Toluene Decomposition in Plasma–Catalytic Systems with Nickel Catalysts on CaO-Al2O3 Carrier
Abstract
:1. Introduction
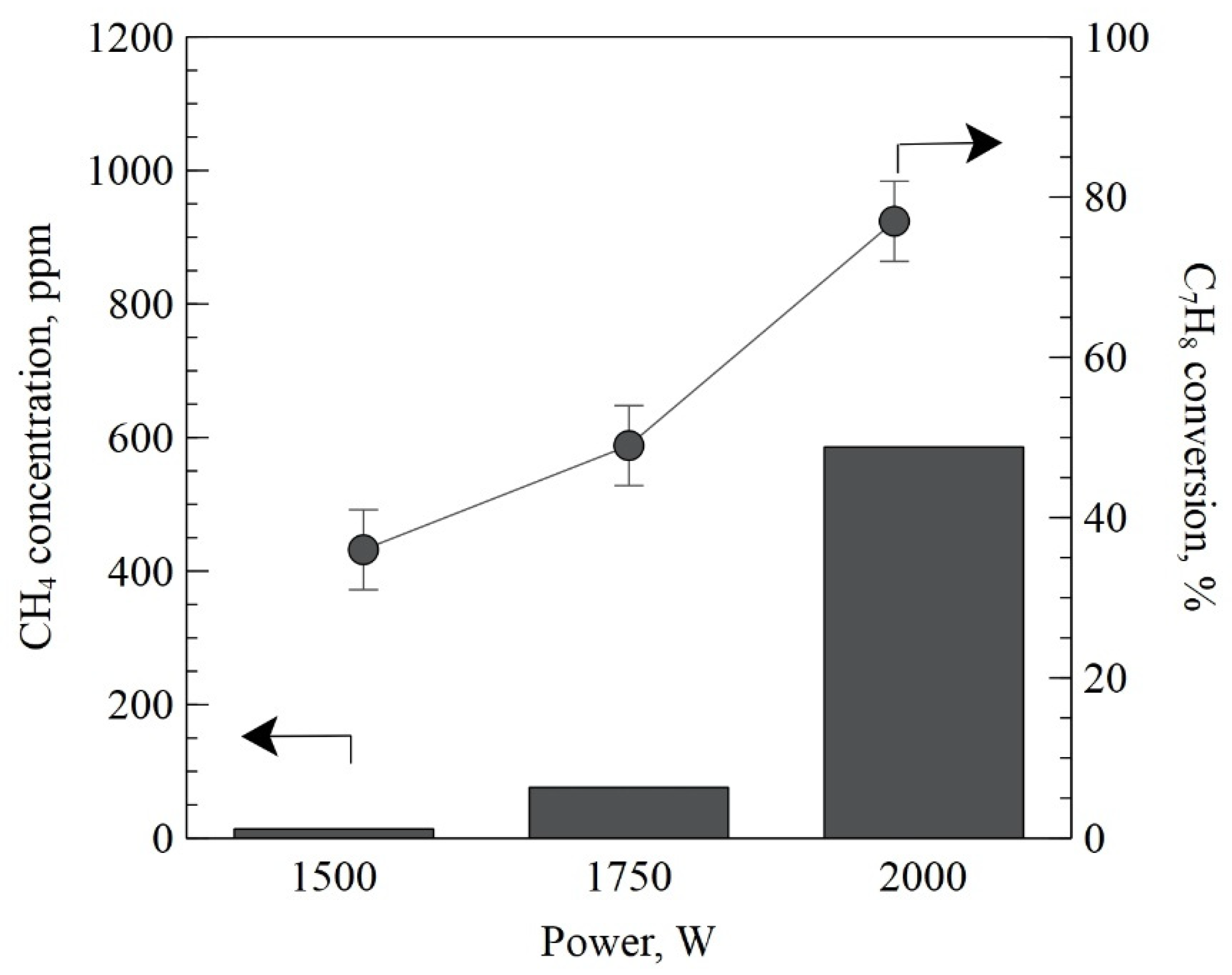
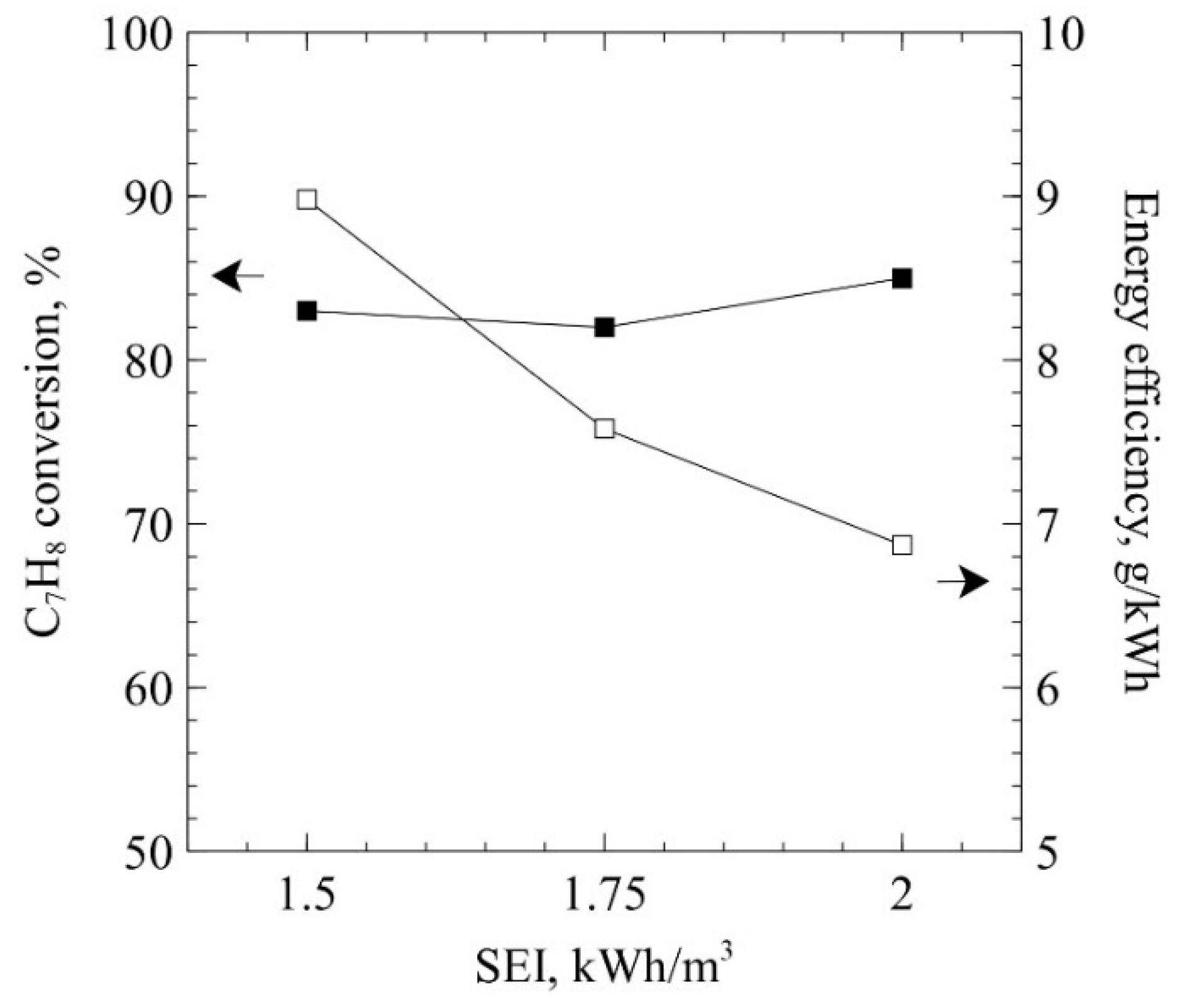
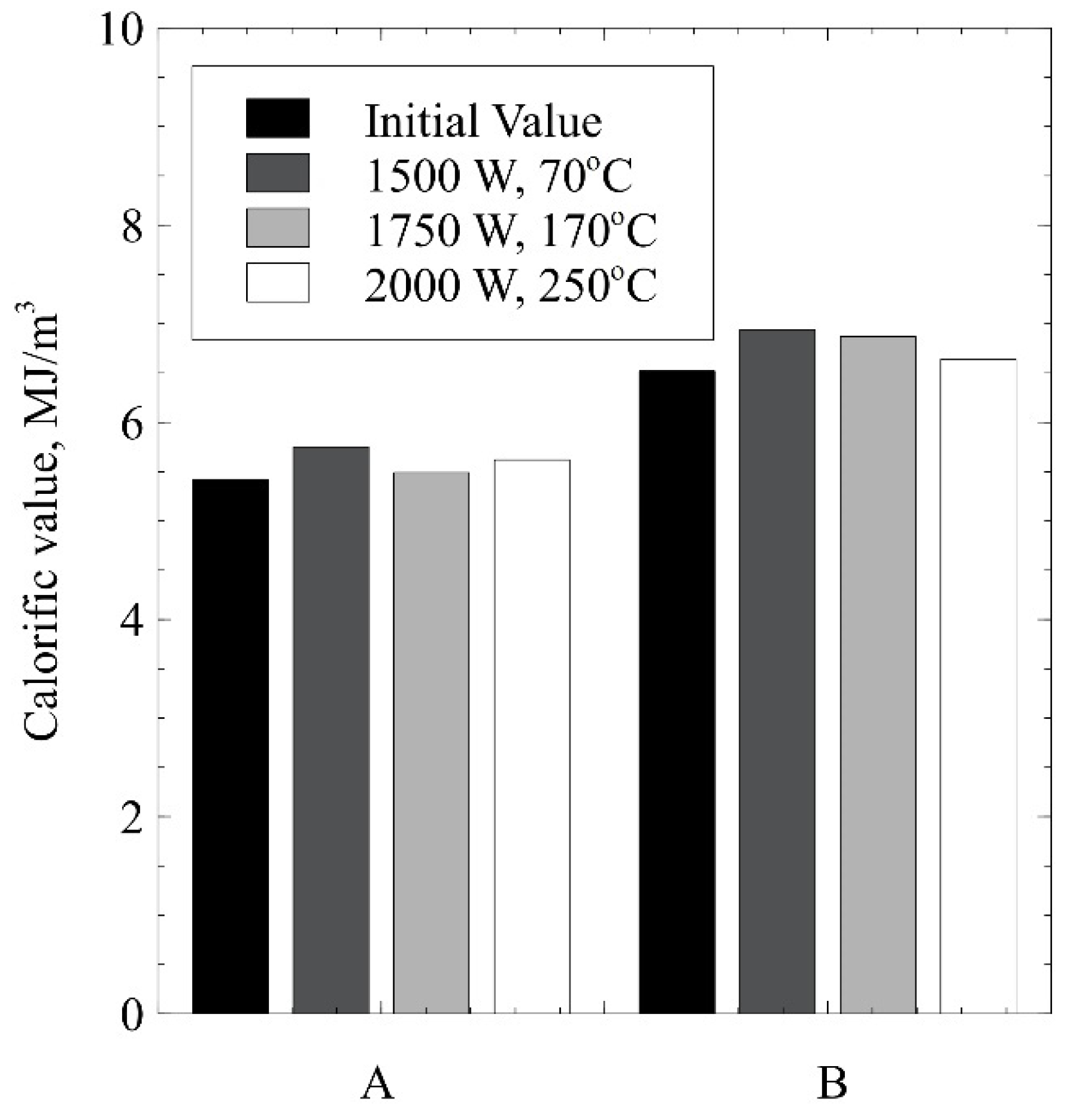
2. Results and Discussion
3. Experimental
3.1. Catalysts Preparation
3.2. Methods
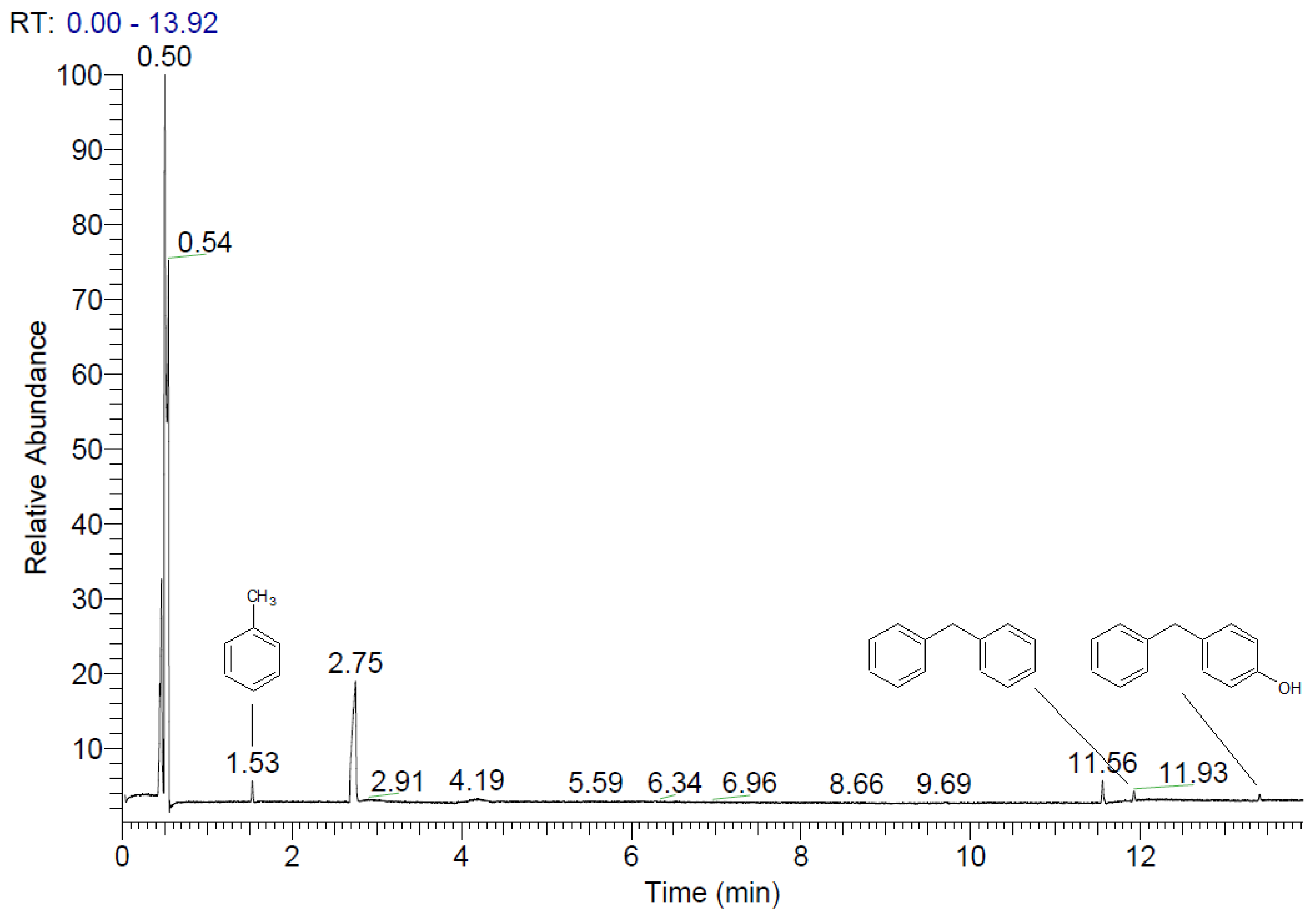
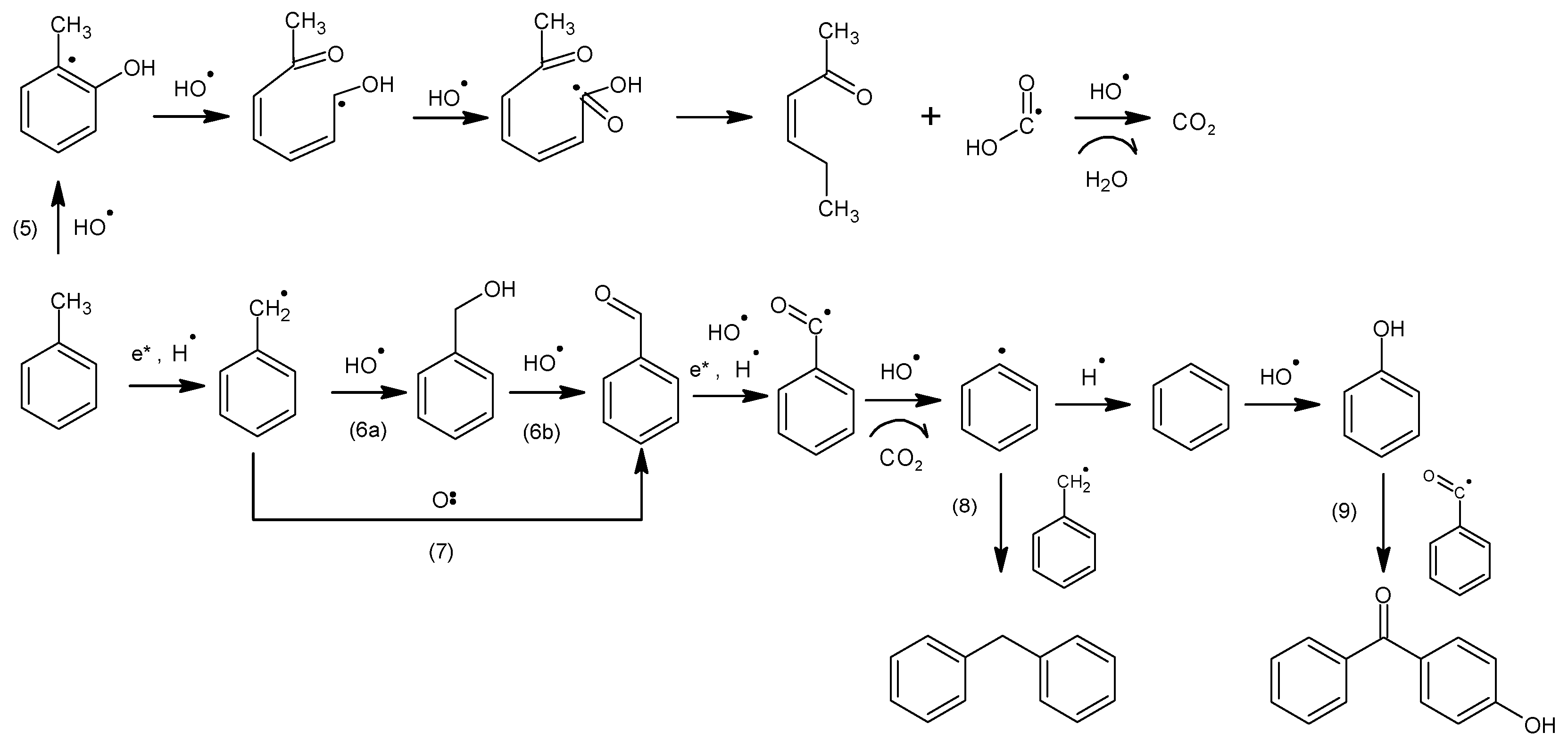
4. Reaction Mechanism
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Z.; Pang, S. Experimental investigation of tar formation and producer gas composition in biomass steam gasification in a 100kW dual fluidized bed gasifier. Renew. Energy 2019, 132, 416–424. [Google Scholar] [CrossRef]
- Di Carlo, A.; Borello, D.; Sisinni, M.; Savuto, E.; Venturini, P.; Bocci, E.; Kuramoto, K. Reforming of tar contained in a raw fuel gas from biomass gasification using nickel-mayenite catalyst. Int. J. Hydrogen Energy 2015, 40, 9088–9095. [Google Scholar] [CrossRef]
- Heidenreich, S.; Foscolo, P. New concepts in biomass gasification. Prog. Energy Combust. Sci. 2015, 46, 72–95. [Google Scholar] [CrossRef]
- Qin, Y.; Campen, A.; Wiltowski, T.; Feng, J.; Li, W. The influence of different chemical compositions in biomass on gasification tar formation. Biomass Bioenergy 2015, 83, 77–84. [Google Scholar] [CrossRef]
- Bhaduri, S.; Contino, F.; Jeanmart, H.; Breuer, E. The effects of biomass syngas composition, moisture, tar loading and operating conditions on the combustion of a tar-tolerant HCCI (Homogeneous Charge Compression Ignition) engine. Energy 2015, 87, 289–302. [Google Scholar] [CrossRef]
- Das, S.; Kashyap, D.; Kalita, P.; Kulkarni, V.; Itaya, Y. Clean gaseous fuel application in diesel engine: A sustainable option for rural electrification in India. Renew. Sustain. Energy Rev. 2020, 117, 109485. [Google Scholar] [CrossRef]
- Fjellerup, J.; Ahrenfeldt, J.; Henriksen, U.; Gobel, B. Formation, Decomposition and Cracking of Biomass Tars in Gasification; Technical University of Denmark, Department of Mechanical Engineering: Kgs. Lyngby, Denmark, 2005; pp. 1–60. [Google Scholar]
- Shen, Y. Chars as carbonaceous adsorbents/catalysts for tar elimination during biomass pyrolysis or gasification. Renew. Sustain. Energy Rev. 2015, 43, 281–295. [Google Scholar] [CrossRef]
- Richardson, Y.; Blin, J.; Julbe, A. A short overview on purification and conditioning of syngas produced by biomass gasification: Catalytic strategies, process intensification and new concepts. Prog. Energy Combust. Sci. 2012, 38, 761–781. [Google Scholar] [CrossRef]
- Riosa, M.-L.-V.; González, A.-M.; Lora, E.-E.-S. Almazán del Olmoc OA. Reduction of tar generated during biomass gasification: A review. Biomass Bioenergy 2018, 108, 345–370. [Google Scholar]
- Huang, Z.; Zheng, A.; Deng, Z.; Wei, G.; Zhao, K.; Chen, D.; He, F.; Zhao, Z.; Lia, H.; Li, F. In-situ removal of toluene as a biomass tar model compound using NiFe2O4 for application in chemical looping gasification oxygen carrier. Energy 2020, 190, 116360. [Google Scholar] [CrossRef]
- Paethanom, A.; Nakahara, S.; Kobayashi, M.; Prawisudha, P.; Yoshikawa, K. Performance of tar removal by absorption and adsorption for biomass gasification. Fuel Process. Technol. 2012, 104, 144–154. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Shen, B.; Wu, C. Preparation, modification and development of Ni-based catalysts for catalytic reforming of tar produced from biomass gasification. Renew. Sust. Energy Rev. 2018, 94, 1086–1109. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Su, Y.; Wang, P.; Zhang, S.; Xiong, Y. In-situ catalytic conversion of tar from biomass gasification over carbon nanofibers- supported Fe-Ni bimetallic catalysts. Fuel Process. Technol. 2018, 182, 77–87. [Google Scholar] [CrossRef]
- Duman, G.; Watanabe, T.; Uddin, M.-A.; Yanik, J. Steam gasification of safflower seed cake and catalytic tar decomposition over ceria modified iron oxide catalysts. Fuel Process. Technol. 2014, 126, 276–283. [Google Scholar] [CrossRef] [Green Version]
- Tan, R.-S.; Abdullah, T.-A.-T.; Ripin, A.; Ahmad, A.; Isac, K.M. Hydrogen-rich gas production by steam reforming of gasified biomass tar over Ni/dolomite/La2O3 catalyst. J. Environ. Chem. Eng. 2019, 7, 103490. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Tu, X. Plasma reforming of naphthalene as a tar model compound of biomass gasification. Energy Convers. Manag. 2019, 187, 593–604. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Z.; Das, S.; Kawi, S. Reforming of tar from biomass gasification in a hybrid catalysis-plasma system: A review. Appl. Catal. B 2019, 250, 250–272. [Google Scholar] [CrossRef]
- Saleem, F.; Zhang, K.; Harvey, A. Plasma-assisted decomposition of a biomass gasification tar analogue into lower hydrocarbons in a synthetic product gas using a dielectric barrier discharge reactor. Fuel 2019, 235, 1412–1419. [Google Scholar] [CrossRef] [Green Version]
- Woroszył-Wojno, J.; Młotek, M.; Perron, M.; Jóźwik, P.; Ulejczyk, B.; Krawczyk, K. Decomposition of Tars on a Nickel Honeycomb Catalyst. Catalysts 2021, 11, 860. [Google Scholar] [CrossRef]
- Młotek, M.; Woroszył, J.; Ulejczyk, B.; Krawczyk, K. Coupled Plasma-Catalytic System with Rang 19PR Catalyst for Conversion of Tar. Sci. Rep. 2019, 9, 13562. [Google Scholar] [CrossRef] [Green Version]
- Młotek, M.; Ulejczyk, B.; Woroszył, J.; Walerczak, I.; Krawczyk, K. Purification of the gas after pyrolysis in coupled plasma-catalytic system. Pol. J. Chem. Technol. 2017, 19, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Młotek, M.; Ulejczyk, B.; Woroszył, J.; Krawczyk, K. Decomposition of Toluene in Coupled Plasma-Catalytic System. Ind. Eng. Chem. Res. 2020, 59, 4239–4244. [Google Scholar] [CrossRef]
- Woroszył-Wojno, J.; Młotek, M.; Ulejczyk, B.; Krawczyk, K. Nickel catalyst in coupled plasma-catalytic system for tar removal. Pol. J. Chem. Technol. 2021, 23, 24–29. [Google Scholar] [CrossRef]
- Tao, K.; Ohta, N.; Liu, G.; Yoneyama, Y.; Wang, T.; Tsubaki, N. Plasma enhanced catalytic reforming of biomass tar model compound to syngas. Fuel 2013, 104, 53–57. [Google Scholar] [CrossRef]
- Hou, Z.; Yokota, O.; Tanaka, T.; Yashima, T. Characterization of Ca-promoted Ni/α-Al2O3 catalyst for CH4 reforming with CO2. Appl. Catal. A 2003, 253, 381–387. [Google Scholar] [CrossRef]
- Li, C.; Hirabayashi, D.; Suzuki, K. Development of new nickel based catalyst for biomass tar steam reforming producing H2-rich syngas. Fuel Process. Technol. 2009, 90, 790–796. [Google Scholar] [CrossRef]
- Franczyk, E.; Gołębiowski, A.; Borowiecki, T.; Kowalik, P.; Wróbel, W. Influence of Steam Reforming Catalyst Geometry On The Performance Of Tubular Reformer—Simulation Calculations. Chem. Eng. Process. 2015, 36, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Ashok, J.; Kathiraser, Y.; Ang, M.-L.; Kawi, S. Bi-functional hydrotalcite-derived NiO–CaO–Al2O3 catalysts for steam reforming of biomass and/or tar model compound at low steam-to-carbon conditions. Appl. Catal. B 2015, 172–173, 116–128. [Google Scholar] [CrossRef]
- Choong, C.-K.-S.; Zhong, Z.; Huang, L.; Wang, Z.; Ang, T.-P.; Borgna, A.; Lin, J.; Hong, L.; Chen, L. Effect of calcium addition on catalytic ethanol steam reforming of Ni/Al2O3: I. Catalytic stability, electronic properties and coking mechanism. Appl. Catal. A 2011, 407, 145–154. [Google Scholar] [CrossRef]
- Meia, D.; Liu, S.; Wang, Y.; Yang, H.; Bo, Z.; Tu, X. Enhanced reforming of mixed biomass tar model compounds using a hybrid gliding arc plasma catalytic process. Catal. Today 2019, 337, 225–233. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, H.; Li, X.; Xu, R.; Mubeen, I.; Li, L.; Yan, J. Destruction of Toluene, Naphthalene and Phenanthrene as Model Tar Compounds in a Modified Rotating Gliding Arc Discharge Reactor. Catalysts 2019, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Li, X.; Tu, X.; Wang, Y.; Lu, S.; Yan, J. Decomposition of Naphthalene by dc Gliding Arc Gas Discharge. J. Phys. Chem. A 2010, 114, 360–368. [Google Scholar] [CrossRef]
- Liu, S.; Mei, D.; Tu, X. Steam reforming of toluene as biomass tar model compound in a gliding arc discharge reactor. Chem. Eng. J. 2017, 307, 793–802. [Google Scholar] [CrossRef] [Green Version]
- Du, C.-M.; Yan, J.-H.; Cheron, B. Decomposition of toluene in a gliding arc discharge plasma reactor. Plasma Sources Sci. Technol. 2007, 16, 791–797. [Google Scholar] [CrossRef]
- Colket, M.-B.; Seery, D.-J. Reaction mechanisms for toluene pyrolysis. Symp. Int. Combust. Proc. 1994, 25, 883–891. [Google Scholar] [CrossRef]
- Benstaali, B.; Boubert, P.; Cheron, B.-G.; Addou, A.; Brisset, J.-L. Density and Rotational Temperature Measurements of the OH° and NO° Radicals Produced by a Gliding Arc in Humid Air. Plasma Chem. Plasma Process. 2002, 22, 553–571. [Google Scholar] [CrossRef]
- Aghamir, F.-M.; Matin, N.-S.; Jalili, A.-H.; Esfarayeni, M.-H.; Khodagholi, M.-A.; Ahmadi, R. Conversion of methane to methanol in an ac dielectric barrier discharge. Plasma Sources Sci. Technol. 2004, 13, 707–711. [Google Scholar] [CrossRef]
- Gołębiowski, A.; Stołecki, K. The kinetics of methanation of CO and CO2 under industrial conditions. Przem. Chem. 2001, 80, 514–516. [Google Scholar]
- Chang, J.-S.; Kostov, K.-G.; Urashima, K.; Yamamoto, T.; Okayasu, Y.; Kato, T.; Iwaizumi, T.; Yoshimura, K. Removal of NF3 from semiconductor-process flue gases by tandem packed-bed plasma and adsorbent hybrid systems. IEEE Trans. Ind. Appl. 2000, 36, 1251–1259. [Google Scholar] [CrossRef]
- Bogaerts, A.; Zhang, Q.-Z.; Zhang, Y.-R.; Laer, K.-V.; Wang, W. Burning questions of plasma catalysis: Answers by modeling. Catal. Today 2019, 337, 3–14. [Google Scholar] [CrossRef]
- Holzer, F.; Kopinke, F.-D.; Roland, U. Influence of Ferroelectric Materials and Catalysts on the Performance of Non-Thermal Plasma (NTP) for the Removal of Air Pollutants. Plasma Chem. Plasma Process. 2005, 25, 595–611. [Google Scholar] [CrossRef]

| SBET (m2/g) | |||||
|---|---|---|---|---|---|
| NiO/(CaO-Al2O3) | Ni/(CaO-Al2O3) | NiO/Al2O3 [24] | |||
| Before | After | Before | After | Before | After |
| 4.2 | 3.6 | 2.7 | 2.5 | 8.6 | 8.0 |
| Composition | H2 | N2 | CO | CO2 |
|---|---|---|---|---|
| A | 0.28 | 0.44 | 0.13 | 0.15 |
| B | 0.38 | 0.34 | 0.13 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woroszył-Wojno, J.; Młotek, M.; Ulejczyk, B.; Krawczyk, K. Toluene Decomposition in Plasma–Catalytic Systems with Nickel Catalysts on CaO-Al2O3 Carrier. Catalysts 2022, 12, 635. https://doi.org/10.3390/catal12060635
Woroszył-Wojno J, Młotek M, Ulejczyk B, Krawczyk K. Toluene Decomposition in Plasma–Catalytic Systems with Nickel Catalysts on CaO-Al2O3 Carrier. Catalysts. 2022; 12(6):635. https://doi.org/10.3390/catal12060635
Chicago/Turabian StyleWoroszył-Wojno, Joanna, Michał Młotek, Bogdan Ulejczyk, and Krzysztof Krawczyk. 2022. "Toluene Decomposition in Plasma–Catalytic Systems with Nickel Catalysts on CaO-Al2O3 Carrier" Catalysts 12, no. 6: 635. https://doi.org/10.3390/catal12060635






