Preparation, Characterization, and Catalytic Properties of Pd-Graphene Quantum Dot Catalysts
Abstract
:1. Introduction
2. Results
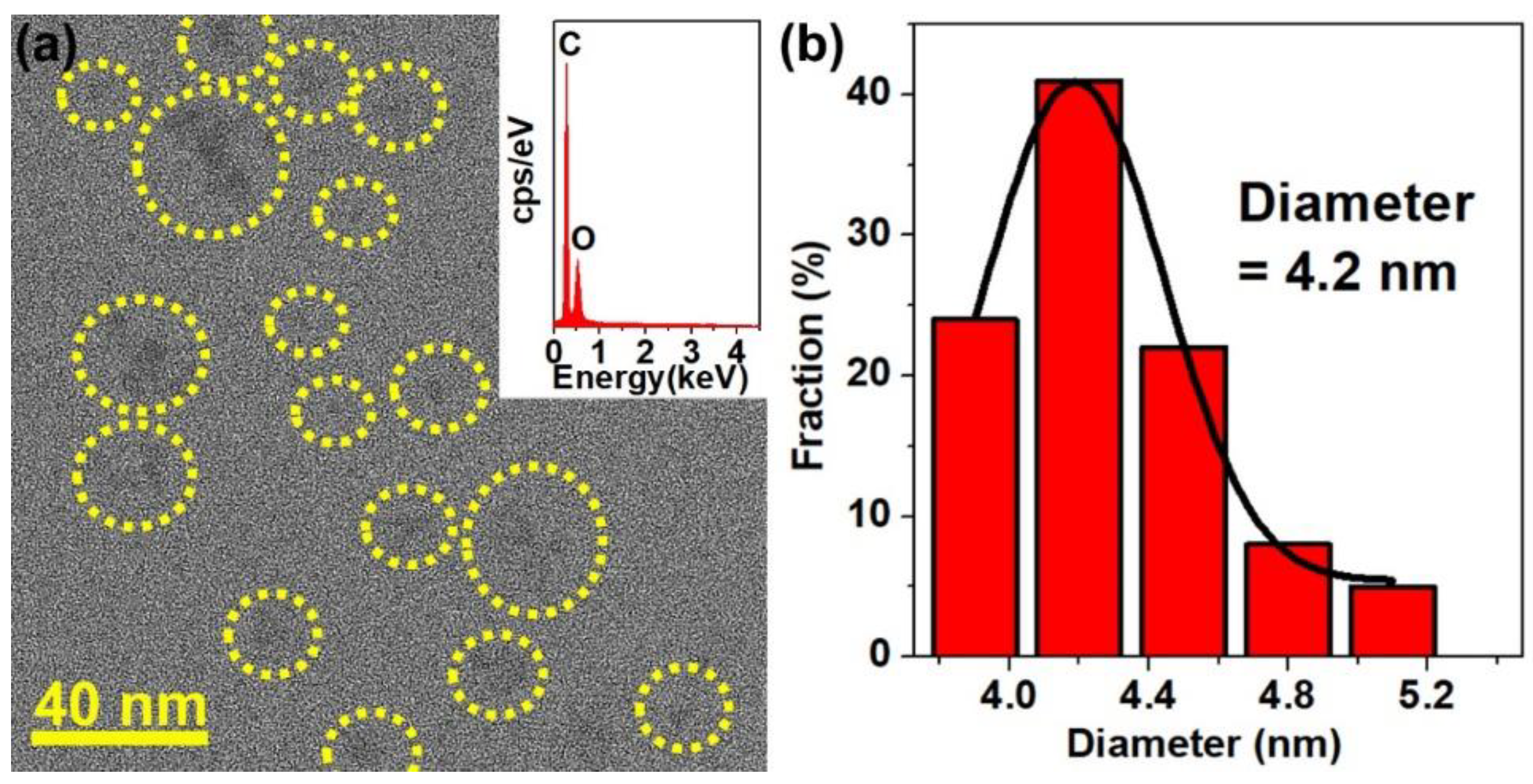
2.1. Characterization of the Catalyst
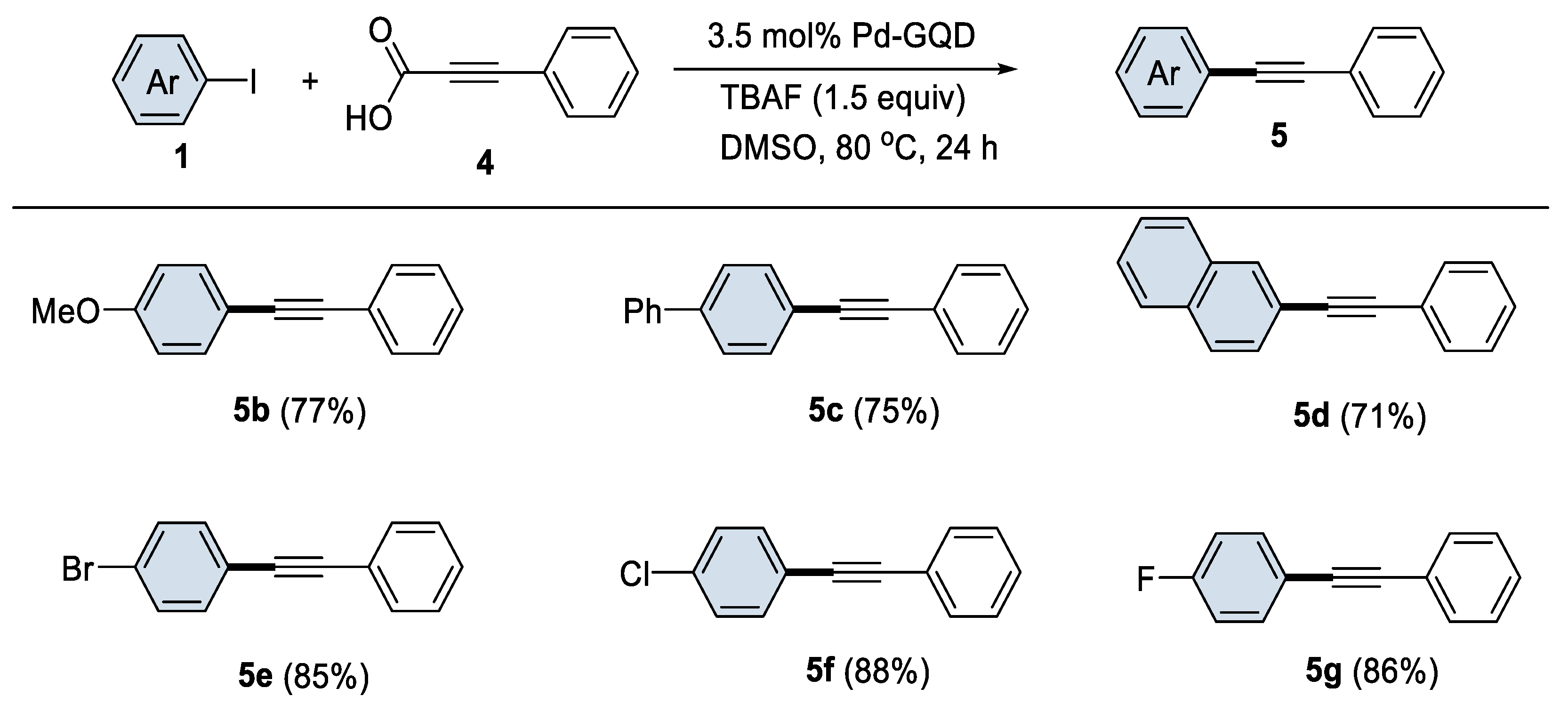
2.2. Organic Catalysis Study
3. Materials and Methods
3.1. Materials
3.2. Preparation of GQDs
3.3. Preparation of Pd-GQDs
3.4. Characterization
3.5. Catalytic Activity Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, B.; Zhang, Y.; Li, L.; Shao, Q.; Huang, X. Recent progress in low-dimensional palladium-based nanostructures for electrocatalysis and beyond. Coord. Chem. Rev. 2022, 459, 214388. [Google Scholar] [CrossRef]
- Sanij, F.D.; Balakrishnan, P.; Leung, P.; Shah, A.; Su, H.; Xu, Q. Advanced Pd-based nanomaterials for electrocatalytic oxygen reduction in fuel cells: A review. Int. J. Hydrog. Energy 2021, 46, 14596–14627. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Dadashi, J.; Ghafuri, H. Pd-based nanoparticles: Plant-assisted biosynthesis, characterization, mechanism, stability, catalytic and antimicrobial activities. Adv. Colloid Interface Sci. 2020, 276, 102103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; He, Y.; Zhao, J.; Qiu, Y.; Zheng, L.; Hu, J.; Yang, Y.; Liu, X.; Liang, Y. Palladium-Catalyzed Regioselective Difluoroalkylation and Carbonylation of Alkynes. Org. Lett. 2016, 18, 2664–2667. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, A.M.; Ziółkowski, J.J. Monomolecular, nanosized and heterogenized palladium catalysts for the Heck reaction. Coord. Chem. Rev. 2007, 251, 1281–1293. [Google Scholar] [CrossRef]
- Jose, D.E.; Kanchana, U.S.; Mathew, T.V.; Anilkumar, G. Recent Studies in Suzuki-Miyaura cross-coupling reactions with the aid of phase transfer catalysts. J. Organomet. Chem. 2020, 927, 121538. [Google Scholar] [CrossRef]
- Heravi, M.M.; Mohammadkhani, L. Recent applications of Stille reaction in total synthesis of natural products: An update. J. Organomet. Chem. 2018, 869, 106–200. [Google Scholar] [CrossRef]
- McCarthy, S.; Braddock, D.C.; Wilton-Ely, J.D.E.T. Strategies for sustainable palladium catalysis. Coord. Chem. Rev. 2021, 442, 213925. [Google Scholar] [CrossRef]
- Cavinato, G.; Toniolo, L. High molecular weight polyketones by CO-ethene copolymerization catalysed with Pd(II)-phosphine complexes in homogeneous phase in formic acid. Inorg. Chem. Commun. 2021, 123, 108358. [Google Scholar] [CrossRef]
- Sunsandee, N.; Phatanasri, S.; Pancharoen, U. Separation of homogeneous palladium catalysts from pharmaceutical industry wastewater by using synergistic recovery phase via HFSLM system. Arab. J. Chem. 2021, 14, 103024. [Google Scholar] [CrossRef]
- Boztepe, C.; Künkül, A.; Yaşar, S.; Gürbüz, N. Heterogenization of homogeneous NHC-Pd-pyridine catalysts and investigation of their catalytic activities in Suzuki-Miyaura coupling reactions. J. Organomet. Chem. 2018, 872, 123–134. [Google Scholar] [CrossRef]
- Li, M.; Li, Z.; Lin, Q.; Cao, J.; Liu, F.; Kawi, S. Synthesis strategies of carbon nanotube supported and confined catalysts for thermal catalysis. Chem. Eng. J. 2022, 431, 133970. [Google Scholar] [CrossRef]
- Gopinath, A.; Pisharody, L.; Popat, A.; Nidheesh, P.V. Supported catalysts for heterogeneous electro-Fenton processes: Recent trends and future directions. Curr. Opin. Solid State Mater. Sci. 2022, 26, 100981. [Google Scholar] [CrossRef]
- Chernyshev, V.M.; Khazipov, O.V.; Eremin, D.B.; Denisova, E.A.; Ananikov, V.P. Formation and stabilization of nanosized Pd particles in catalytic systems: Ionic nitrogen compounds as catalytic promoters and stabilizers of nanoparticles. Coord. Chem. Rev. 2021, 437, 213860. [Google Scholar] [CrossRef]
- Li, C.; Zhang, C.; Liu, R.; Wang, L.; Zhang, X.; Li, G. Heterogeneously supported active Pd(0) complex on silica mediated by PEG as efficient dimerization catalyst for the production of high energy density fuel. Mol. Catal. 2022, 520, 112170. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Q.; Luo, M.; Yang, Z.; Wang, F.; Li, H. Dendritic mesoporous silica nanosphere supported highly dispersed Pd-CoOx catalysts for catalytic oxidation of toluene. Mol. Catal. 2022, 519, 112123. [Google Scholar] [CrossRef]
- Ariannezhad, M.; Pourmorteza, N.; Yousefi, A.; Esperi, M. Catalytic reduction of nitroarenes and Suzuki-Miyaura reactions using Pd complex stabilized on the functionalized polymeric support. Chem. Phys. Lett. 2022, 793, 139431. [Google Scholar] [CrossRef]
- Liguori, F.; Oldani, C.; Capozzoli, L.; Calisi, N.; Barbaro, P. Liquid-phase synthesis of methyl isobutyl ketone over bifunctional heterogeneous catalysts comprising cross-linked perfluorinated sulfonic acid Aquivion polymers and supported Pd nanoparticles. Appl. Catal. A Gen. 2021, 610, 117957. [Google Scholar] [CrossRef]
- Kim, H.J.; Song, C. A refined design concept for sulfur-tolerant Pd catalyst supported on zeolite by shape-selective exclusion and hydrogen spillover for hydrogenation of aromatics. J. Catal. 2021, 403, 203–214. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Baran, T.; Baran, N.Y.; Sajjadi, M.; Tahsili, M.R.; Shokouhimehr, M. Pd nanocatalyst stabilized on amine-modified zeolite: Antibacterial and catalytic activities for environmental pollution remediation in aqueous medium. Sep. Purif. Technol. 2020, 239, 116542. [Google Scholar] [CrossRef]
- Alkabli, J.; Rizk, M.A.; Elshaarawy, R.F.M.; El-Sayed, W.N. Ionic chitosan Schiff bases supported Pd(II) and Ru(II) complexes; production, characterization, and catalytic performance in Suzuki cross-coupling reaction. Int. J. Biol. Macromol. 2021, 184, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Kargar, S.; Elhamifar, D.; Elhamifar, D. Ionic liquid-containing polyethylene supported palladium: A green, highly efficient and stable catalyst for Suzuki reaction. Mater. Today Chem. 2020, 17, 100318. [Google Scholar] [CrossRef]
- Ponomarenko, L.A.; Schedin, F.; Katsnelson, M.I.; Yang, R.; Hill, E.E.; Novoselov, K.S.; Geim, A.K. Chaotic dirac billiard in graphene quantum dots. Science 2008, 320, 356–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Shen, J.; Chen, S.; Yan, J.; Zhang, N.; Zheng, K.; Liu, X. Synthesis of graphene quantum dot-stabilized gold nanoparticles and their application. RSC Adv. 2019, 9, 21215–21219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Yu, X.; Yu, X.; Du, R.; Liu, L.; Zhang, Y. Graphene quantum dots decorated ZnO-ZnFe2O4 nanocages and their visible light photocatalytic activity. Appl. Surf. Sci. 2019, 478, 991–997. [Google Scholar] [CrossRef]
- Tian, P.; Tang, L.; Teng, K.S.; Lau, S.P. Graphene quantum dots from chemistry to applications. Mater. Today Chem. 2018, 10, 221–258. [Google Scholar] [CrossRef]
- Du, Z.; Shen, S.; Tang, Z.; Yang, J. Graphene quantum dots-based heterogeneous catalysts. New Carbon Mater. 2021, 36, 449–467. [Google Scholar] [CrossRef]
- Roy, E.; Nagar, A.; Sharma, A.; Roy, S.; Pal, S. Graphene quantum dots and its modified application for energy storage and conversion. J. Energy Storage 2021, 39, 102606. [Google Scholar] [CrossRef]
- Cheng, C.; Liang, Q.; Yan, M.; Liu, Z.; He, Q.; Wu, T.; Luo, S.; Pan, Y.; Zhao, C.; Liu, Y. Advances in preparation, mechanism and applications of graphene quantum dots/semiconductor composite photocatalysts: A review. J. Hazard. Mater. 2022, 424, 127721. [Google Scholar] [CrossRef]
- Jeong, Y.N.; Choi, M.Y.; Choi, H.C. Preparation of Pt- and Pd-decorated CNTs by DCC-activated amidation and investigation of their electrocatalytic activities. Electrochim. Acta 2012, 60, 78–84. [Google Scholar] [CrossRef]
- Kim, J.D.; Palani, T.; Kumar, M.R.; Lee, S.; Choi, H.C. Preparation of reusable Ag-decorated graphene oxide catalysts for decarboxylative cycloaddition. J. Mater. Chem. 2012, 22, 20665–20670. [Google Scholar] [CrossRef]
- Kim, S.P.; Choi, M.Y.; Choi, H.C. Characterization and photocatalytic performance of SnO2–CNT nanocomposites. Appl. Surf. Sci. 2015, 357, 302–308. [Google Scholar] [CrossRef]
- Lim, J.; Lim, J.D.; Choi, H.C.; Lee, S. CNT-CuO catalyzed C−N bond formation for N-arylation of 2-phenylindoles. J. Organomet. Chem. 2019, 902, 120970. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, K.; Bae, S.Y.; Kim, G.C.; Lee, S.; Choi, H.C. Preparation, characterization and catalytic properties of Pd-decorated carbon nanotubes possessing different linkers. J. Mater. Chem. 2011, 21, 5999–6005. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jo, Y.; Kook, S.; Lee, S.; Choi, H.C. Synthesis of carbon nanotube supported Pd catalysts and evaluation of their catalytic properties for C-C bond forming reactions. J. Mol. Catal. A 2010, 323, 28–32. [Google Scholar] [CrossRef]
- Kalita, H.; Palaparthy, V.S.; Baghini, M.S.; Aslam, M. Electrochemical synthesis of graphene quantum dots from graphene oxide at room temperature and its soil moisture sensing properties. Carbon 2020, 165, 9–17. [Google Scholar] [CrossRef]
- Mukherjee, D.; Das, P.; Kundu, S.; Mandal, B. Engineering of graphene quantum dots by varying the properties of graphene oxide for fluorescence detection of picric acid. Chemosphere 2022, 300, 134432. [Google Scholar] [CrossRef]
- Ma, Q.; Song, J.; Wang, S.; Yang, J.; Guo, Y.; Dong, C. A general sensing strategy for detection of Fe3+ by using aminoacid-modified graphene quantum dots as fluorescent probe. Appl. Surf. Sci. 2016, 389, 995–1002. [Google Scholar] [CrossRef]
- Yan, Y.; Manickam, S.; Lester, E.; Wu, T.; Pang, C.H. Synthesis of graphene oxide and graphene quantum dots from miscanthus via ultrasound-assisted mechano-chemical cracking method. Ultrason. Sonochem. 2021, 73, 105519. [Google Scholar] [CrossRef]
- Ying, Z.; Diao, J.; Wang, S.; Cai, X.; Cai, Y.; Liu, H.; Wang, N. In situ atomic-scale studies of thermal stability and surface reconstruction of ZnO nanowires based Pd nanocatalysts. Mater. Des. 2021, 209, 109947. [Google Scholar] [CrossRef]
- Ma, K.; Liao, W.; Shi, W.; Xu, F.; Zhou, Y.; Tang, C.; Lu, J.; Shen, W.; Zhang, Z. Ceria-supported Pd catalysts with different size regimes ranging from single atoms to nanoparticles for the oxidation of CO. J. Catal. 2022, 407, 104–114. [Google Scholar] [CrossRef]
- Kim, J.D.; Choi, M.Y.; Choi, H.C. Graphene-oxide-supported Pt nanoparticles with high activity and stability for hydrazine electro-oxidation in a strong acidic solution. Appl. Surf. Sci. 2017, 420, 700–706. [Google Scholar] [CrossRef]
- Ritter, U.; Scharff, P.; Siegmund, C.; Dmytrenko, O.P.; Kulish, N.P.; Prylutskyy, Y.I.; Belyi, N.M.; Gubanov, V.A.; Komarova, L.I.; Lizunova, S.V.; et al. Radiation damage to multi-walled carbon nanotubes and their Raman vibrational modes. Carbon 2006, 44, 2694–2700. [Google Scholar] [CrossRef]
- Pierlot, A.P.; Woodhead, A.L.; Church, J.S. Thermal annealing effects on multi-walled carbon nanotube yarns probed by Raman spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 117, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Fan, H.; Jiang, W.; Ni, G.; Qu, S. Amino-functionalized graphene quantum dots prepared using high-softening point asphalt and their application in Fe3+ detection. Appl. Surf. Sci. 2019, 467–468, 446–455. [Google Scholar] [CrossRef]
- Liu, Z.; Tan, L.; Hou, P.; Jin, X.; Li, M.; Zhou, Q.; Liao, P.; Zeng, Z.; Deng, S.; Dai, G. One-pot synthesis of single-component graphene quantum dots for stable and bright white luminescence films as a phosphor. Opt. Mater. 2022, 127, 112368. [Google Scholar] [CrossRef]
- Moon, J.; Jeong, M.; Nam, H.; Ju, J.; Moon, J.H.; Jung, H.M.; Lee, S. One-pot synthesis of diarylalkynes using palladium-catalyzed Sonogashira reaction and decarboxylative coupling of sp carbon and sp2 carbon. Org. Lett. 2008, 10, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yan, X.; Chen, J.; Xue, Q. Superior micro-supercapacitors based on graphene quantum dots. Adv. Funct. Mater. 2013, 23, 4111–4122. [Google Scholar] [CrossRef]
- Xie, J.; Lai, G.; Huq, M.M. Hydrothermal route to graphene quantum dots: Effects of precursor and temperature. Diam. Relat. Mater. 2017, 79, 112–118. [Google Scholar] [CrossRef]



 | ||
| Entry | Pd Catalyst | Yield (%) |
|---|---|---|
| 1 | Pd-GQD | 84 |
| 2 | Pd-GO | 72 |
| 3 | Pd-CNT | 49 |
| 4 | Commercial Pd/C | 15 |
| 5 | GQD | Not detected |
| 6 | GO | Not detected |
| 7 | CNT | Not detected |
| 8 | - | Not detected |
 | ||
| Entry | Pd Catalyst | Yield (%) |
|---|---|---|
| 1 | Pd-GQD | 82 |
| 2 | Pd-GO | 41 |
| 3 | Pd-CNT | 35 |
| 4 | Commercial Pd/C | 34 |
| 5 | GQD | Not detected |
| 6 | - | Not detected |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lim, J.; Kim, J.D.; Choi, M.Y.; Lee, S.; Choi, H.C. Preparation, Characterization, and Catalytic Properties of Pd-Graphene Quantum Dot Catalysts. Catalysts 2022, 12, 619. https://doi.org/10.3390/catal12060619
Kim J, Lim J, Kim JD, Choi MY, Lee S, Choi HC. Preparation, Characterization, and Catalytic Properties of Pd-Graphene Quantum Dot Catalysts. Catalysts. 2022; 12(6):619. https://doi.org/10.3390/catal12060619
Chicago/Turabian StyleKim, Jisoo, Jeongah Lim, Ji Dang Kim, Myong Yong Choi, Sunwoo Lee, and Hyun Chul Choi. 2022. "Preparation, Characterization, and Catalytic Properties of Pd-Graphene Quantum Dot Catalysts" Catalysts 12, no. 6: 619. https://doi.org/10.3390/catal12060619
APA StyleKim, J., Lim, J., Kim, J. D., Choi, M. Y., Lee, S., & Choi, H. C. (2022). Preparation, Characterization, and Catalytic Properties of Pd-Graphene Quantum Dot Catalysts. Catalysts, 12(6), 619. https://doi.org/10.3390/catal12060619








