Producing Natural Flavours from Isoamyl Alcohol and Fusel Oil by Using Immobilised Rhizopus oryzae Lipase
Abstract
:1. Introduction
2. Results and Discussion
2.1. Reaction Solvent for Butyric and Acetic Acid Esterification with Isoamyl Alcohol
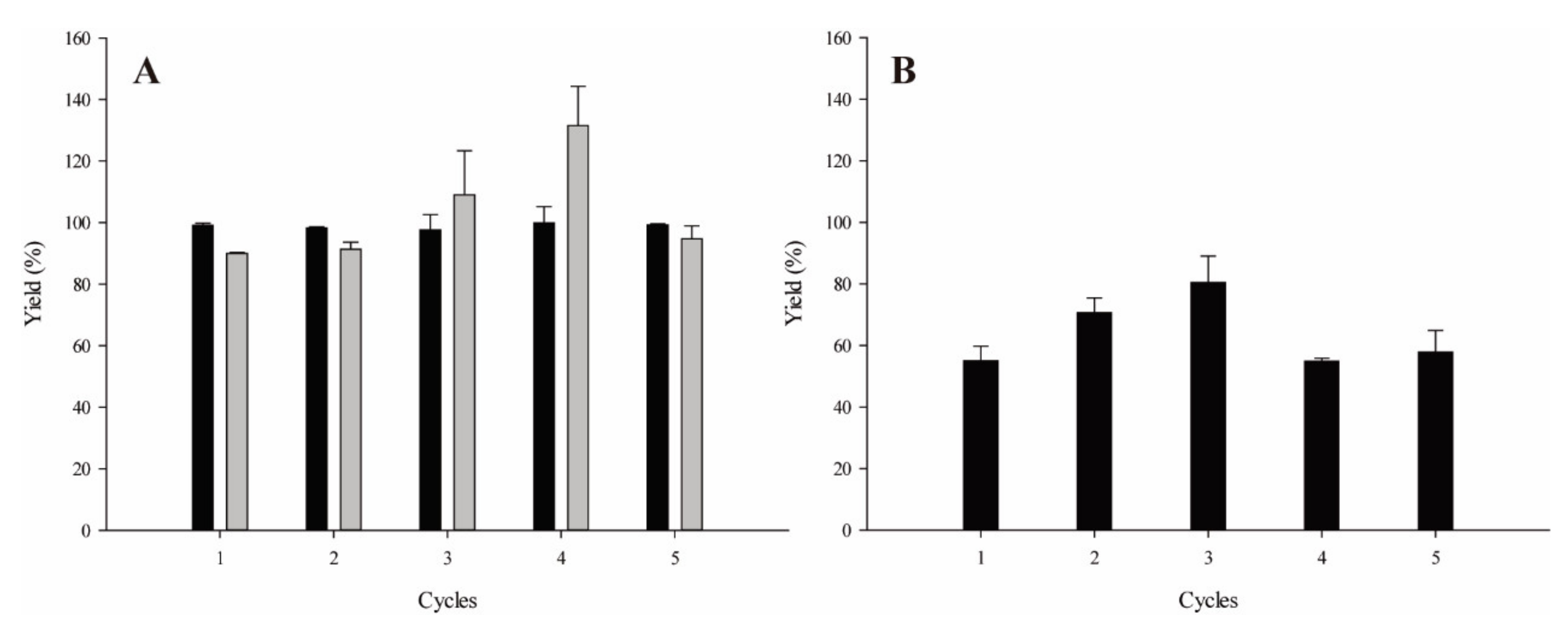
2.2. Experimental Design: Optimisation of Isoamyl Butyrate Single-Batch and Cumulative Production
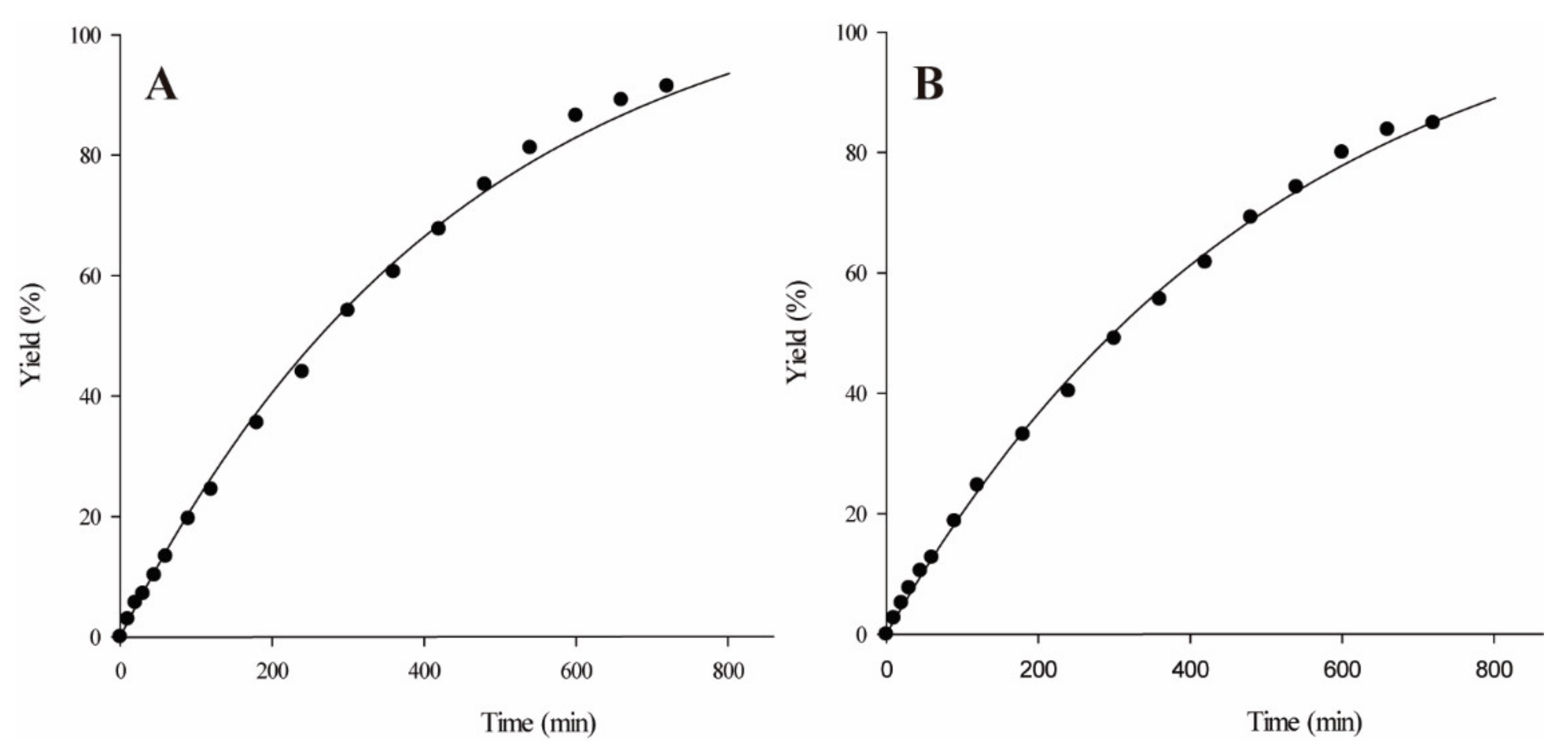
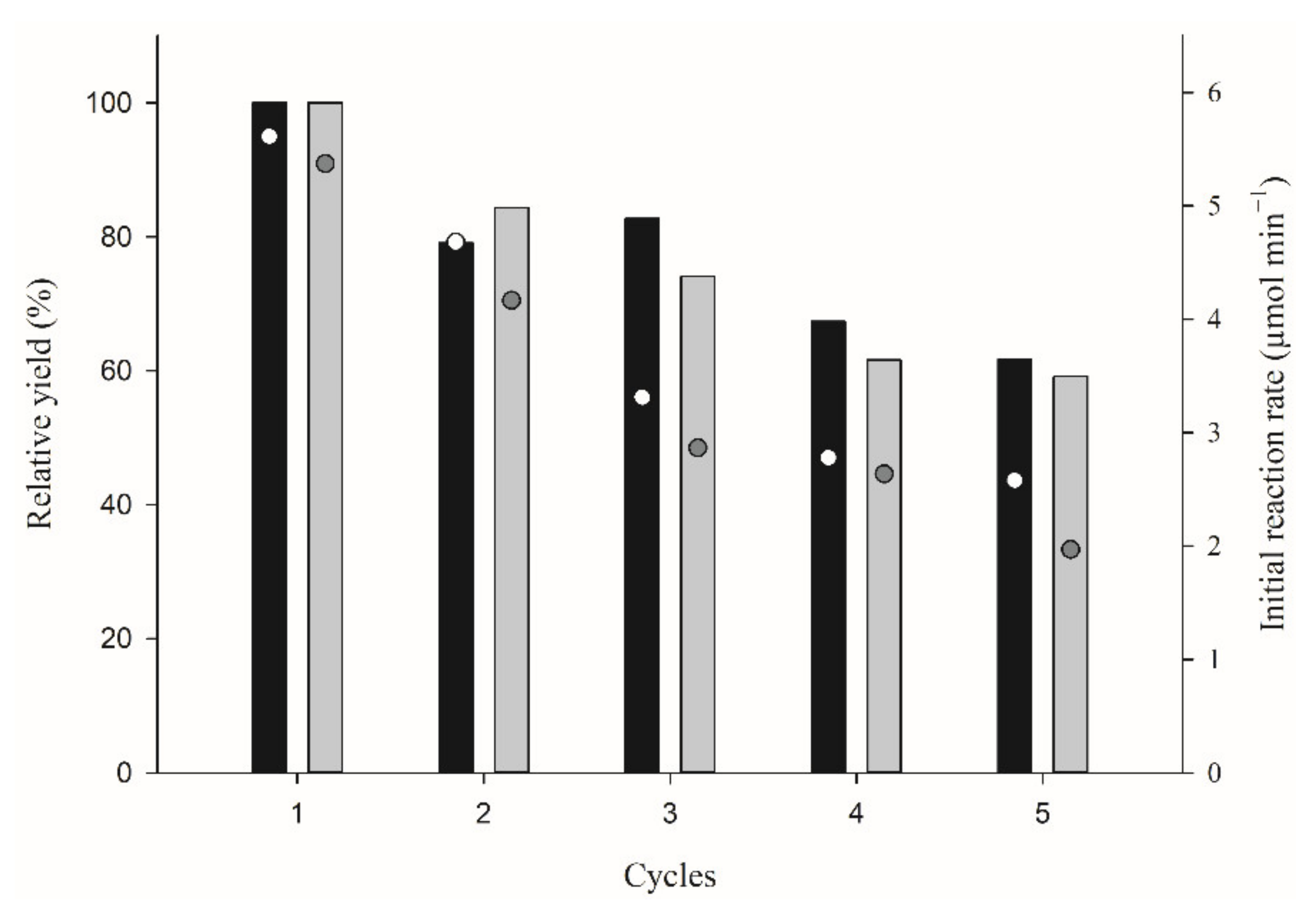
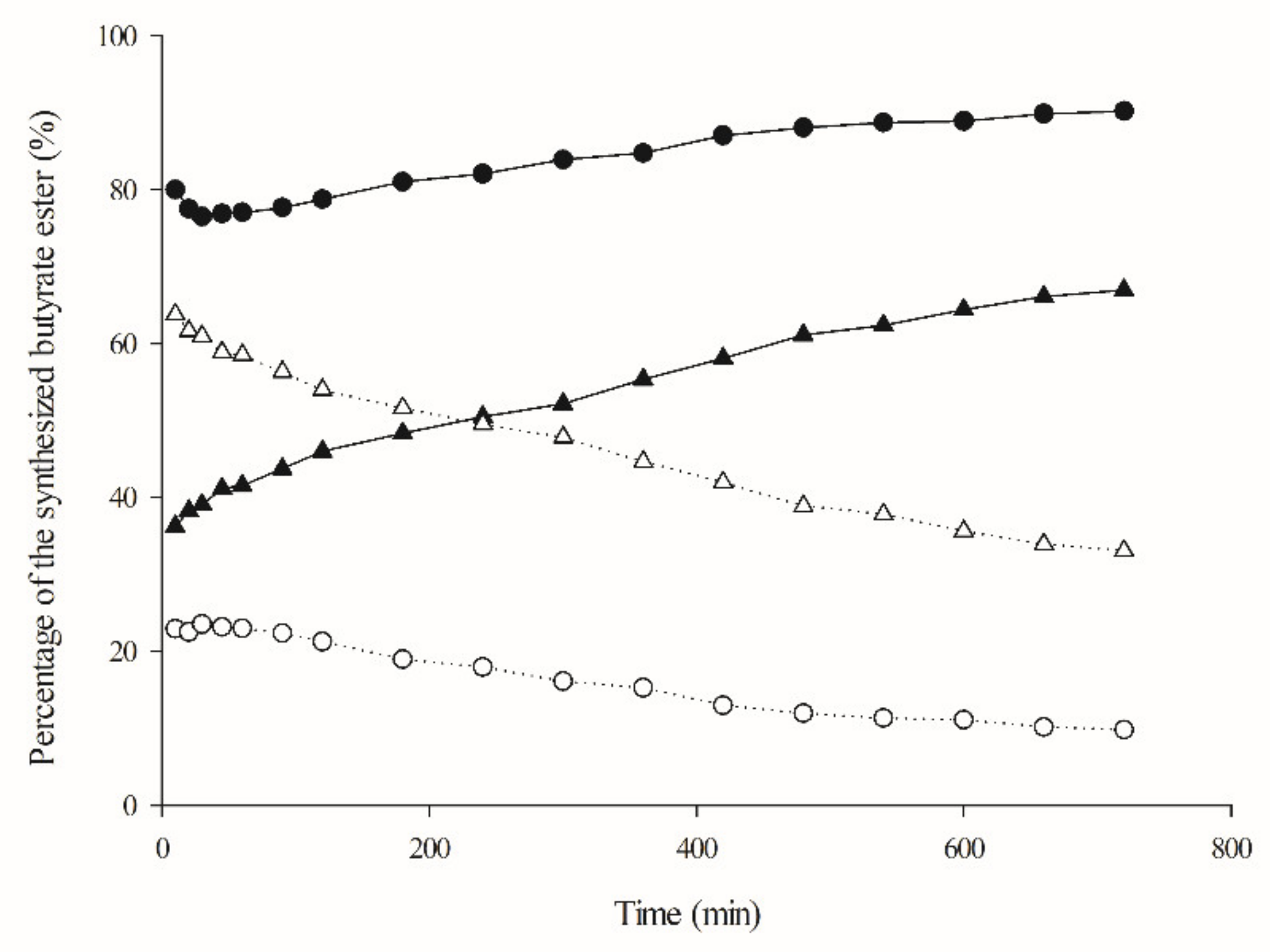
2.3. Reaction Scale Up: Isoamyl Butyrate Production, Fusel Oil Use and Half-Lives
2.4. Structural Isomers: 3-Methylbutanol and 2-Methylbutanol
3. Materials and Methods
3.1. Chemicals
3.2. Lipase Production and Downstream Processing
3.3. Lipase Activity
3.4. Lipase Immobilisation
3.5. Esterification Reactions
3.5.1. Influence of the Acid Concentration and Acid:Alcohol Mole Ratio
3.5.2. Reaction Scale Up
3.6. Statistical Analysis
3.7. Gas Chromatography/Mass Spectrometry Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cong, S.; Tian, K.; Zhang, X.; Lu, F.; Singh, S.; Prior, B.; Wang, Z.X. Synthesis of flavor esters by a novel lipase from Aspergillusniger in a soybean-solvent system. 3 Biotech 2019, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- SÁ, A.; de Meneses, A.C.; Hermes de Araújo, P.H.; de Oliveira, D. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci. Technol. 2017, 69, 95–105. [Google Scholar] [CrossRef]
- Flavors and Fragrance Market Size, Share & Trends|Forecast by 2027. Available online: https://www.alliedmarketresearch.com/flavors-and-fragrances-market (accessed on 11 December 2021).
- Brault, G.; Shareck, F.; Hurtubise, Y.; Lépine, F.; Doucet, N. Short-chain flavor ester synthesis in organic media by an E. coli whole-cell biocatalyst expressing a newly characterized heterologous lipase. PLoS ONE 2014, 9, e91872. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-M.; Huang, H.-Y.; Chen, Y.-M.; Kuo, C.-H.; Shieh, C.-J. Continuous production of 2-phenylethyl acetate in a solvent-free system using a packed-bed reactor with Novozym® 435. Catalysts 2020, 10, 714. [Google Scholar] [CrossRef]
- Dudu, A.I.; Lăcătuş, M.A.; Bencze, L.C.; Paizs, C.; Toşa, M.I. Green Process for the enzymatic synthesis of aroma compounds mediated by lipases entrapped in tailored sol–gel matrices. ACS Sustain. Chem. Eng. 2021, 9, 5461–5469. [Google Scholar] [CrossRef]
- Bayout, I.; Bouzemi, N.; Guo, N.; Mao, X.; Serra, S.; Riva, S.; Secundo, F. Natural flavor ester synthesis catalyzed by lipases. Flavour Fragr. J. 2020, 35, 209–218. [Google Scholar] [CrossRef]
- Náray-Szabó, G.; Mika, L.T. Conservative evolution and industrial metabolism in Green Chemistry. Green Chem. 2018, 20, 2171–2191. [Google Scholar] [CrossRef]
- Zare, M.; Golmakani, M.T.; Niakousari, M. Lipase synthesis of isoamyl acetate using different acyl donors: Comparison of novel esterification techniques. LWT 2019, 101, 214–219. [Google Scholar] [CrossRef]
- Dhake, K.P.; Thakare, D.D.; Bhanage, B.M. Lipase: A potential biocatalyst for the synthesis of valuable flavour and fragrance ester compounds. Flavour Fragr. J. 2013, 28, 71–83. [Google Scholar] [CrossRef]
- Sarmah, N.; Revathi, D.; Sheelu, G.; Yamuna Rani, K.; Sridhar, S.; Mehtab, V.; Sumana, C. Recent advances on sources and industrial applications of lipases. Biotechnol. Prog. 2018, 34, 5–28. [Google Scholar] [CrossRef]
- Melani, N.B.; Tambourgi, E.B.; Silveira, E. Lipases: From production to applications. Sep. Purif. Rev. 2020, 49, 143–158. [Google Scholar] [CrossRef]
- López-Fernández, J.; Benaiges, M.D.; Valero, F. Rhizopus oryzae lipase, a promising industrial enzyme: Biochemical characteristics, production and biocatalytic applications. Catalysts 2020, 10, 1277. [Google Scholar] [CrossRef]
- Kovalenko, G.; Perminova, L.; Pykhtina, M.; Beklemishev, A. Lipase-active heterogeneous biocatalysts for enzymatic synthesis of short-chain aroma esters. Biocatal. Agric. Biotechnol. 2021, 36, 102124. [Google Scholar] [CrossRef]
- Ismail, A.R.; Kashtoh, H.; Baek, K.H. Temperature-resistant and solvent-tolerant lipases as industrial biocatalysts: Biotechnological approaches and applications. Int. J. Biol. Macromol. 2021, 187, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, H.; Yasinian, A.; Taghizadeh, A. Covalent immobilization of lipase from Candida rugosa on epoxy-activated cloisite 30B as a new heterofunctional carrier and its application in the synthesis of banana flavor and production of biodiesel. Int. J. Biol. Macromol. 2021, 178, 569–579. [Google Scholar] [CrossRef]
- Yang, M.; Yu, X.W.; Zheng, H.; Sha, C.; Zhao, C.; Qian, M.; Xu, Y. Role of N-linked glycosylation in the secretion and enzymatic properties of Rhizopus chinensis lipase expressed in Pichia pastoris. Microb. Cell Fact. 2015, 14, 40–54. [Google Scholar] [CrossRef]
- López-Fernández, J.; Barrero, J.J.; Benaiges, M.D.; Valero, F. Truncated prosequence of Rhizopus oryzae lipase: Key factor for production improvement and biocatalyst stability. Catalysts 2019, 9, 961. [Google Scholar] [CrossRef]
- Beer, H.D.; Wohlfahrt, G.; Schmid, R.D.; McCarthy, J.E. The folding and activity of the extracellular lipase of Rhizopus oryzae are modulated by a prosequence. Biochem. J. 1996, 319, 351–359. [Google Scholar] [CrossRef]
- Yu, X.W.; Sha, C.; Guo, Y.L.; Xiao, R.; Xu, Y. High-level expression and characterization of a chimeric lipase from Rhizopus oryzae for biodiesel production. Biotechnol. Biofuels 2013, 6, 29–41. [Google Scholar] [CrossRef]
- Mendes, A.A.; de Castro, H.F.; Giordano, R.L.C. Covalent attachment of lipases on glyoxyl-agarose beads: Application in fruit flavor and biodiesel synthesis. Int. J. Biol. Macromol. 2014, 70, 78–85. [Google Scholar] [CrossRef]
- Asmat, S.; Anwer, A.H.; Husain, Q. Immobilization of lipase onto novel constructed polydopamine grafted multiwalled carbon nanotube impregnated with magnetic cobalt and its application in synthesis of fruit flavours. Int. J. Biol. Macromol. 2019, 140, 484–495. [Google Scholar] [CrossRef]
- Ismail, A.R.; Baek, K.H. Lipase immobilization with support materials, preparation techniques, and applications: Present and future aspects. Int. J. Biol. Macromol. 2020, 163, 1624–1639. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández, J.; Dolors Benaiges, M.; Valero, F. Second- and third-generation biodiesel production with immobilised recombinant Rhizopus oryzae lipase: Influence of the support, substrate acidity and bioprocess scale-up. Bioresour. Technol. 2021, 334, 125233. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.W.; Xu, Y.; Xiao, R. Lipases from the genus Rhizopus: Characteristics, expression, protein engineering and application. Prog. Lipid Res. 2016, 64, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Juturu, V.; Wu, J.C. Heterologous protein expression in Pichia pastoris: Latest research progress and applications. ChemBioChem 2018, 19, 7–21. [Google Scholar] [CrossRef]
- García-Ortega, X.; Cámara, E.; Ferrer, P.; Albiol, J.; Montesinos-Seguí, J.L.; Valero, F. Rational development of bioprocess engineering strategies for recombinant protein production in Pichia pastoris (Komagataella phaffii) using the methanol-free GAP promoter. Where do we stand? N. Biotechnol. 2019, 53, 24–34. [Google Scholar] [CrossRef]
- Chahed, H.; Boumaiza, M.; Ezzine, A.; Marzouki, M.N. Heterologous expression and biochemical characterization of a novel thermostable Sclerotiniasclerotiorum GH45 endoglucanase in Pichia pastoris. Int. J. Biol. Macromol. 2018, 106, 629–635. [Google Scholar] [CrossRef]
- Sturmberger, L.; Chappell, T.; Geier, M.; Krainer, F.; Day, K.J.; Vide, U.; Trstenjak, S.; Schiefer, A.; Richardson, T.; Soriaga, L.; et al. Refined Pichia pastoris reference genome sequence. J. Biotechnol. 2016, 235, 121–131. [Google Scholar] [CrossRef]
- Grosso, C.; Ferreira-Dias, S.; Pires-Cabral, P. Modelling and optimization of ethyl butyrate production catalysed by Rhizopus oryzae lipase. J. Food Eng. 2013, 115, 475–480. [Google Scholar] [CrossRef]
- Ghamgui, H.; Karra-Chaâbouni, M.; Bezzine, S.; Miled, N.; Gargouri, Y. Production of isoamyl acetate with immobilized Staphylococcus simulans lipase in a solvent-free system. Enzyme Microb. Technol. 2006, 38, 788–794. [Google Scholar] [CrossRef]
- Hari Krishna, S.; Prapulla, S.G.; Karanth, N.G. Enzymatic synthesis of isoamyl butyrate using immobilized Rhizomucor miehei lipase in non-aqueous media. J. Ind. Microbiol. Biotechnol. 2000, 25, 147–154. [Google Scholar] [CrossRef]
- Macedo, G.A.; Pastore, G.M.; Rodrigues, M.I. Optimising the synthesis of isoamyl butyrate using Rhizopus sp. lipase with a central composite rotatable design. Process Biochem. 2004, 39, 687–693. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Meirelles, A.J.A.; Batista, E.A.C. Study of the Fusel Oil Distillation Process. Ind. Eng. Chem. Res. 2013, 52, 2336–2351. [Google Scholar] [CrossRef]
- Vilas Bôas, R.N.; Ceron, A.A.; Bento, H.B.S.; de Castro, H.F. Application of an immobilized Rhizopus oryzae lipase to batch and continuous ester synthesis with a mixture of a lauric acid and fusel oil. Biomass Bioenergy 2018, 119, 61–68. [Google Scholar] [CrossRef]
- Hari Krishna, S.; Divakar, S.; Prapulla, S.G.; Karanth, N.G. Enzymatic synthesis of isoamyl acetate using immobilized lipase from Rhizomucor miehei. J. Biotechnol. 2001, 87, 193–201. [Google Scholar] [CrossRef]
- Huang, S.Y.; Chang, H.L.; Goto, M. Preparation of surfactant-coated lipase for the esterification of geraniol and acetic acid in organic solvents. Enzym. Microb. Technol. 1998, 22, 552–557. [Google Scholar] [CrossRef]
- Trivedi, J.; Aila, M.; Sharma, C.D.; Gupta, P.; Kaul, S. Clean synthesis of biolubricant range esters using novel liquid lipase enzyme in solvent free medium. SpringerPlus 2015, 4, 165. [Google Scholar] [CrossRef]
- Larios, A.; García, H.S.; Oliart, R.M.; Valerio-Alfaro, G. Synthesis of flavor and fragrance esters using Candida antarctica lipase. Appl. Microbiol. Biotechnol. 2004, 65, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.-J.; Park, J.-B.; Park, S. Esterification of secondary alcohols and multi-hydroxyl compounds by Candida antarctica Lipase B and subtilisin. Biotechnol. Bioprocess Eng. 2019, 24, 41–47. [Google Scholar] [CrossRef]
- Djoudi, W.; Aissani-Benissad, F.; Bourouina-Bacha, S. Optimization of copper cementation process by iron using central composite design experiments. Chem. Eng. J. 2007, 133, 1–6. [Google Scholar] [CrossRef]
- Box, G.E.P.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenters: Design, Innovation and Discovery, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2005. [Google Scholar]
- Lorenzoni, A.S.G.; Graebin, N.G.; Martins, A.B.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Optimization of pineapple flavour synthesis by esterification catalysed by immobilized lipase from Rhizomucor miehei. Flavour Fragr. J. 2012, 27, 196–200. [Google Scholar] [CrossRef]
- Fabbri, F.; Bertolini, F.A.; Guebitz, G.M.; Pellis, A. Biocatalyzed synthesis of flavor esters and polyesters: A Design of Experiments (DoE) approach. Int. J. Mol. Sci. 2021, 22, 8493. [Google Scholar] [CrossRef] [PubMed]
- Anschau, A.; Aragão, V.C.; Porciuncula, B.D.A.; Kalil, S.J.; Burkert, C.A.V.; Burkert, J.F.M. Enzymatic synthesis optimization of isoamyl butyrate. J. Braz. Chem. Soc. 2011, 22, 2148–2156. [Google Scholar] [CrossRef]
- Guillén, M.; Benaiges, M.D.; Valero, F. Improved ethyl butyrate synthesis catalyzed by an immobilized recombinant Rhizopus oryzae lipase: A comprehensive statistical study by production, reaction rate and yield analysis. J. Mol. Catal. B Enzym. 2016, 133, S371–S376. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Nidetzky, B. The microenvironment in immobilized enzymes: Methods of characterization and its role in determining enzyme performance. Molecules 2019, 24, 3460. [Google Scholar] [CrossRef]
- Hari Krishna, S.; Karanth, N.G. Lipase-catalyzed synthesis of isoamyl butyrate: A kinetic study. Biochim. Biophys. Acta-Protein Struct. Mol. Enzymol. 2001, 1547, 262–267. [Google Scholar] [CrossRef]
- Ben Salah, R.; Ghamghui, H.; Miled, N.; Mejdoub, H.; Gargouri, Y. Production of butyl acetate ester by lipase from novel strain of Rhizopus oryzae. J. Biosci. Bioeng. 2007, 103, 368–372. [Google Scholar] [CrossRef]
- Lopresto, C.G.; Calabrò, V.; Woodley, J.M.; Tufvesson, P. Kinetic study on the enzymatic esterification of octanoic acid and hexanol by immobilized Candida antarctica lipase B. J. Mol. Catal. B Enzym. 2014, 110, 64–71. [Google Scholar] [CrossRef]
- Bezbradica, D.; Mijin, D.; Šiler-Marinković, S.; Knežević, Z. The effect of substrate polarity on the lipase-catalyzed synthesis of aroma esters in solvent-free systems. J. Mol. Catal. B Enzym. 2007, 45, 97–101. [Google Scholar] [CrossRef]
- Matte, C.R.; Bordinhaõ, C.; Poppe, J.K.; Rodrigues, R.C.; Hertz, P.F.; Ayub, M.A.Z. Synthesis of butyl butyrate in batch and continuous enzymatic reactors using Thermomyces lanuginosus lipase immobilized in Immobead 150. J. Mol. Catal. B Enzym. 2016, 127, 67–75. [Google Scholar] [CrossRef]
- Graebin, N.G.; Martins, A.B.; Lorenzoni, A.S.; Garcia-Galan, C.; Fernandez-Lafuente, R.; Ayub, M.A.; Rodrigues, R.C. Immobilization of lipase B from Candida antarctica on porous styrene-divinylbenzene beads improves butyl acetate synthesis. Biotechnol. Prog. 2012, 28, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.H.; Raghavendra, T.; Madamwar, D. An alkaline lipase from organic solvent tolerant Acinetobacter sp. EH28: Application for ethyl caprylate synthesis. Bioresour. Technol. 2010, 101, 3628–3634. [Google Scholar] [CrossRef] [PubMed]
- Pires-Cabral, P.; da Fonseca, M.M.R.; Ferreira-Dias, S. Synthesis of ethyl butyrate in organic media catalyzed by Candida rugosa lipase immobilized in polyurethane foams: A kinetic study. Biochem. Eng. J. 2009, 43, 327–332. [Google Scholar] [CrossRef]
- Sun, J.; Yu, B.; Curran, P.; Liu, S.Q. Lipase-catalysed ester synthesis in solvent-free oil system: Is it esterification or transesterification? Food Chem. 2013, 141, 2828–2832. [Google Scholar] [CrossRef]
- Guillén, M.; Benaiges, M.D.; Valero, F. Biosynthesis of ethyl butyrate by immobilized recombinant Rhizopus oryzae lipase expressed in Pichia pastoris. Biochem. Eng. J. 2012, 65, 1–9. [Google Scholar] [CrossRef]
- Webb, A.D.; Kepner, R.E.; Ikeda, R.M. Composition of typical grape brandy fusel oil. Anal. Chem. 2002, 24, 1944–1949. [Google Scholar] [CrossRef]
- Bezbradica, D.; Karalazic, I.; Ognjanovic, N.; Knezevi, Z. Studies on the specificity of Candida rugosa lipase catalyzed esterification reactions in organic media. J. Serb. Chem. Soc 2006, 71, 31–41. [Google Scholar] [CrossRef]
- López-Fernández, J.; Benaiges, M.D.; Valero, F. Constitutive expression in Komagataella phaffii of mature Rhizopus oryzae lipase jointly with its truncated prosequence improves production and the biocatalyst operational stability. Catalysts 2021, 11, 1192. [Google Scholar] [CrossRef]
- Chang, S.W.; Lee, G.C.; Shaw, J.F. Codon optimization of Candida rugosa lip1 gene for improving expression in Pichia pastoris and biochemical characterization of the purified recombinant LIP1 lipase. J. Agric. Food Chem. 2006, 54, 815–822. [Google Scholar] [CrossRef]
- Bonet-Ragel, K.; Canet, A.; Benaiges, M.D.; Valero, F. Synthesis of biodiesel from high FFA alperujo oil catalysed by immobilised lipase. Fuel 2015, 161, 12–17. [Google Scholar] [CrossRef]
- Salihu, A.; Alam, M.Z.; AbdulKarim, M.I.; Salleh, H.M. Esterification for butyl butyrate formation using Candida cylindracea lipase produced from palm oil mill effluent supplemented medium. Arab. J. Chem. 2014, 7, 1159–1165. [Google Scholar] [CrossRef]
- Rodrigues, J.; Canet, A.; Rivera, I.; Osório, N.M.; Sandoval, G.; Valero, F.; Ferreira-Dias, S. Biodiesel production from crude Jatropha oil catalyzed by non-commercial immobilized heterologous Rhizopus oryzae and Carica papaya lipases. Bioresour. Technol. 2016, 213, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Aymard, C.; Belarbi, A. Kinetics of thermal deactivation of enzymes: A simple three parameters phenomenological model can describe the decay of enzyme activity, irrespectively of the mechanism. Enzym. Microb. Technol. 2000, 27, 612–618. [Google Scholar] [CrossRef]

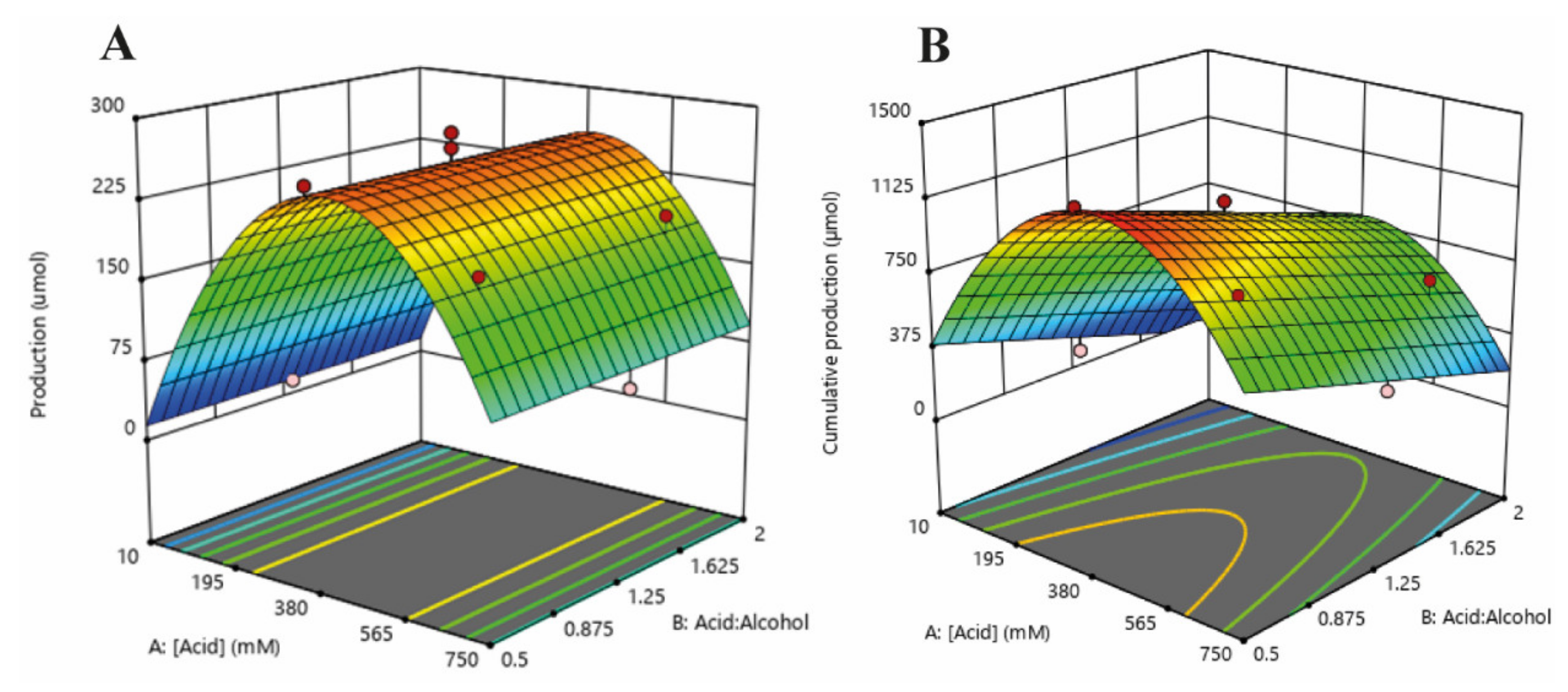
| Box-Hunter Design | |||||
|---|---|---|---|---|---|
| Experimental Run | Variables | Production Response | |||
| [Butyrate] (mM) | Acid:Alcohol Mole Ratio | [Isoamyl Alcohol] * (mM) | Single-Batch (µmol) | Cumulative (µmol) | |
| 1 | 118.37 | 1.78 | 66.49 | 105.2 | 537.14 |
| 2 | 10 | 1.25 | 8 | 12.7 | 78.74 |
| 3 | 641.63 | 0.72 | 891.56 | 194.6 | 968.8 |
| 4 | 380 | 1.25 | 304 | 241.2 | 1092.4 |
| 5 | 380 | 2 | 190 | 200.6 | 686.9 |
| 6 | 380 | 0.5 | 760 | 263.2 | 1293.2 |
| 7 | 750 | 1.25 | 600 | 75.8 | 383.7 |
| 8 | 118.37 | 0.72 | 164.48 | 153.9 | 742.7 |
| 9 | 380 | 1.25 | 304 | 263.1 | 972.9 |
| 10 | 641.63 | 1.78 | 360.40 | 199.6 | 672 |
| 11 | 380 | 1.25 | 304 | 281.4 | 1006.14 |
| Statistical Analysis | |||||
| Production | F Test p-Value | LOF Test p-Value | R2 | Adjusted-R2 | Predicted-R2 |
| Single-batch | <0.01 | 0.3593 | 0.9193 | 0.8991 | 0.8566 |
| Cumulative | <0.01 | 0.3085 | 0.9541 | 0.9345 | 0.8620 |
| Model | Single-Batch Production | Cumulative Production | |||
| Parameters | Coefficient | p-value | Coefficient | p-value | |
| β0 | 260 | <0.05 | 1036 | <0.05 | |
| β1 | 28 | 0.02 | 99 | 0.016 | |
| β2 | NS | >0.05 | −170 | <0.01 | |
| β12 | NS | >0.05 | NS | >0.05 | |
| β11 | 104 | <0.01 | −370 | <0.01 | |
| β22 | NS | >0.05 | NS | >0.05 | |
| Alcohol | k1 | k2 | c | R2 |
|---|---|---|---|---|
| Commercial isoamyl alcohol | 2.498 | 0.0926 | 99.986 | 0.9571 |
| Isoamyl alcohol (Fusel oil) | 0.1122 | 0.9124 | 26.629 | 0.9944 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Fernández, J.; Benaiges, M.D.; Sebastian, X.; Bueno, J.M.; Valero, F. Producing Natural Flavours from Isoamyl Alcohol and Fusel Oil by Using Immobilised Rhizopus oryzae Lipase. Catalysts 2022, 12, 639. https://doi.org/10.3390/catal12060639
López-Fernández J, Benaiges MD, Sebastian X, Bueno JM, Valero F. Producing Natural Flavours from Isoamyl Alcohol and Fusel Oil by Using Immobilised Rhizopus oryzae Lipase. Catalysts. 2022; 12(6):639. https://doi.org/10.3390/catal12060639
Chicago/Turabian StyleLópez-Fernández, Josu, Maria Dolors Benaiges, Xavier Sebastian, Jose María Bueno, and Francisco Valero. 2022. "Producing Natural Flavours from Isoamyl Alcohol and Fusel Oil by Using Immobilised Rhizopus oryzae Lipase" Catalysts 12, no. 6: 639. https://doi.org/10.3390/catal12060639
APA StyleLópez-Fernández, J., Benaiges, M. D., Sebastian, X., Bueno, J. M., & Valero, F. (2022). Producing Natural Flavours from Isoamyl Alcohol and Fusel Oil by Using Immobilised Rhizopus oryzae Lipase. Catalysts, 12(6), 639. https://doi.org/10.3390/catal12060639








