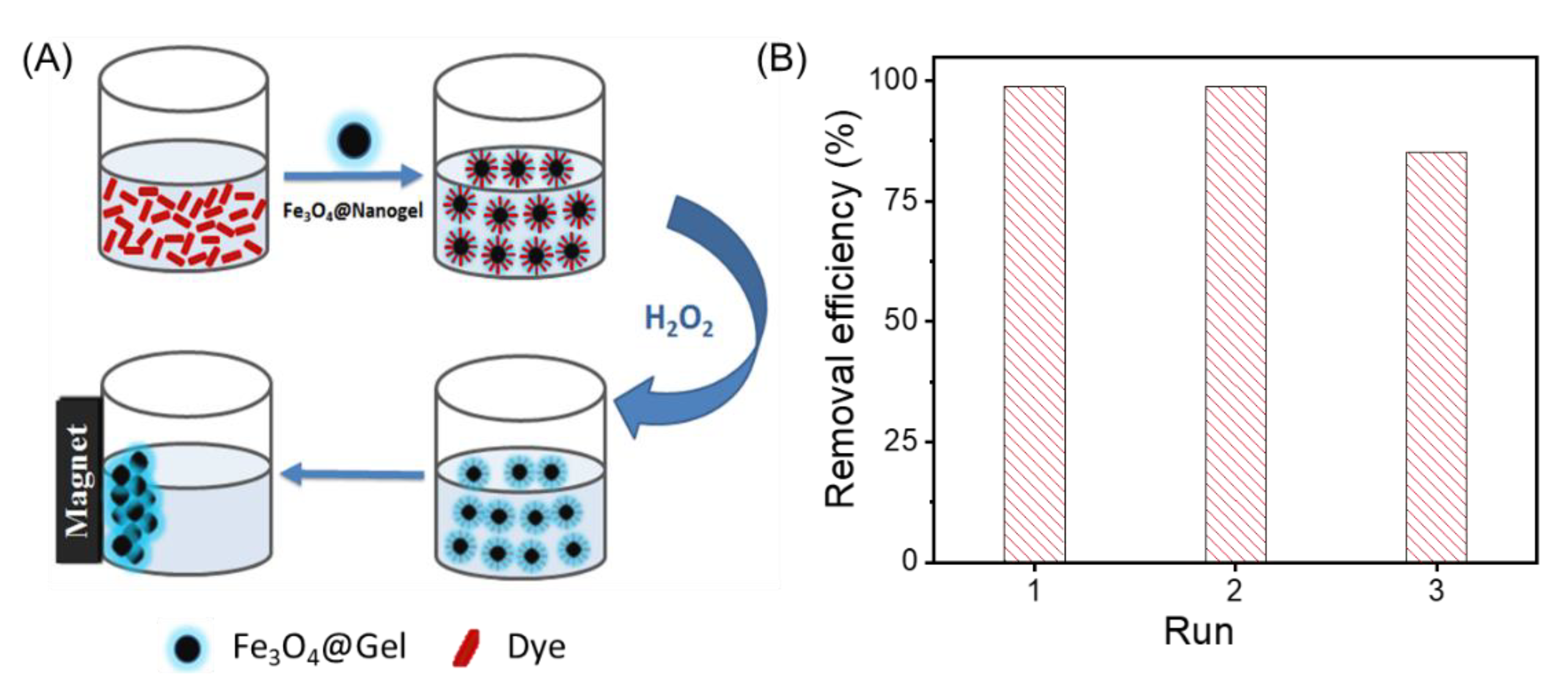
2.4. Efficient Removal of Organic Dyes with H2O2
2.5. Efficient Removal of Organic Dyes with Na2S2O8
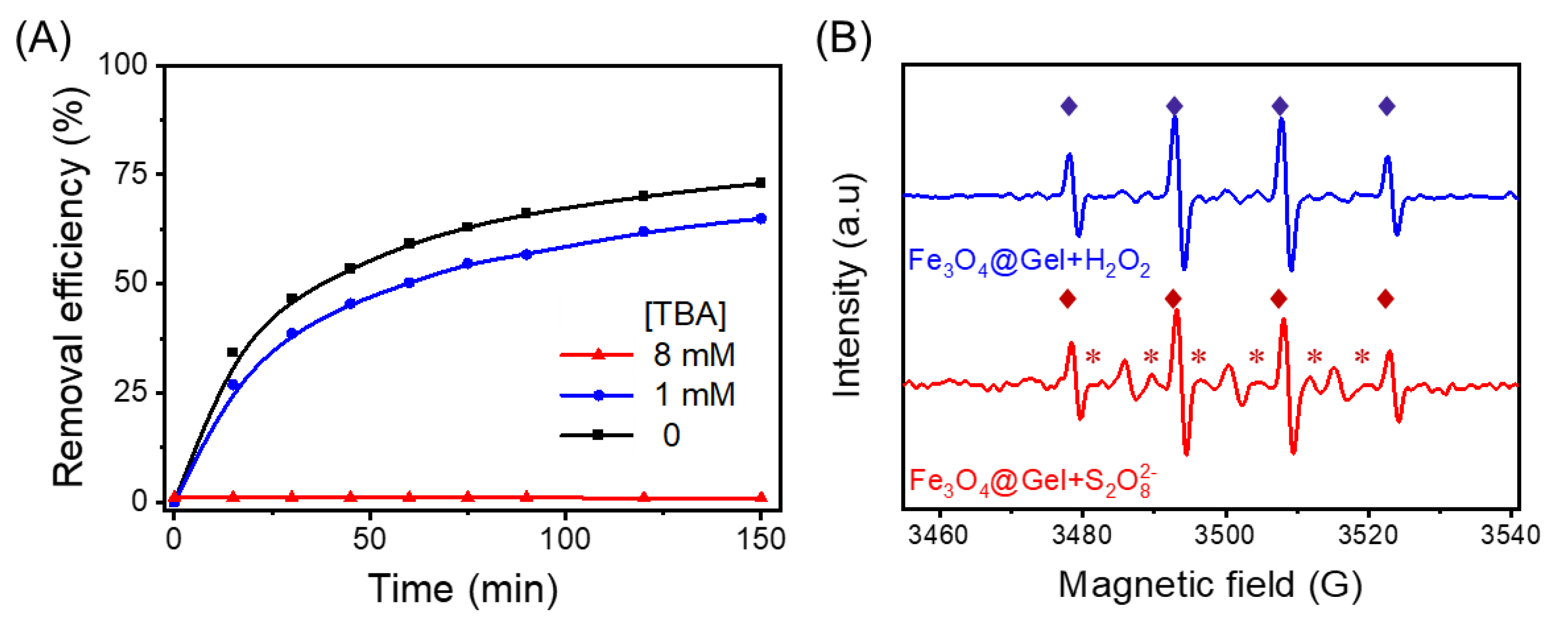
2.6. Identification of Primary Reactive Oxidants
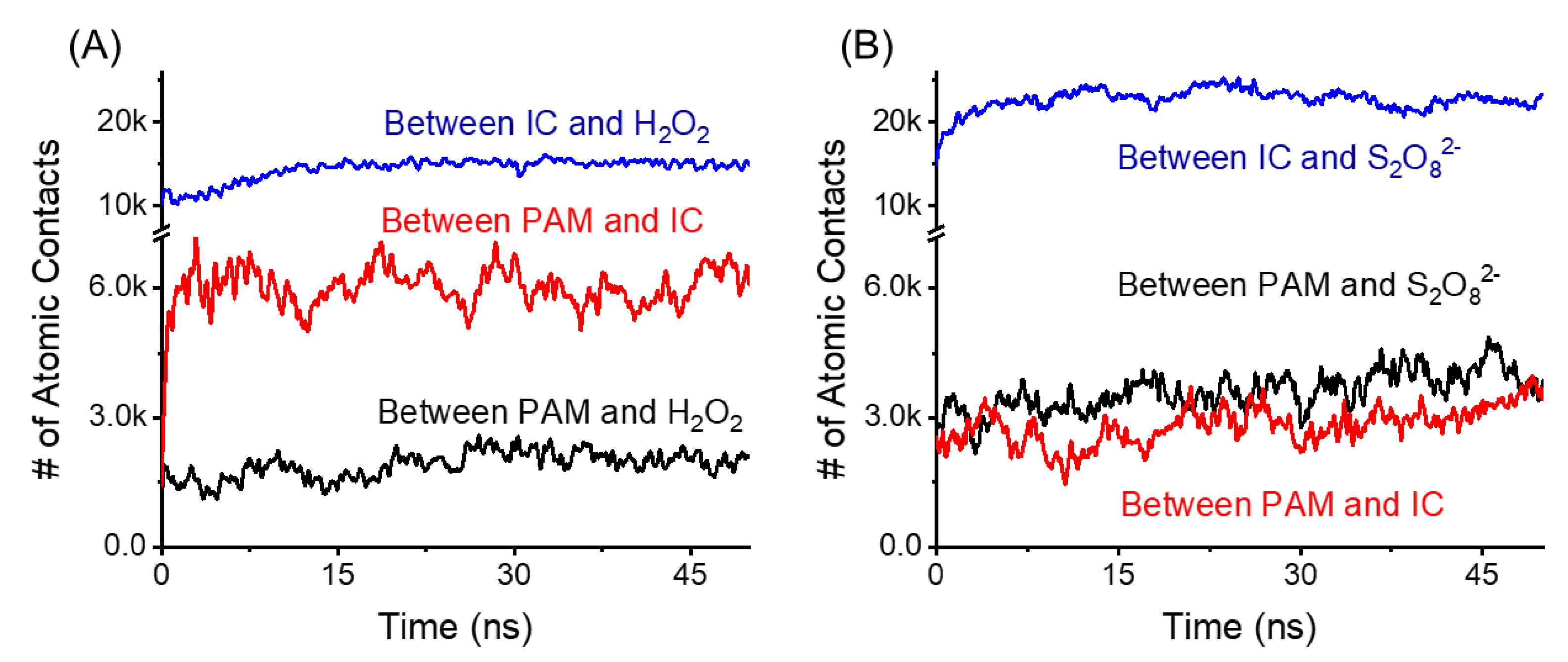
2.7. An Adsorption Mechanism as Revealed by Molecular Simulation
3. Materials and Methods
3.1. Materials
3.2. Preparation of Fe3O4 Nanoparticles
3.2.1. Naked Fe3O4 NP
3.2.2. DA-Fe3O4 NP
3.3. Encapsulation of Fe3O4 Nanoparticles
3.4. Characterization of Fe3O4 Nanoparticles
3.5. Peroxidase-Mimic Activity Assessment
3.6. Batch Experiments for Dye Degradation
3.7. Molecular Modeling and Computer Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, X.; Zhang, H. Persulfate activation by Fe(III) with bioelectricity at acidic and near-neutral pH regimes: Homogeneous versus heterogeneous mechanism. J. Hazard. Mater. 2019, 374, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Xu, W.; Dong, C.; Bai, Y.; Zeng, J.; Zhou, Y.; Zhang, J.; Yin, Y. Metal Sulfides as Excellent Co-catalysts for H2O2 Decomposition in Advanced Oxidation Processes. Chem 2018, 4, 1359–1372. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Ji, J.; Shen, B.; Xing, M.; Zhang, J. Enhancement of H2O2 Decomposition by the Co-catalytic Effect of WS2 on the Fenton Reaction for the Synchronous Reduction of Cr(VI) and Remediation of Phenol. Environ. Sci. Technol. 2018, 52, 11297–11308. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Bethi, B.; Sonawane, S.H.; Bhanvase, B.A.; Gumfekar, S.P. Nanomaterials-based advanced oxidation processes for wastewater treatment: A review. Chem. Eng. Process. Process Intensif. 2016, 109, 178–189. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Fan, J.; Gu, L.; Wu, D.; Liu, Z. Mackinawite (FeS) activation of persulfate for the degradation of p -chloroaniline: Surface reaction mechanism and sulfur-mediated cycling of iron species. Chem. Eng. J. 2018, 333, 657–664. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, H.; Liu, C.; Luo, S.; Liu, Y.; Yu, X.; Ma, J.; Yin, K.; Feng, H. Fast and efficient removal of As(III) from water by CuFe2O4 with peroxymonosulfate: Effects of oxidation and adsorption. Water Res. 2019, 150, 182–190. [Google Scholar] [CrossRef]
- Li, Z.; Yang, X.; Yang, Y.; Tan, Y.; He, Y.; Liu, M.; Liu, X.; Yuan, Q. Peroxidase-Mimicking Nanozyme with Enhanced Activity and High Stability based on Metal-Support Interaction. Chemistry 2017, 24, 409–415. [Google Scholar] [CrossRef]
- Pan, F.; Ji, H.; Du, P.; Huang, T.; Wang, C.; Liu, W. Insights into catalytic activation of peroxymonosulfate for carbamazepine degradation by MnO2 nanoparticles in-situ anchored titanate nanotubes: Mechanism, ecotoxicity and DFT study. J. Hazard. Mater. 2021, 402, 123779. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, Y.; Zhang, X.; Zhou, X.; Teng, X.; Yan, M.; Bi, H. Horseradish peroxidase-immobilized magnetic mesoporous silica nanoparticles as a potential candidate to eliminate intracellular reactive oxygen species. Nanoscale 2015, 7, 2941–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, Y.; Chen, C.S.; Tu, Y.J.; Huang, Y.H.; Zhang, H. Heterogeneous Degradation of Organic Pollutants by Persulfate Activated by CuO-Fe3O4: Mechanism, Stability, and Effects of pH and Bicarbonate Ions. Environ. Sci. Technol. 2015, 49, 6838–6845. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Shen, N.; Zhao, J.; Su, Y.; Ren, H. Glutathione-coated Fe3O4 nanoparticles with enhanced Fenton-like activity at neutral pH for degrading 2,4-dichlorophenol. J. Mater. Chem. A 2018, 6, 1275–1283. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, J.; Ding, Z.; Zhao, Z.; Xu, X.; Fang, Z. Ultrasound irritation enhanced heterogeneous activation of peroxymonosulfate with Fe3O4 for degradation of azo dye. Ultrason. Sonochem. 2017, 34, 953–959. [Google Scholar] [CrossRef]
- Sun, C.; Zhou, R.; E, J.; Sun, J.; Su, Y.; Ren, H. Ascorbic acid-coated Fe3O4 nanoparticles as a novel heterogeneous catalyst of persulfate for improving the degradation of 2,4-dichlorophenol. RSC Adv. 2016, 6, 10633–10640. [Google Scholar] [CrossRef]
- He, L.; Li, M.-X.; Chen, F.; Yang, S.-S.; Ding, J.; Ding, L.; Ren, N.-Q. Novel coagulation waste-based Fe-containing carbonaceous catalyst as peroxymonosulfate activator for pollutants degradation: Role of ROS and electron transfer pathway. J. Hazard. Mater. 2021, 417, 126113. [Google Scholar] [CrossRef]
- Xiao, S.; Cheng, M.; Zhong, H.; Liu, Z.F.; Liu, Y.; Yang, X.; Liang, Q.H. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review. Chem. Eng. J. 2020, 384, 123265. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Surface modification of nanozymes. Nano Res. 2017, 10, 1125–1148. [Google Scholar] [CrossRef]
- An, P.; Zuo, F.; Wu, Y.P.; Zhang, J.H.; Zheng, Z.H.; Ding, X.B.; Peng, Y.X. Fast synthesis of dopamine-coated Fe3O4nanoparticles through ligand-exchange method. Chin. Chem. Lett. 2012, 23, 1099–1102. [Google Scholar] [CrossRef]
- Xie, Y.; Yan, B.; Xu, H.; Chen, J.; Liu, Q.; Deng, Y.; Zeng, H. Highly regenerable mussel-inspired Fe(3)O(4)@polydopamine-Ag core-shell microspheres as catalyst and adsorbent for methylene blue removal. ACS Appl Mater. Interfaces 2014, 6, 8845–8852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, X.; Liu, B.; Liu, J. Molecular Imprinting on Inorganic Nanozymes for Hundred-fold Enzyme Specificity. J. Am. Chem Soc. 2017, 139, 5412–5419. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, W.; Lv, H.; Li, H.; Wang, Y.; Wang, P. Spacer arm-facilitated tethering of laccase on magnetic polydopamine nanoparticles for efficient biocatalytic water treatment. Chem. Eng. J. 2018, 350, 949–959. [Google Scholar] [CrossRef]
- Xiao, F.; Xiao, P.; Jiang, W.; Wang, D. Immobilization of horseradish peroxidase on Fe3O4 nanoparticles for enzymatic removal of endocrine disrupting chemicals. Environ. Sci. Pollut. Res. Int. 2020, 27, 24357–24368. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Zhu, Q.; Song, J.; Li, S.; Liu, X. Improved performance of immobilized laccase on Fe3O4@C-Cu2+ nanoparticles and its application for biodegradation of dyes. J. Hazard. Mater. 2020, 399, 123088. [Google Scholar] [CrossRef]
- Liu, S.; Huang, B.; Zheng, G.; Zhang, P.; Li, J.; Yang, B.; Chen, Y.T.; Liang, L. Nanocapsulation of horseradish peroxidase (HRP) enhances enzymatic performance in removing phenolic compounds. Int. J. Biol. Macromol. 2020, 150, 814–822. [Google Scholar] [CrossRef]
- Zheng, G.; Liu, S.; Zha, J.; Zhang, P.; Xu, X.; Chen, Y.; Jiang, S. Protecting Enzymatic Activity via Zwitterionic Nanocapsulation for the Removal of Phenol Compound from Wastewater. Langmuir 2019, 35, 1858–1863. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Deng, Y.; Deng, J.; Zhou, S.; Li, J.; Xin, X. Radical induced degradation of acetaminophen with Fe3O4magnetic nanoparticles as heterogeneous activator of peroxymonosulfate. J. Hazard. Mater. 2014, 276, 452–460. [Google Scholar] [CrossRef]
- De Laat, J.; Dao, Y.H.; El Najjar, N.H.; Daou, C. Effect of some parameters on the rate of the catalysed decomposition of hydrogen peroxide by iron(III)-nitrilotriacetate in water. Water Res. 2011, 45, 5654–5664. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Y.; Zha, J.; Han, H.; Chen, Y.; Jia, Z. Enhancing the peroxidase-mimicking activity of hemin by covalent immobilization in polymer nanogels. Polym. Chem. 2021, 12, 858–866. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Liu, Z.L.; Wang, H.B.; Lu, Q.H.; Du, G.H.; Peng, L.; Du, Y.Q.; Zhang, S.M.; Yao, K.L. Synthesis and characterization of ultrafine well-dispersed magnetic nanoparticles. J. Magn. Magn. Mater. 2004, 283, 258–262. [Google Scholar] [CrossRef]
- Ghosh, R.; Pradhan, L.; Devi, Y.P.; Meena, S.S.; Tewari, R.; Kumar, A.; Sharma, S.; Gajbhiye, N.S.; Vatsa, R.K.; Pandey, B.N.; et al. Induction heating studies of Fe3O4 magnetic nanoparticles capped with oleic acid and polyethylene glycol for hyperthermia. J. Mater. Chem. 2011, 21, 13388–13398. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, L.; He, J.; Guo, W.; Zhou, Z.; Zhang, X.; Nie, S.; Wei, H. Integrated Nanozymes with Nanoscale Proximity for in Vivo Neurochemical Monitoring in Living Brains. Anal. Chem. 2016, 88, 5489–5497. [Google Scholar] [CrossRef] [PubMed]
- Malde, A.K.; Zuo, L.; Breeze, M.; Stroet, M.; Poger, D.; Nair, P.C.; Oostenbrink, C.; Mark, A.E. An Automated Force Field Topology Builder (ATB) and Repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. [Google Scholar] [CrossRef] [PubMed]
- Reif, M.M.; Hunenberger, P.H.; Oostenbrink, C. New Interaction Parameters for Charged Amino Acid Side Chains in the GROMOS Force Field. J. Chem. Theory Comput. 2012, 8, 3705–3723. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Pronk, S.; Pall, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef]
- DeLano, W.L. The PyMOL Molecular Graphics System; DeLano Scientific: San Carlos, CA, USA, 2002. [Google Scholar]
- Jacinto, M.J.; Souto, R.S.; Silva, V.C.P.; Prescilio, I.C.; Kauffmann, A.C.; Soares, M.A.; de Souza, J.R.; Bakuzis, A.F.; Fontana, L.C. Biosynthesis of Cube-Shaped Fe3O4Nanoparticles for Removal of Dyes Using Fenton Process. Water Air Soil Pollut. 2021, 232, 270. [Google Scholar] [CrossRef]
- Kang, Y.-G.; Yoon, H.; Lee, C.-S.; Kim, E.-J.; Chang, Y.-S. Advanced oxidation and adsorptive bubble separation of dyes using MnO2-coated Fe3O4 nanocomposite. Water Res. 2019, 151, 413–422. [Google Scholar] [CrossRef]
- Yang, R.; Peng, Q.; Yu, B.; Shen, Y.; Cong, H. Yolk-shell Fe3O4@MOF-5 nanocomposites as a heterogeneous Fenton-like catalyst for organic dye removal. Sep. Purif. Technol. 2021, 267, 118620. [Google Scholar] [CrossRef]
- Li, K.; Zhao, Y.; Song, C.; Guo, X. Magnetic ordered mesoporous Fe3O4/CeO2 composites with synergy of adsorption and Fenton catalysis. Appl. Surf. Sci. 2017, 425, 526–534. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Li, S.; Tang, W.; Chen, Y. Magnetic porous Fe3O4/carbon octahedra derived from iron-based metal-organic framework as heterogeneous Fenton-like catalyst. Appl. Surf. Sci. 2018, 436, 252–262. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zha, J.; Wu, W.; Xie, P.; Han, H.; Fang, Z.; Chen, Y.; Jia, Z. Polymeric Nanocapsule Enhances the Peroxidase-like Activity of Fe3O4 Nanozyme for Removing Organic Dyes. Catalysts 2022, 12, 614. https://doi.org/10.3390/catal12060614
Zha J, Wu W, Xie P, Han H, Fang Z, Chen Y, Jia Z. Polymeric Nanocapsule Enhances the Peroxidase-like Activity of Fe3O4 Nanozyme for Removing Organic Dyes. Catalysts. 2022; 12(6):614. https://doi.org/10.3390/catal12060614
Chicago/Turabian StyleZha, Junqi, Wugao Wu, Peng Xie, Honghua Han, Zheng Fang, Yantao Chen, and Zhongfan Jia. 2022. "Polymeric Nanocapsule Enhances the Peroxidase-like Activity of Fe3O4 Nanozyme for Removing Organic Dyes" Catalysts 12, no. 6: 614. https://doi.org/10.3390/catal12060614
APA StyleZha, J., Wu, W., Xie, P., Han, H., Fang, Z., Chen, Y., & Jia, Z. (2022). Polymeric Nanocapsule Enhances the Peroxidase-like Activity of Fe3O4 Nanozyme for Removing Organic Dyes. Catalysts, 12(6), 614. https://doi.org/10.3390/catal12060614






