α-Fe2O3/Reduced Graphene Oxide Composites as Cost-Effective Counter Electrode for Dye-Sensitized Solar Cells
Abstract
:1. Introduction
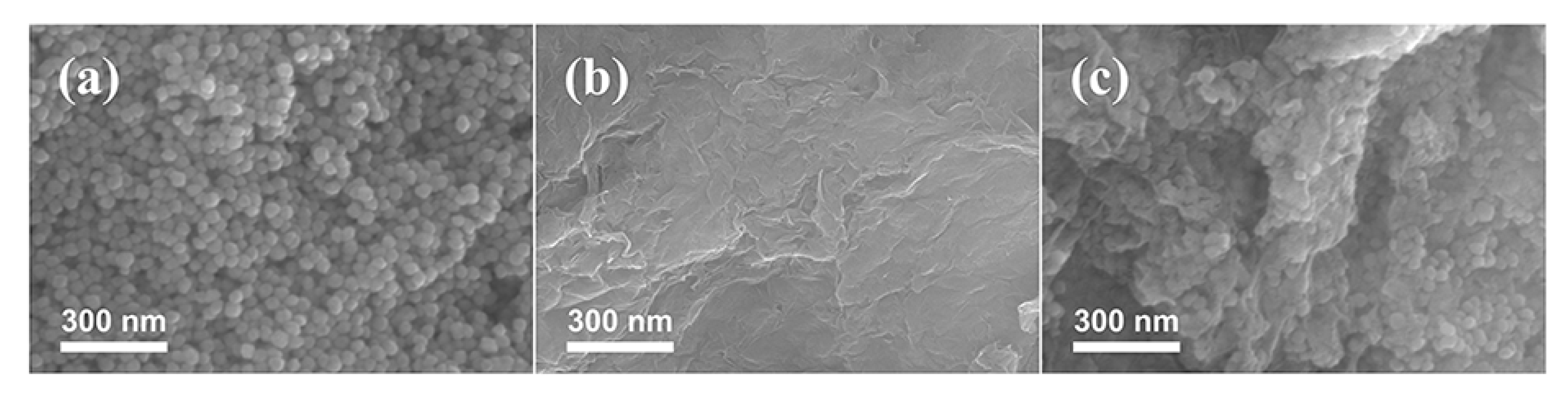
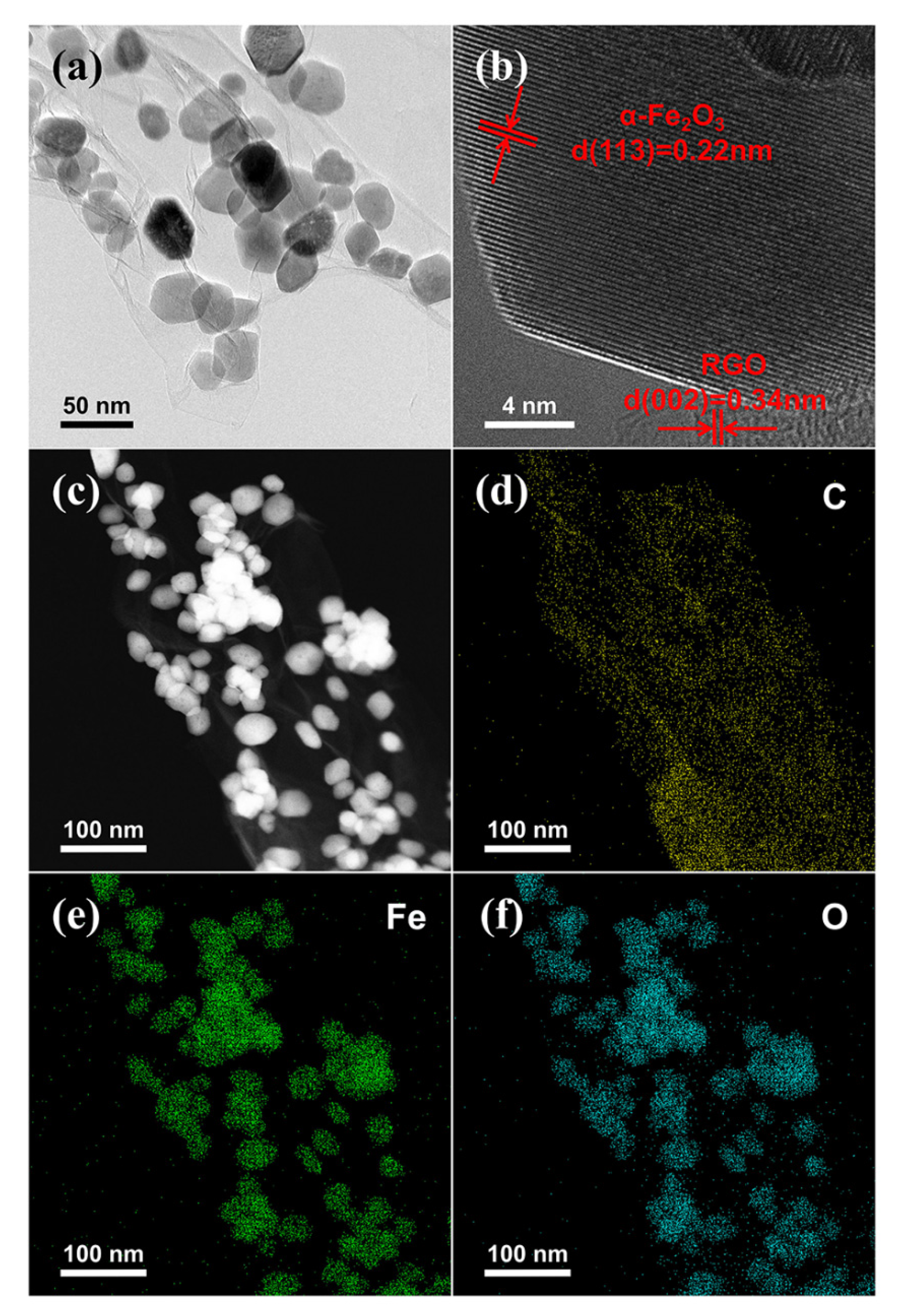
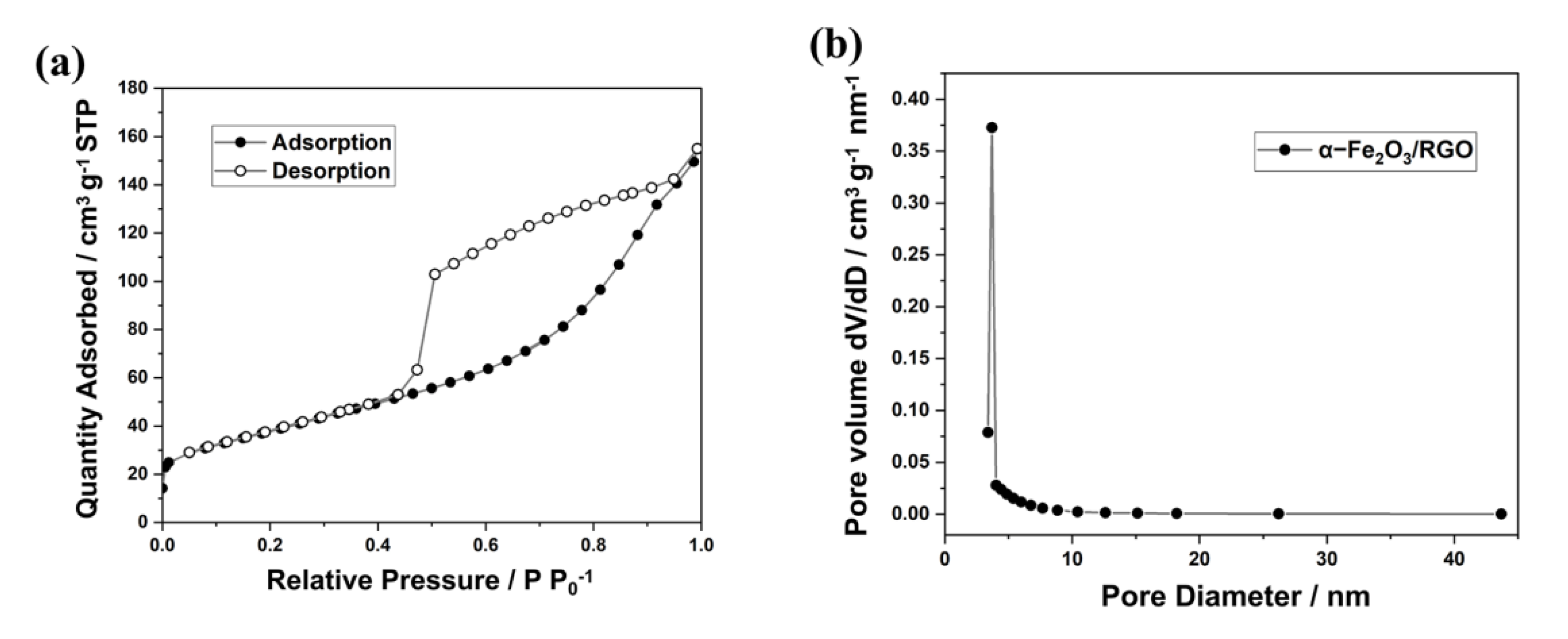
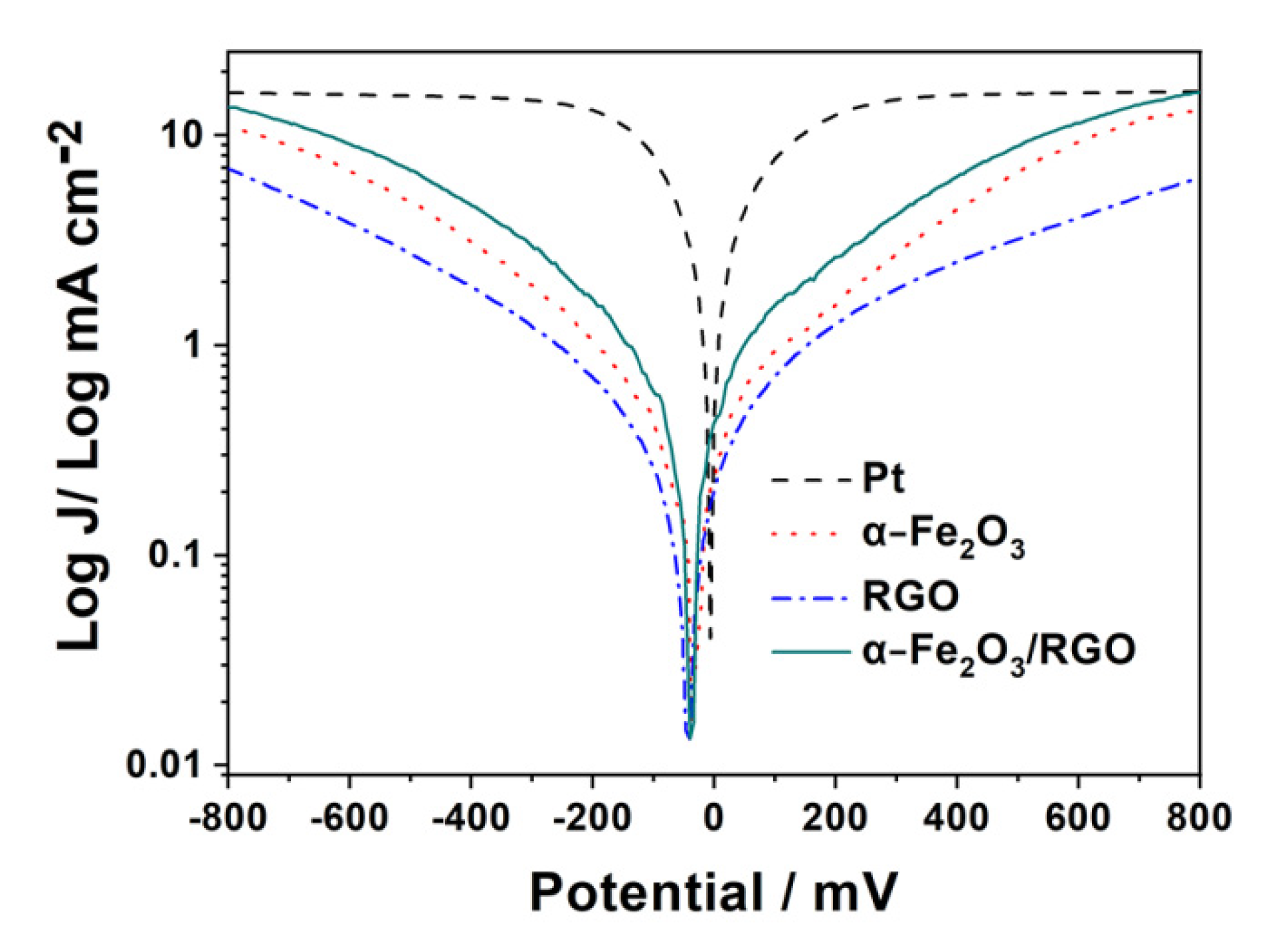
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Materials
3.2. Fabrication of CEs and DSSCs
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Saranya, K.; Rameez, M.; Subramania, A. Developments in conducting polymer based counter electrodes for dye-sensitized solar cells–An overview. Eur. Polym. J. 2015, 66, 207–227. [Google Scholar] [CrossRef]
- Kang, J.S.; Kim, J.; Kim, J.Y.; Lee, M.J.; Kang, J.; Son, Y.J.; Jeong, J.; Park, S.H.; Ko, M.J.; Sung, Y.E. Highly efficient bifacial dye-sensitized solar cells employing polymeric counter electrodes. ACS Appl. Mater. Interfaces 2018, 10, 8611–8620. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Duan, Y.; Fu, N.; Liu, Q.; Fang, Y.; Sun, Q.; Lin, Y. Mn3O4/graphene composite as counter electrode in dye-sensitized solar cells. RSC Adv. 2014, 4, 15091–15097. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, K.; Hasija, V.; Sharma, V.; Sharma, S.; Raizada, P.; Singh, M.; Saini, A.K.; Hosseini-Bandegharaei, A.; Thakur, V.K. Systematic review on applicability of magnetic iron oxides-integrated photocatalysts for degradation of organic pollutants in water. Mater. Today Chem. 2019, 14, 100186. [Google Scholar] [CrossRef]
- Ye, H.; Wang, Y.; Liu, X.J.; Xu, D.D.; Yuan, H.; Sun, H.Q.; Wang, S.B.; Ma, X. Magnetically steerable iron oxides-manganese dioxide core-shell micromotors for organic and microplastic removals. J. Colloid Interface Sci. 2021, 588, 510–521. [Google Scholar] [CrossRef]
- Kumar, Y.; Kumar, R.; Raizada, P.; Khan, A.A.P.; Singh, A.; Le, Q.V.; Nguyen, V.H.; Selvasembian, R.; Thakur, S.; Singh, P. Current status of hematite (α-Fe2O3) based Z-scheme photocatalytic systems for environmental and energy applications. J. Environ. Chem. Eng. 2022, 10, 107427. [Google Scholar] [CrossRef]
- Gao, R.J.; Wang, J.; Huang, Z.F.; Zhang, R.R.; Wang, W.; Pan, L.; Zhang, J.F.; Zhu, W.K.; Zhang, X.W.; Shi, C.X.; et al. Pt/Fe2O3 with Pt-Fe pair sites as a catalyst for oxygen reduction with ultralow Pt loading. Nat. Energy 2021, 6, 614–623. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, Q.; Liao, Q.; Ma, M.; Gao, F.; Zhao, X.; Song, Y.; Song, L.; Xun, X.; Zhang, Y. Amphiphobic hydraulic triboelectric nanogenerator for self-cleaning/charging power system. Adv. Funct. Mater. 2018, 28, 1803117. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, D.; Yang, X.H.; Fang, W.Q.; Zhang, B.; Wang, H.F.; Lu, G.Z.; Hu, P.; Zhao, H.; Yang, H. Rational screening low-cost counter electrodes for dye-sensitized solar cells. Nat. Commun. 2013, 4, 1583. [Google Scholar] [CrossRef] [Green Version]
- Niu, H.; Zhang, S.; Ma, Q.; Qin, S.; Wan, L.; Xu, J.; Miao, S. Dye-sensitized solar cells based on flower-shaped α-Fe2O3 as a photoanode and reduced graphene oxide–polyaniline composite as a counter electrode. RSC Adv. 2013, 3, 17228–17235. [Google Scholar] [CrossRef]
- Wang, M.; Huang, M.; Luo, D.; Li, Y.; Choe, M.; Seong, W.K.; Kim, M.; Jin, S.; Wang, M.; Chatterjee, S.; et al. Single-crystal, large-area, fold-free monolayer graphene. Nature 2021, 596, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Qing, M.; Wang, Y.; Chen, S. Defects in graphene: Generation, healing, and their effects on the properties of graphene: A review. J. Mater. Sci. Technol. 2015, 31, 599–606. [Google Scholar] [CrossRef]
- Fang, B.; Chang, D.; Xu, Z.; Gao, C. A review on graphene fibers: Expectations, advances, and prospects. Adv. Mater. 2020, 32, 1902664. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, Q.; Liao, Q.; Yi, F.; Zheng, X.; Ma, M.; Gao, F.; Zhang, Y. Service behavior of multifunctional triboelectric nanogenerators. Adv. Mater. 2017, 29, 1606703. [Google Scholar] [CrossRef]
- Kweon, D.H.; Baek, J.B. Edge-functionalized graphene nanoplatelets as metal-Free electrocatalysts for dye-sensitized solar cells. Adv. Mater. 2019, 31, 1804440. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, Y.; Murali, S.; Stoller, M.D.; Ruoff, R.S. Nanostructured reduced graphene oxide/Fe2O3 composite as a high-performance anode material for lithium ion batteries. ACS Nano 2011, 5, 3333–3338. [Google Scholar] [CrossRef]
- Yang, W.; Xu, X.W.; Li, Z.; Yang, F.; Zhang, L.Q.; Li, Y.F.; Wang, A.J.; Chen, S.L. Construction of efficient counter electrodes for dye-sensitized solar cells: Fe2O3 nanoparticles anchored onto graphene frameworks. Carbon 2016, 96, 947–954. [Google Scholar] [CrossRef]
- Zhao, G.M.; Xu, G.J.; Jin, S. α-Fe2O3 hollow meso-microspheres grown on graphene sheets function as a promising counter electrode in dye-sensitized solar cells. Rsc Adv. 2019, 9, 24164–24170. [Google Scholar] [CrossRef] [Green Version]
- Du, M.; Xu, C.; Sun, J.; Gao, L. Synthesis of α-Fe2O3 nanoparticles from Fe(OH)3 sol and their composite with reduced graphene oxide for lithium ion batteries. J. Mater. Chem. A 2013, 1, 7154–7158. [Google Scholar] [CrossRef]
- Wang, G. Preparation of α-Fe2O3/graphene composite and its electrochemical performance as an anode material for lithium ion batteries. J. Alloys Compd. 2011, 509, 216–220. [Google Scholar] [CrossRef]
- Pradhan, G.K.; Padhi, D.K.; Parida, K.M. Fabrication of α-Fe2O3 nanorod/RGO composite: A novel hybrid photocatalyst for phenol degradation. ACS Appl. Mater. Interfaces 2013, 5, 9101–9110. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Venâncio Silva, S.; De Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman. Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Yi, H.; Wei, H.; Guo, Z.; Wang, X. One-step preparation of single-crystalline Fe2O3 particles/graphene composite hydrogels as high performance anode materials for supercapacitors. Nano Energy 2014, 7, 86–96. [Google Scholar] [CrossRef]
- Sun, B.; Horvat, J.; Kim, H.S.; Kim, W.-S.; Ahn, J.; Wang, G. Synthesis of mesoporous α-Fe2O3 nanostructures for highly sensitive gas sensors and high capacity anode materials in lithium ion batteries. J. Phys. Chem. C 2010, 114, 18753–18761. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, J.; Zhu, B.; Yu, J.; Xu, J. CuInS2 sensitized TiO2 hybrid nanofibers for improved photocatalytic CO2 reduction. Appl. Catal. B Environ. 2018, 230, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.-L.; Chen, H.-Y.; Su, C.-Y.; Kuang, D.-B. A novel TCO-and Pt-free counter electrode for high efficiency dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 1724–1730. [Google Scholar] [CrossRef]
- Gong, F.; Wang, H.; Xu, X.; Zhou, G.; Wang, Z.-S. In situ growth of Co0.85Se and Ni0.85Se on conductive substrates as high-performance counter electrodes for dye-sensitized solar cells. J. Am. Chem. Soc. 2012, 134, 10953–10958. [Google Scholar] [CrossRef]
- Fu, N.Q.; Xiao, X.; Zhou, X.W.; Zhang, J.; Lin, Y. Electrodeposition of platinum on plastic substrates as counter electrodes for flexible dye-sensitized solar cells. J. Phys. Chem. C 2012, 116, 2850–2857. [Google Scholar] [CrossRef]
- Tai, S.-Y.; Liu, C.-J.; Chou, S.-W.; Chien, F.S.-S.; Lin, J.-Y.; Lin, T.-W. Few-layer MoS2 nanosheets coated onto multi-walled carbon nanotubes as a low-cost and highly electrocatalytic counter electrode for dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 24753–24759. [Google Scholar] [CrossRef]
- Velten, J.; Mozer, A.J.; Li, D.; Officer, D.; Wallace, G.; Baughman, R.; Zakhidov, A. Carbon nanotube/graphene nanocomposite as efficient counter electrodes in dye-sensitized solar cells. Nanotechnology 2012, 23, 085201. [Google Scholar] [CrossRef] [Green Version]
- Alkhouzaam, A.; Abdelrazeq, H.; Khraisheh, M.; AlMomani, F.; Hameed, B.H.; Hassan, M.K.; Al-Ghouti, M.A.; Selvaraj, R. Spectral and structural properties of high-quality reduced graphene oxide produced via a simple approach using tetraethylenepentamine. Nanomaterials 2022, 12, 1240. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Duan, Y.; Fu, N.; Zhang, Q.; Fang, Y.; Zhou, X.; Lin, Y. Influence of Sn source on the performance of dye-sensitized solar cells based on Sn-doped TiO2 photoanodes: A strategy for choosing an appropriate doping source. Electrochim. Acta 2013, 107, 473–480. [Google Scholar] [CrossRef]
- Duan, Y.; Fu, N.; Liu, Q.; Fang, Y.; Zhou, X.; Zhang, J.; Lin, Y. Sn-doped TiO2 photoanode for dye-sensitized solar cells. J. Phys. Chem. C 2012, 116, 8888–8893. [Google Scholar] [CrossRef]



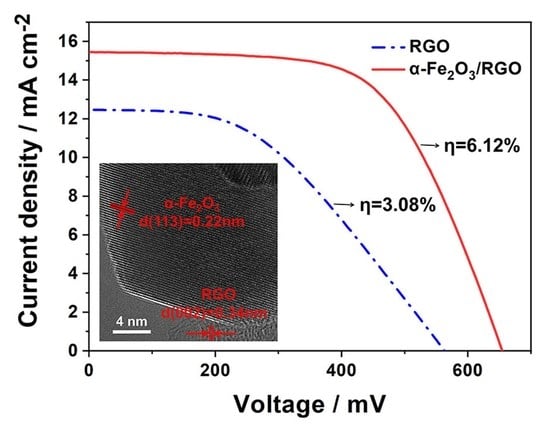
| CEs | Rct | jsc | Voc | FF | η |
|---|---|---|---|---|---|
| (Ω) | (mA cm−2) | (mV) | (%) | ||
| Pt | 1.78 | 16.33 | 635 | 0.67 | 6.93 |
| ±0.06 | ±0 | ±0.00 | ±0.05 | ||
| α-Fe2O3 | 5.42 | 13.3 | 635 | 0.55 | 4.69 |
| ±0.05 | ±5 | ±0.01 | ±0.03 | ||
| RGO | 11.02 | 12.47 | 555 | 0.45 | 3.08 |
| ±0.05 | ±5 | ±0.01 | ±0.05 | ||
| α-Fe2O3/RGO | 3.81 | 15.43 | 645 | 0.61 | 6.12 |
| ±0.00 | ±0 | ±0.00 | ±0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Zhang, Q.; Liang, Q.; Li, W.; Li, X.; Liu, S.; Shuai, J. α-Fe2O3/Reduced Graphene Oxide Composites as Cost-Effective Counter Electrode for Dye-Sensitized Solar Cells. Catalysts 2022, 12, 645. https://doi.org/10.3390/catal12060645
Sun L, Zhang Q, Liang Q, Li W, Li X, Liu S, Shuai J. α-Fe2O3/Reduced Graphene Oxide Composites as Cost-Effective Counter Electrode for Dye-Sensitized Solar Cells. Catalysts. 2022; 12(6):645. https://doi.org/10.3390/catal12060645
Chicago/Turabian StyleSun, Lian, Qian Zhang, Qijie Liang, Wenbo Li, Xiangguo Li, Shenghua Liu, and Jing Shuai. 2022. "α-Fe2O3/Reduced Graphene Oxide Composites as Cost-Effective Counter Electrode for Dye-Sensitized Solar Cells" Catalysts 12, no. 6: 645. https://doi.org/10.3390/catal12060645