Quantitative Study of the Enhanced Content and Chemical Stability of Functional Groups in Mesoporous Silica by In-Situ Co-condensation Synthesis
Abstract
1. Introduction
2. Results and Discussions
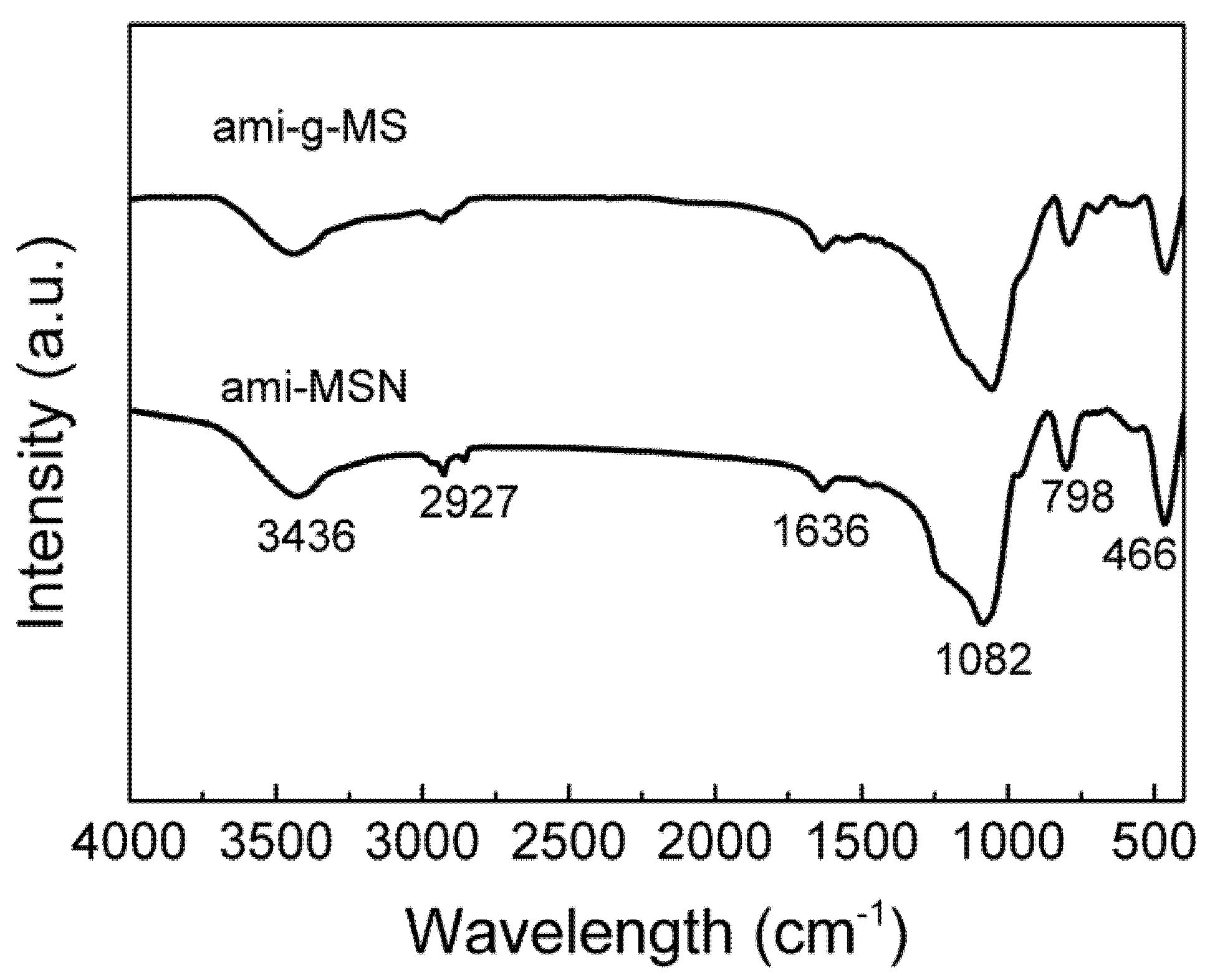
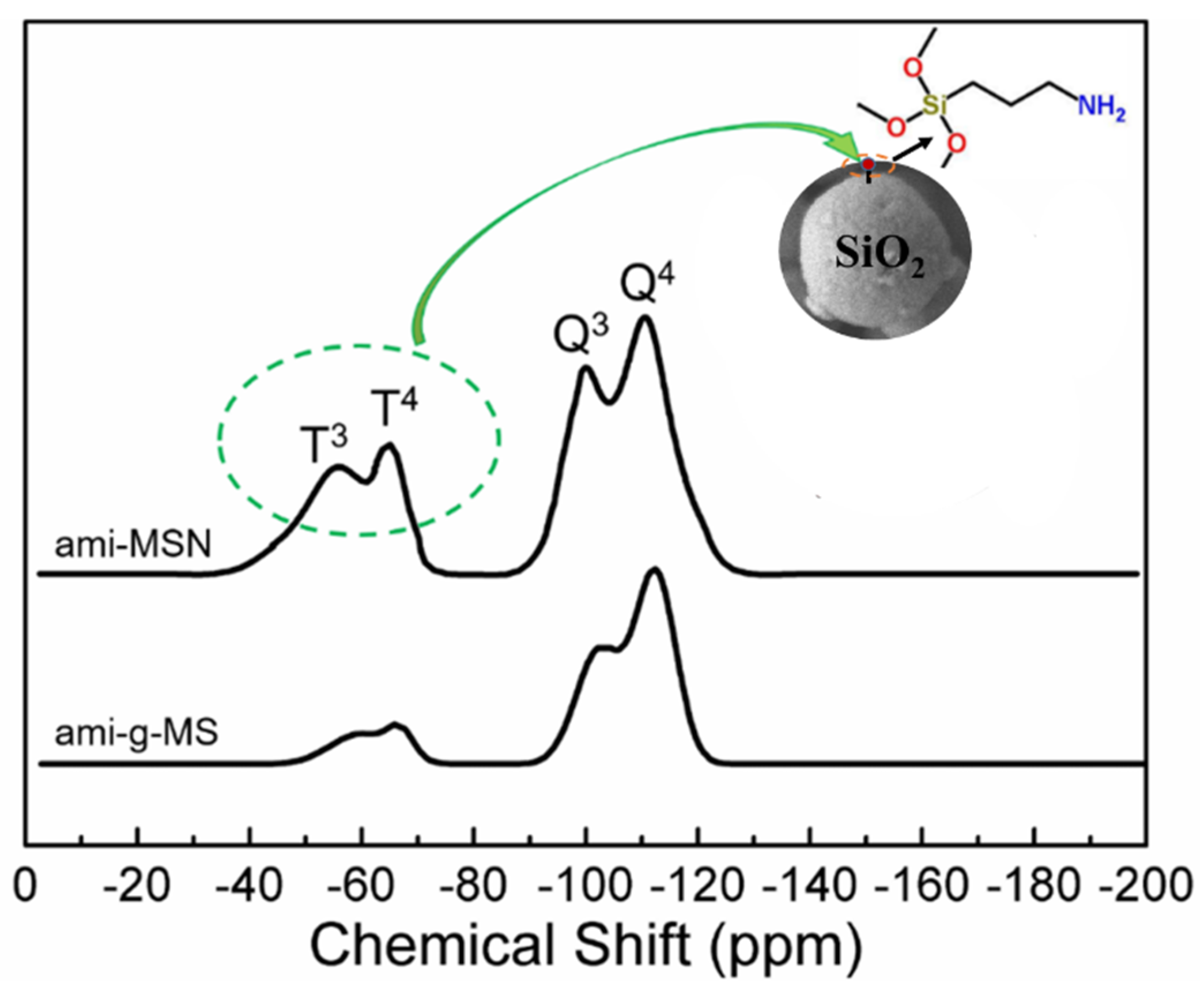
2.1. Material Characterizations
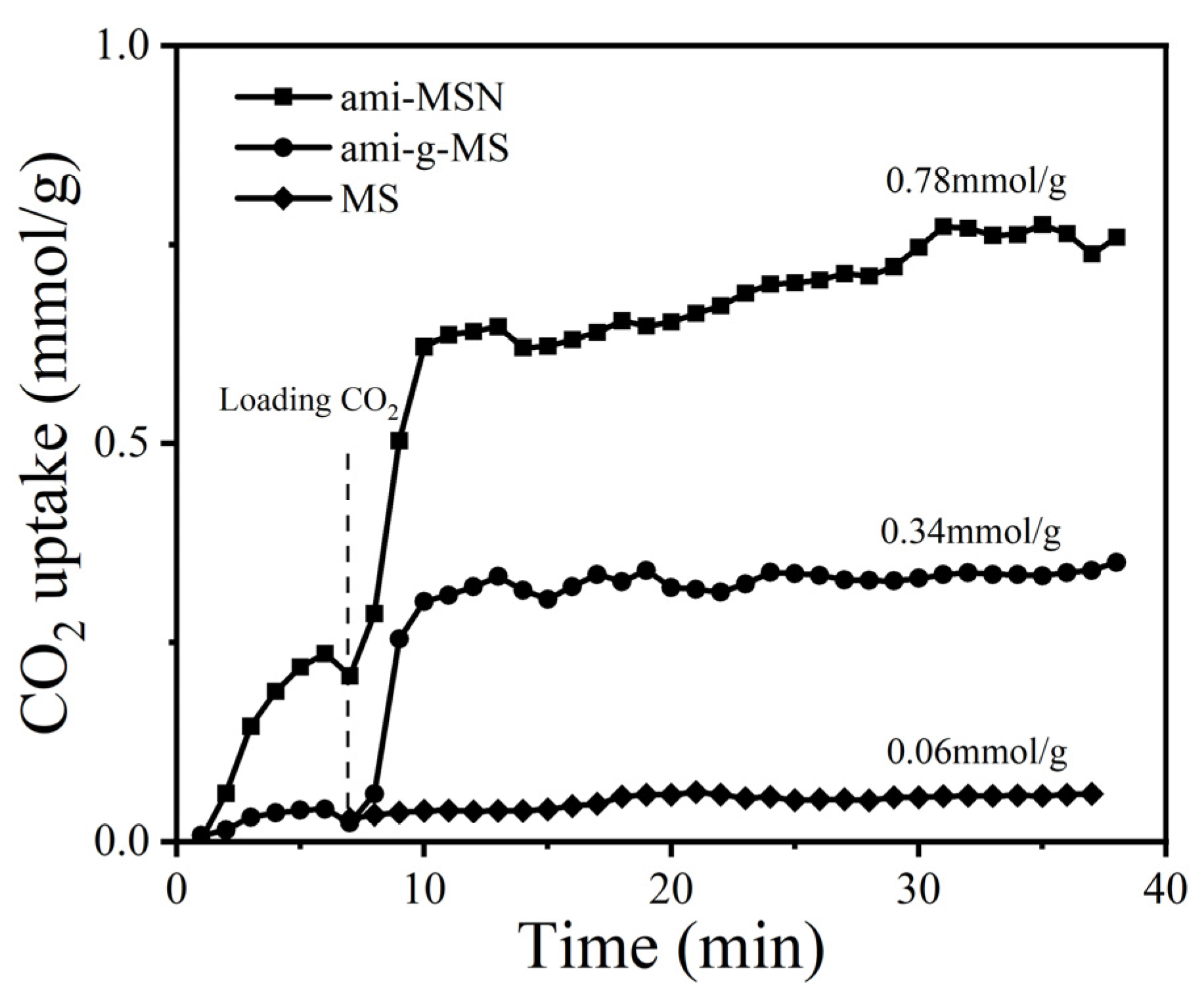
2.2. Sorption Experiments
3. Experimental
3.1. Chemicals
3.2. Preparation of Materials
3.3. Characterization
3.4. Determination of the Removal Efficiency of Template Agent
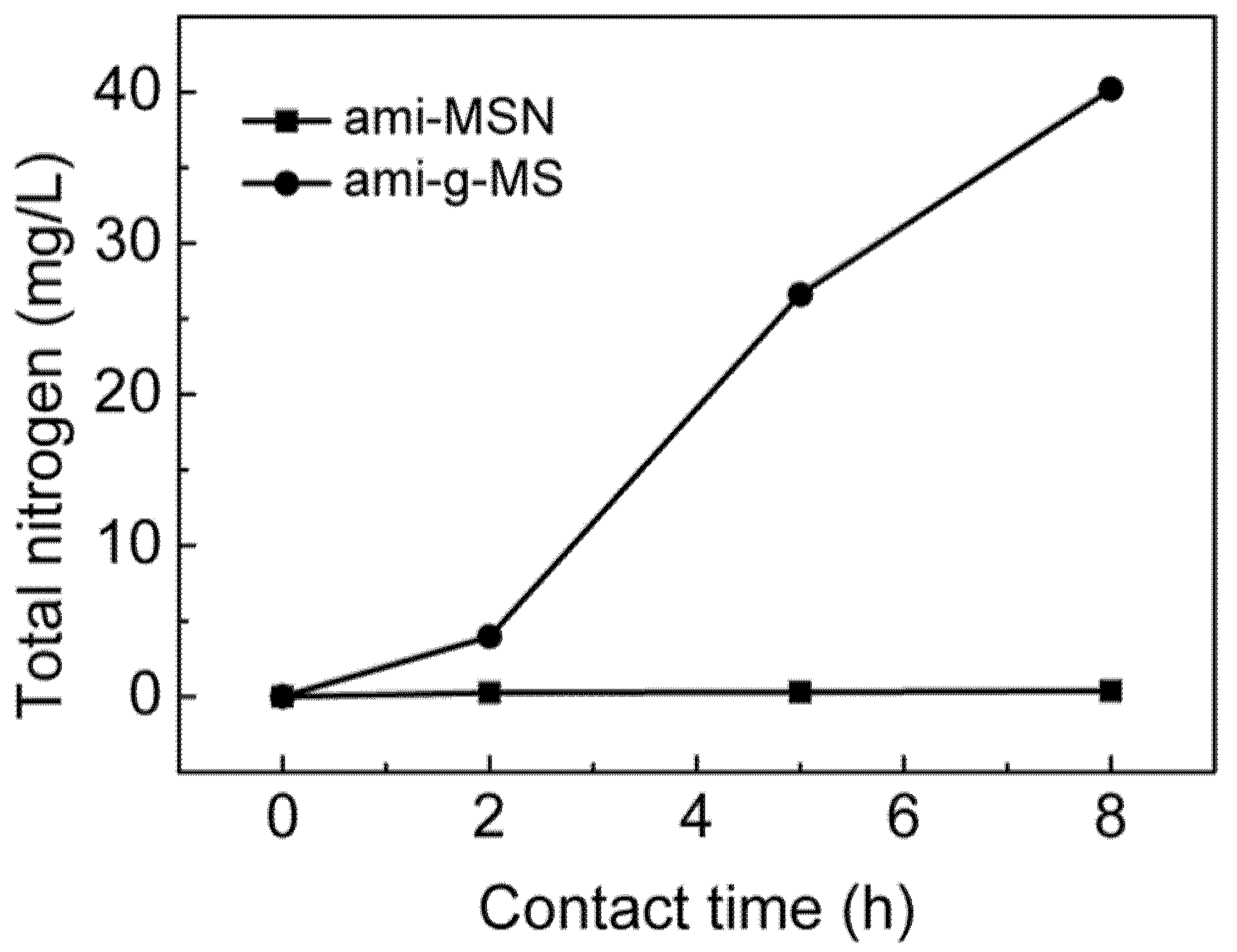
3.5. Determination of the Stability of Functional Groups
3.6. Batch Sorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yuan, T.; Chen, Q.; Shen, X. Adsorption of cesium using mesoporous silica gel evenly doped by Prussian blue nanoparticles. Chin. Chem. Lett. 2020, 31, 2835–2838. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chen, C.H.; Cheng, S.; Li, H.Y. Adsorption of Pb(II) and Cu(II) metal ions on functionalized large-pore mesoporous silica. Int. J. Environ. Sci. Technol. 2016, 13, 65–76. [Google Scholar] [CrossRef]
- Qiao, W.; Zhang, P.; Sun, L.; Ma, S.; Xu, W.; Xu, S.; Niu, Y. Adsorption performance and mechanism of Schiff base functionalized polyamidoamine dendrimer/silica for aqueous Mn(II) and Co(II). Chin. Chem. Lett. 2020, 31, 2742–2746. [Google Scholar] [CrossRef]
- Zamani, C.; Illa, X.; Abdollahzadeh-Ghom, S.; Morante, J.R.; Rodriguez, A.R. Mesoporous Silica: A Suitable Adsorbent for Amines. Nanoscale Res. Lett. 2009, 4, 1303–1308. [Google Scholar] [CrossRef][Green Version]
- Sha, X.; Dai, Y.; Song, X.; Liu, S.; Zhang, S.; Li, J. The Opportunities and Challenges of Silica Nanomaterial for Atherosclerosis. Int. J. Nanomed. 2021, 16, 701–714. [Google Scholar] [CrossRef]
- Li, J.; Miao, X.; Hao, Y.; Zhao, J.; Sun, X.; Wang, L. Synthesis, amino-functionalization of mesoporous silica and its adsorption of Cr(VI). J. Colloid Interface Sci. 2008, 318, 309–314. [Google Scholar] [CrossRef]
- Chung, J.; Chun, J.; Lee, J.; Lee, S.H.; Lee, Y.J.; Hong, S.W. Sorption of Pb(II) and Cu(II) onto multi-amine grafted mesoporous silica embedded with nano-magnetite: Effects of steric factors. J. Hazard. Mater. 2012, 239–240, 183. [Google Scholar] [CrossRef]
- Heidari, A.; Younesi, H.; Mehraban, Z. Removal of Ni(II), Cd(II), and Pb(II) from a ternary aqueous solution by amino functionalized mesoporous and nano mesoporous silica. Chem. Eng. J. 2009, 153, 70–79. [Google Scholar] [CrossRef]
- Ghorbani, M.; Nowee, S.M.; Ramezanian, N.; Raji, F. A new nanostructured material amino functionalized mesoporous silica synthesized via co-condensation method for Pb(II) and Ni(II) ion sorption from aqueous solution. Hydrometallurgy 2016, 161, 117–126. [Google Scholar] [CrossRef]
- Wang, S.; Wang, K.; Dai, C.; Shi, H.; Li, J. Adsorption of Pb 2+ on amino-functionalized core–shell magnetic mesoporous SBA-15 silica composite. Chem. Eng. J. 2015, 262, 897–903. [Google Scholar] [CrossRef]
- Yokoi, T.; Kubota, Y.; Tatsumi, T. Amino-functionalized mesoporous silica as base catalyst and adsorbent. Appl. Catal. A Gen. 2012, 421–422, 14–37. [Google Scholar] [CrossRef]
- Lei, X.; Zhang, A.; Zhang, F.; Liu, J. Preparation and characterization of a novel macroporous silica-bipyridine asymmetric multidentate functional adsorbent and its application for heavy metal palladium removal. J. Hazard. Mater. 2017, 337, 178–188. [Google Scholar]
- Walcarius, A.; Etienne, M.; Lebeau, B. Rate of Access to the Binding Sites in Organically Modified Silicates. 2. Ordered Mesoporous Silicas Grafted with Amine or Thiol Groups. Chem. Mater. 2003, 15, 2161–2173. [Google Scholar] [CrossRef]
- Shephard, D.S.; Zhou, W.; Maschmeyer, T.; Matters, J.M.; Roper, C.L.; Parsons, S.; Johnson, B.F.G.; Duer, M.J. Site-Directed Surface Derivatization of MCM-41: Use of High-Resolution Transmission Electron Microscopy and Molecular Recognition for Determining the Position of Functionality within Mesoporous Materials. Angew. Chem. Int. Ed. 2010, 37, 2719–2723. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, W.; Chai, Z.; Kuraoka, E. Modification of a novel macroporous silica-based crown ether impregnated polymeric composite with 1-dodecanol and its adsorption for some fission and non-fission products contained in high level liquid waste. Eur. Polym. J. 2008, 44, 3899–3907. [Google Scholar] [CrossRef]
- Kang, H.J.H.; Ali, R.F.; Paul, M.T.Y.; Radford, M.J.; Andreu, I.; Lee, A.W.H.; Gates, B.D. Tunable functionalization of silica coated iron oxide nanoparticles achieved through a silanol-alcohol condensation reaction. Chem. Commun. 2019, 55, 10452–10455. [Google Scholar] [CrossRef]
- Estevao, B.M.; Miletto, I.; Hioka, N.; Marchese, L.; Gianotti, E. Mesoporous Silica Nanoparticles Functionalized with Amino Groups for Biomedical Applications. Chemistryopen 2021, 10, 1251–1259. [Google Scholar] [CrossRef]
- Rico, M.; Sepulveda, A.E.; Ruiz, S.; Serrano, E.; Berenguer, J.R.; Lalinde, E.; Garcia-Martinez, J. A stable luminescent hybrid mesoporous copper complex-silica. Chem. Commun. 2012, 48, 8883–8885. [Google Scholar] [CrossRef]
- Tomer, V.K.; Malik, R.; Jangra, S.; Nehra, S.P.; Duhan, S. One pot direct synthesis of mesoporous SnO2/SBA-15 nanocomposite by the hydrothermal method. Mater. Lett. 2014, 132, 228–230. [Google Scholar] [CrossRef]
- Walcarius, A.; Delacôte, C. Rate of Access to the Binding Sites in Organically Modified Silicates. 3. Effect of Structure and Density of Functional Groups in Mesoporous Solids Obtained by the Co-Condensation Route. Chem. Mater. 2003, 15, 4181–4192. [Google Scholar] [CrossRef]
- Kazemzadeh, P.; Sayadi, K.; Toolabi, A.; Sayadi, J.; Zeraati, M.; Chauhan, N.P.S.; Sargazi, G. Structure-Property Relationship for Different Mesoporous Silica Nanoparticles and its Drug Delivery Applications: A Review. Front. Chem. 2022, 10, 823785. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Xu, L.; Chen, M.; Cui, Y.; Wen, X.; Wu, C.-e.; Yang, B.; Wang, F.; Miao, Z.; Hu, X.; et al. Constructing highly dispersed Ni based catalysts supported on fibrous silica nanosphere for low-temperature CO2 methanation. Fuel 2020, 278, 118333. [Google Scholar] [CrossRef]
- Rameli, N.; Jumbri, K.; Wahab, R.A.; Ramli, A.; Huyop, F. Synthesis and characterization of mesoporous silica nanoparticles using ionic liquids as a template. J. Phys. Conf. Ser. 2018, 1123, 012068. [Google Scholar] [CrossRef]
- Walrafen, G.E.; Stone, J. Raman Spectral Characterization of Pure and Doped Fused Silica Optical Fibers. Appl. Spectrosc. 1975, 29, 337–344. [Google Scholar] [CrossRef]
- Giraldo, L.; Moreno-Piraján, J.C. Study on the adsorption of heavy metal ions from aqueous solution on modified SBA-15. Mater. Res. 2013, 16, 745–754. [Google Scholar] [CrossRef][Green Version]
- Long, D.A. Infrared and Raman characteristic group frequencies. Tables and charts George Socrates John Wiley and Sons, Ltd., Chichester, Third Edition, 2001. Price £135. J. Raman Spectrosc. 2004, 35, 905. [Google Scholar] [CrossRef]
- Saraswati, T.E.; Astuti, A.R.; Rismana, N. Quantitative analysis by UV-Vis absorption spectroscopy of amino groups attached to the surface of carbon-based nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2018, 333, 012027. [Google Scholar] [CrossRef]
- Takeda, M.; Iavazzo, R.E.S.; Garfinkel, D.; Scheinberg, I.H.; Edsall, J.T. Raman Spectra of Amino Acids and Related Compounds. IX. Ionization and Deuterium Substitution in Glycine, Alanine and β-Alanine1,2,3. J. Am. Chem. Soc. 1958, 80, 3813–3818. [Google Scholar] [CrossRef]
- Yoncheva, K.; Popova, M.; Szegedi, A.; Mihaly, J.; Tzankov, B.; Lambov, N.; Konstantinov, S.; Tzankova, V.; Pessina, F.; Valoti, M. Functionalized mesoporous silica nanoparticles for oral delivery of budesonide. J. Solid State Chem. 2014, 211, 154–161. [Google Scholar] [CrossRef]
- Ulfa, M.; Miftahul Jannah, A. Synthesis of Mesoporous Fe2o3/SBA-15 and its Application for Nickel (II) Ion Adsorption. Orient. J. Chem. 2018, 34, 420–427. [Google Scholar] [CrossRef]
- Ulfa, M.; Prasetyoko, D. Infrared Spectroscopic and Scanning Electron Microscopy Study of Ibuprofen Loading onto the Molecular Sieve Mesoporous Silica SBA-15 Material. Orient. J. Chem. 2018, 34, 2631–2636. [Google Scholar] [CrossRef]
- Margolese, D.; Melero, J.A.; Christiansen, S.C.; Chmelka, B.F.; Stucky, G.D. Direct Syntheses of Ordered SBA-15 Mesoporous Silica Containing Sulfonic Acid Groups. Chem. Mater. 2000, 12, 2448–2459. [Google Scholar] [CrossRef]
- Ng, E.P.; Mintova, S. Nanoporous materials with enhanced hydrophilicity and high water sorption capacity. Microporous Mesoporous Mater. 2008, 114, 1–26. [Google Scholar] [CrossRef]
- Xuan, W.; Zhu, C.; Liu, Y.; Cui, Y. Mesoporous metal-organic framework materials. Chem. Soc. Rev. 2012, 41, 1677–1695. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Feng, Y.; Xiong, T.; Li, Y.; Wahia, H.; Ma, H. Preparation of corn ACE inhibitory peptide-ferrous chelate by dual-frequency ultrasound and its structure and stability analyses. Ultrason. Sonochem. 2022, 83, 105937. [Google Scholar] [CrossRef] [PubMed]
- Gan, F.; Wang, B.; Jin, Z.; Xie, L.; Dai, Z.; Zhou, T.; Jiang, X. From typical silicon-rich biomass to porous carbon-zeolite composite: A sustainable approach for efficient adsorption of CO2. Sci. Total Environ. 2021, 768, 144529. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhu, M.; Dai, B. Synthesis of Alumina-Containing Hexagonal Mesoporous Silica and Its Application in the Adsorption of Gossypol. Asian J. Chem. 2013, 25, 525–528. [Google Scholar] [CrossRef]
- Cho, H.-H.; Wepasnick, K.; Smith, B.A.; Bangash, F.K.; Fairbrother, D.H.; Ball, W.P. Sorption of AqueousZn II and Cd II by Multiwall Carbon Nanotubes: The Relative Roles of Oxygen-Containing Functional Groups and Graphenic Carbon. Langmuir 2010, 26, 967–981. [Google Scholar] [CrossRef]
- Liu, Y. Some consideration on the Langmuir isotherm equation. Colloids Surf. A-Physicochem. Eng. Asp. 2006, 274, 34–36. [Google Scholar] [CrossRef]
- Obrien, R.W.; Cannon, D.W.; Rowlands, W.N. Electroacoustic determination of particle-sizeand zeta-potential. J. Colloid Interface Sci. 1995, 173, 406–418. [Google Scholar] [CrossRef]
- Barz, D.P.J.; Vogel, M.J.; Steen, P.H. Determination of the Zeta Potential of Porous Substrates byDroplet Deflection. I. The Influence of Ionic Strength and pH Value of an Aqueous Electrolyte in Contact with a Borosilicate Surface. Langmuir 2009, 25, 1842–1850. [Google Scholar] [CrossRef]


| C% | H% | N% | Amino Content | |
|---|---|---|---|---|
| ami-MSN | 12.99 | 3.69 | 4.33 | 2.71 mmol/g |
| ami-g-MS | 10.17 | 2.84 | 1.58 | 0.98 mmol/g |
| Pb | Cd | Ni | Cu | Zn | Mn | ||
|---|---|---|---|---|---|---|---|
| After pretreatment | Cp | 5.60 | 4.10 | 12.20 | 8.90 | 5.60 | 1.70 |
| MS | C1 | 4.10 | 2.80 | 9.10 | 7.90 | 5.20 | 1.10 |
| R1/% | 26.80 | 31.70 | 25.40 | 11.20 | 21.20 | 35.30 | |
| ami-g-MS | C2 | 0.86 | 1.62 | 2.42 | 1.24 | 5.00 | 1.20 |
| R2/% | 84.60 | 60.50 | 80.20 | 86.10 | 24.20 | 29.40 | |
| ami-MSN | C3 | 0.07 | 0.17 | 0.46 | 0.54 | 5.10 | 1.30 |
| R3/% | 98.80 | 95.80 | 96.20 | 93.90 | 27.30 | 23.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zha, H.; Zhou, T.; Gan, F.; Wang, B.; Dai, Z.; Jiang, X. Quantitative Study of the Enhanced Content and Chemical Stability of Functional Groups in Mesoporous Silica by In-Situ Co-condensation Synthesis. Catalysts 2022, 12, 620. https://doi.org/10.3390/catal12060620
Zha H, Zhou T, Gan F, Wang B, Dai Z, Jiang X. Quantitative Study of the Enhanced Content and Chemical Stability of Functional Groups in Mesoporous Silica by In-Situ Co-condensation Synthesis. Catalysts. 2022; 12(6):620. https://doi.org/10.3390/catal12060620
Chicago/Turabian StyleZha, Hao, Tongxiao Zhou, Fengli Gan, Bangda Wang, Zhongde Dai, and Xia Jiang. 2022. "Quantitative Study of the Enhanced Content and Chemical Stability of Functional Groups in Mesoporous Silica by In-Situ Co-condensation Synthesis" Catalysts 12, no. 6: 620. https://doi.org/10.3390/catal12060620
APA StyleZha, H., Zhou, T., Gan, F., Wang, B., Dai, Z., & Jiang, X. (2022). Quantitative Study of the Enhanced Content and Chemical Stability of Functional Groups in Mesoporous Silica by In-Situ Co-condensation Synthesis. Catalysts, 12(6), 620. https://doi.org/10.3390/catal12060620







