Hg0 Removal by a Palygorskite and Fly Ash Supported MnO2-CeO2 Catalyst at Low Temperature
Abstract
:1. Introduction
2. Results and Discussion
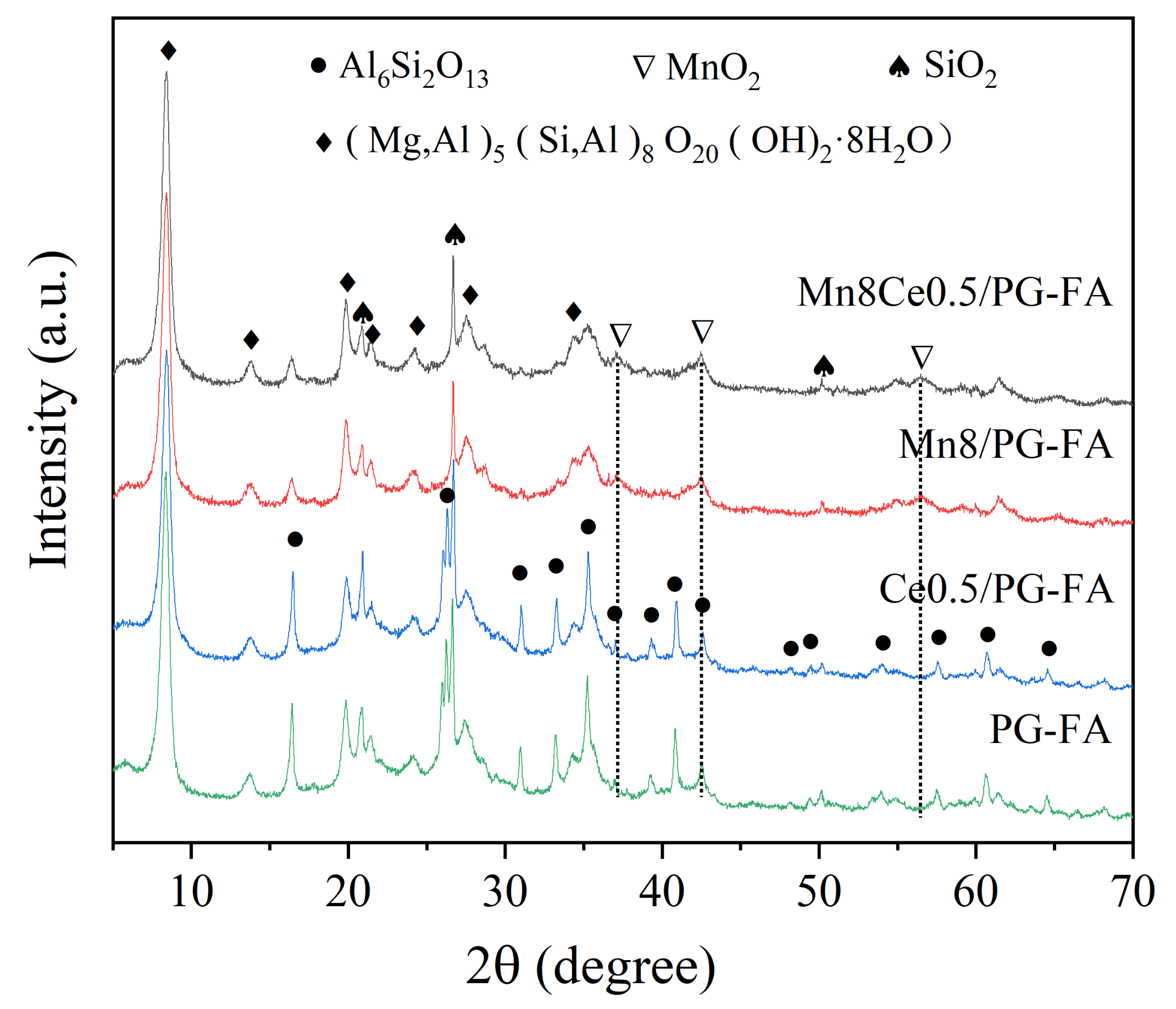
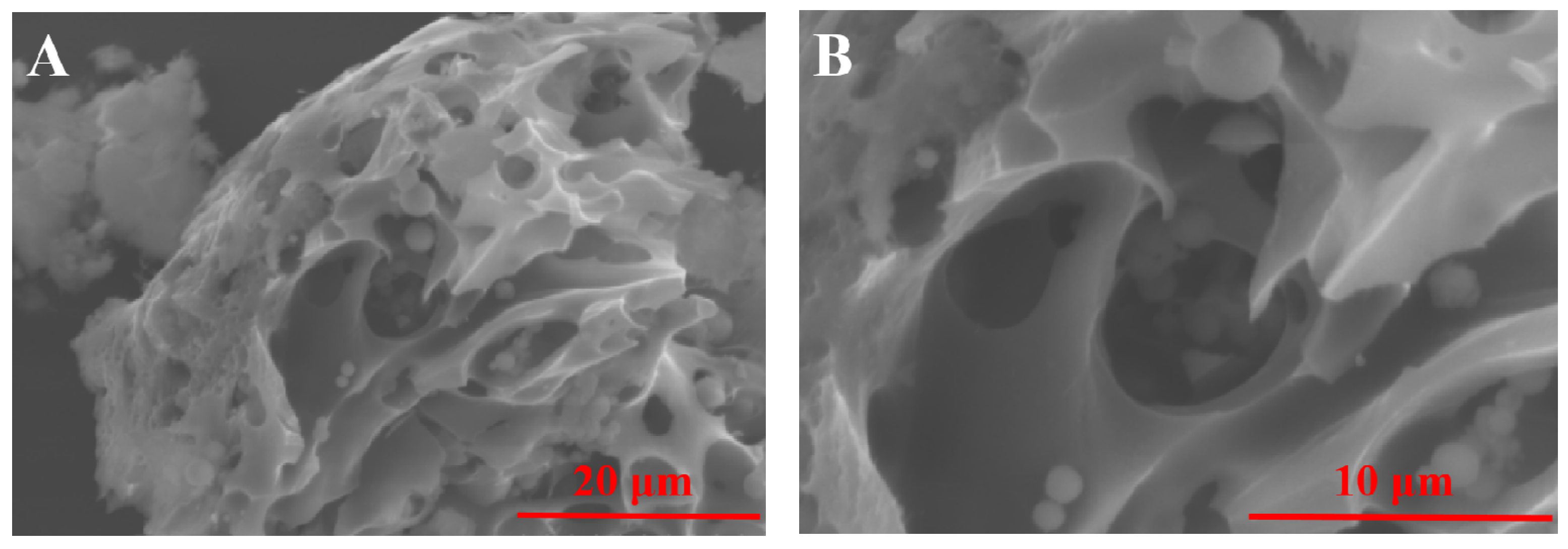
2.1. Characterization of the MnO2-CeO2/PG-FA Catalyst
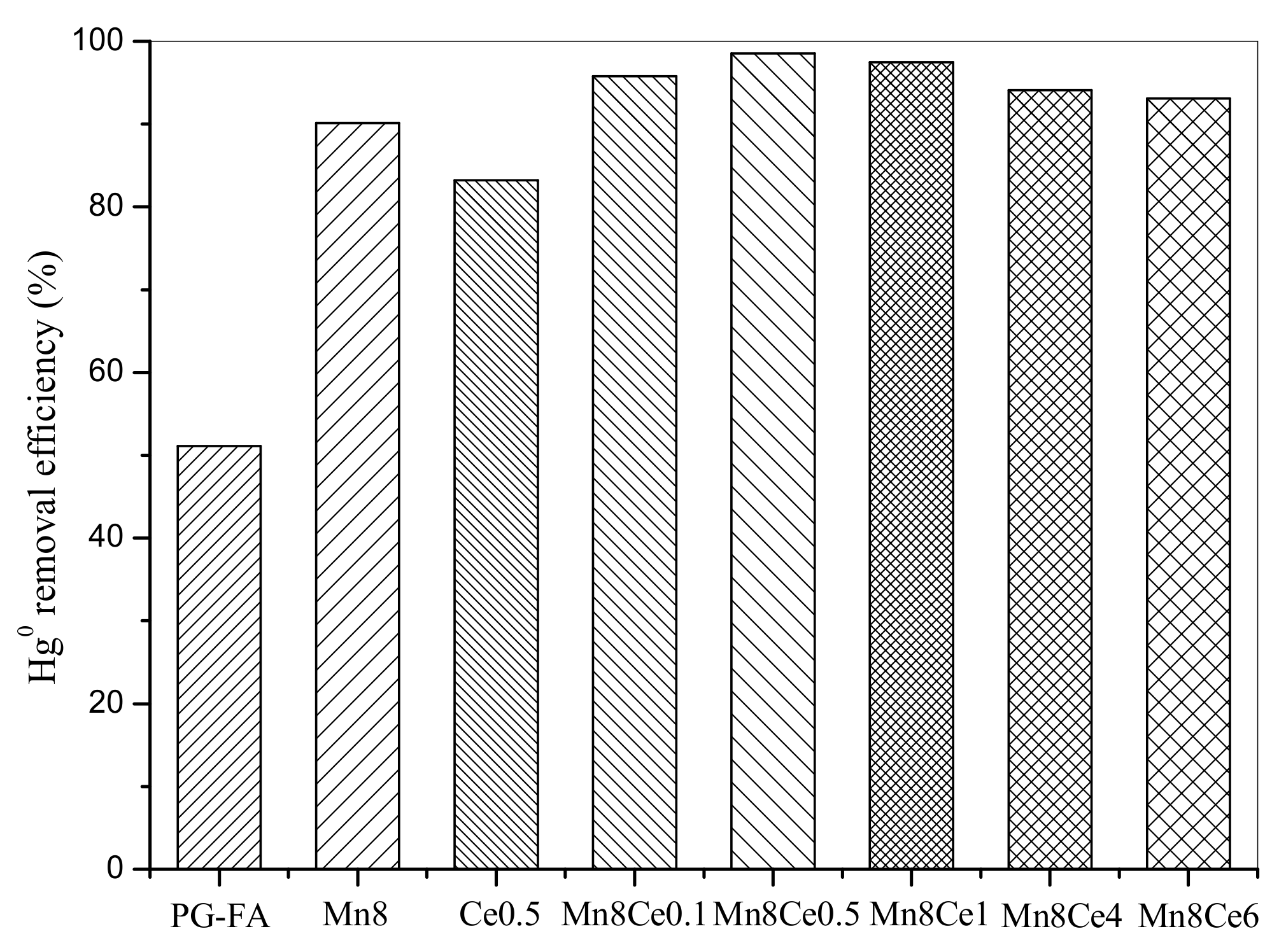
2.2. Effect of MnO2 and CeO2 Loading on Hg0 Removal
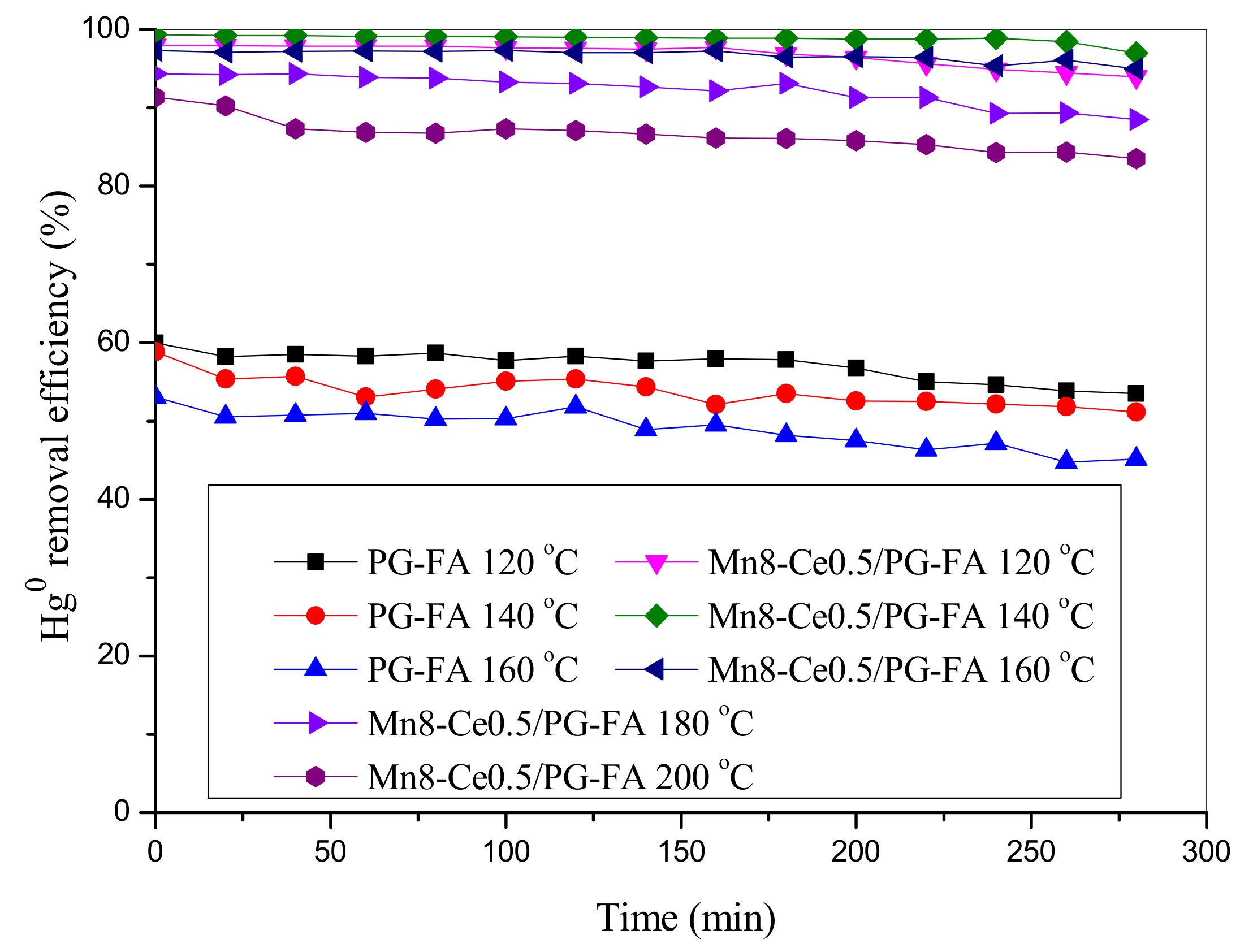
2.3. Effect of Temperature on Hg0 Removal by MnO2-CeO2/PG-FA
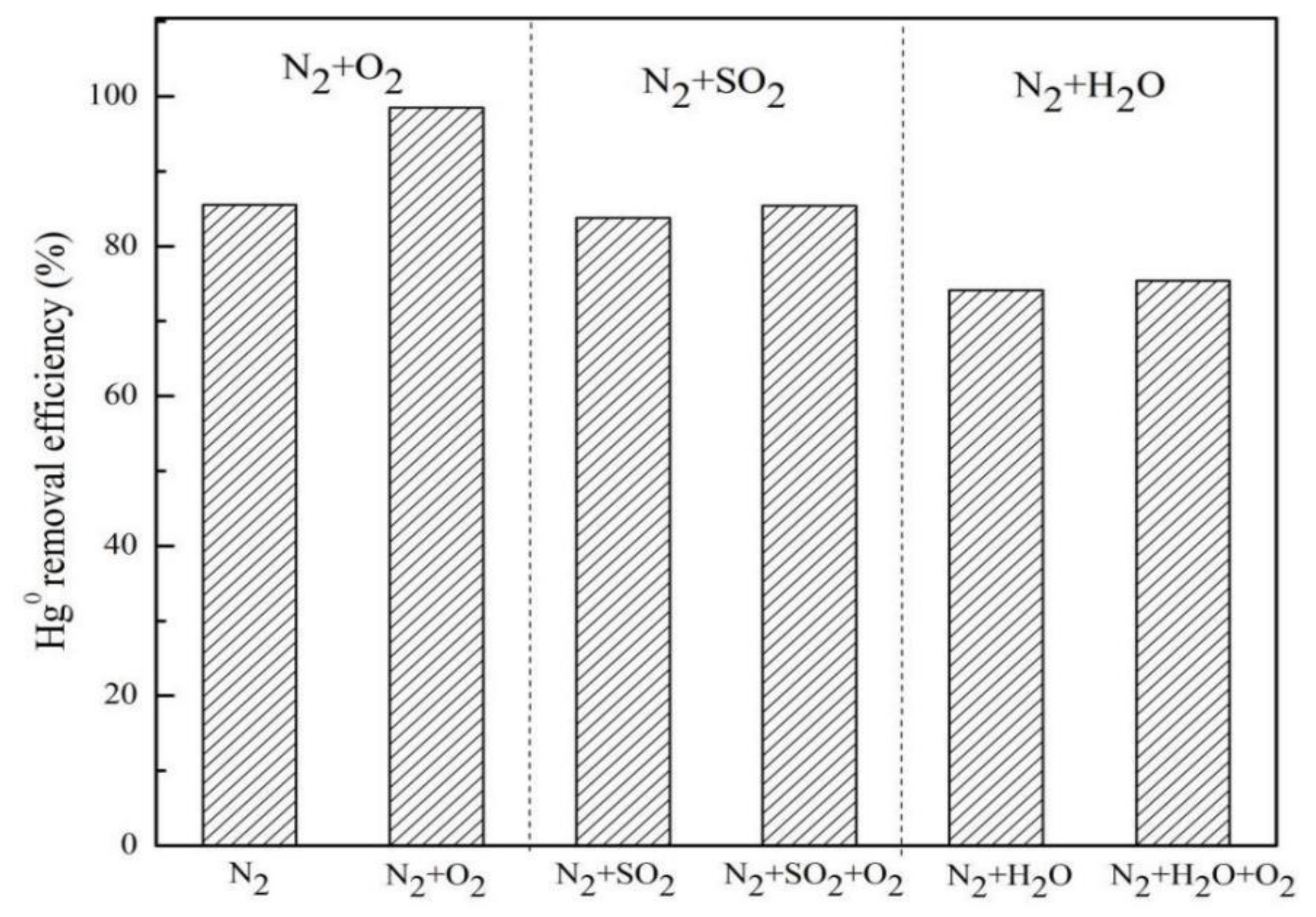
2.4. Effect of Flue Gas Components on Hg0 Removal by MnO2-CeO2/PG-FA
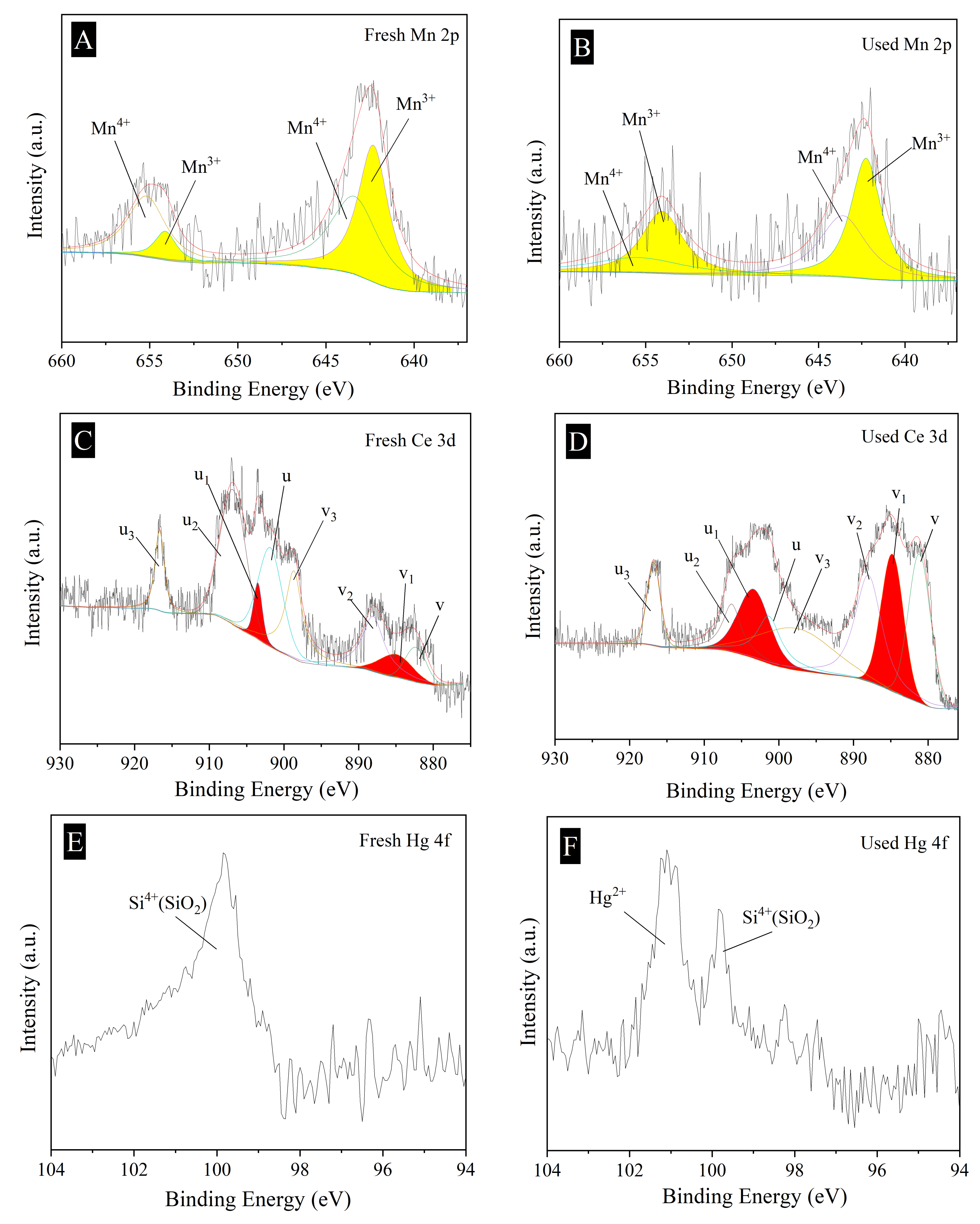
2.5. Speciation of Hg Adsorbed over Mn8-Ce0.5/PG-FA
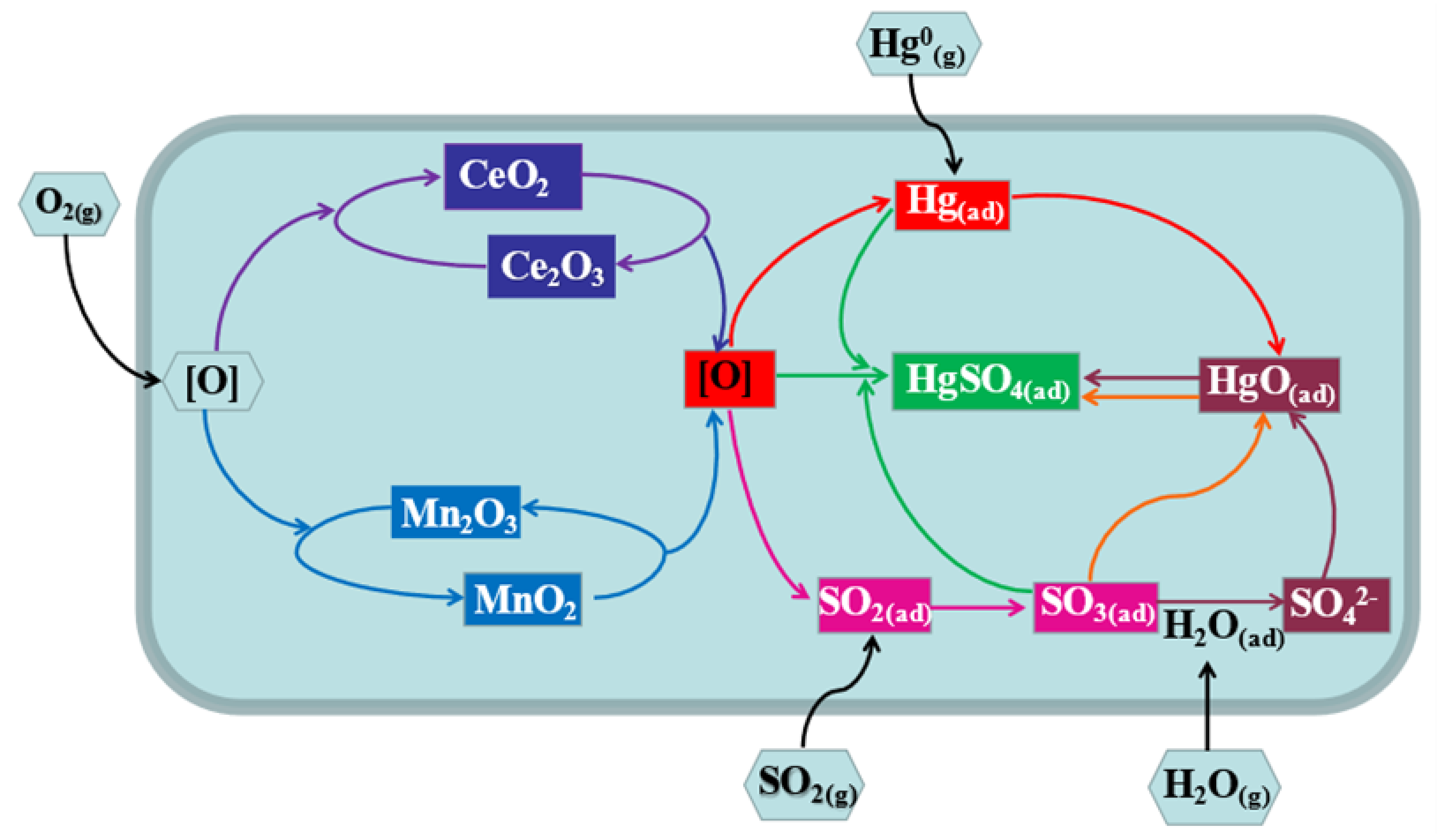
2.6. Hg0 Removal Process over MnO2-CeO2/PG-FA Catalyst
- Hg0 (g) → Hg0 (ad)
- O2 (g) → 2[O] (ad)
- 2CeO2 → Ce2O3 + [O]
- 2MnO2 → Mn2O3 + [O]
- Hg0 (ad) + [O] → HgO (ad)
- SO2 (g) + [O] → SO3 (ad)
- Hg0 (ad) + SO3 (ad) + [O] → HgSO4 (ad)
- SO3 (ad) + HgO (ad) → HgSO4 (ad)
- SO3 (ad) + H2O (ad) → H2SO4 (ad)
- H2SO4 (ad) + HgO (ad) → HgSO4 (ad) + H2O (ad)
- Ce2O3 + [O] → 2CeO2
- Mn2O3 + [O] → 2MnO2
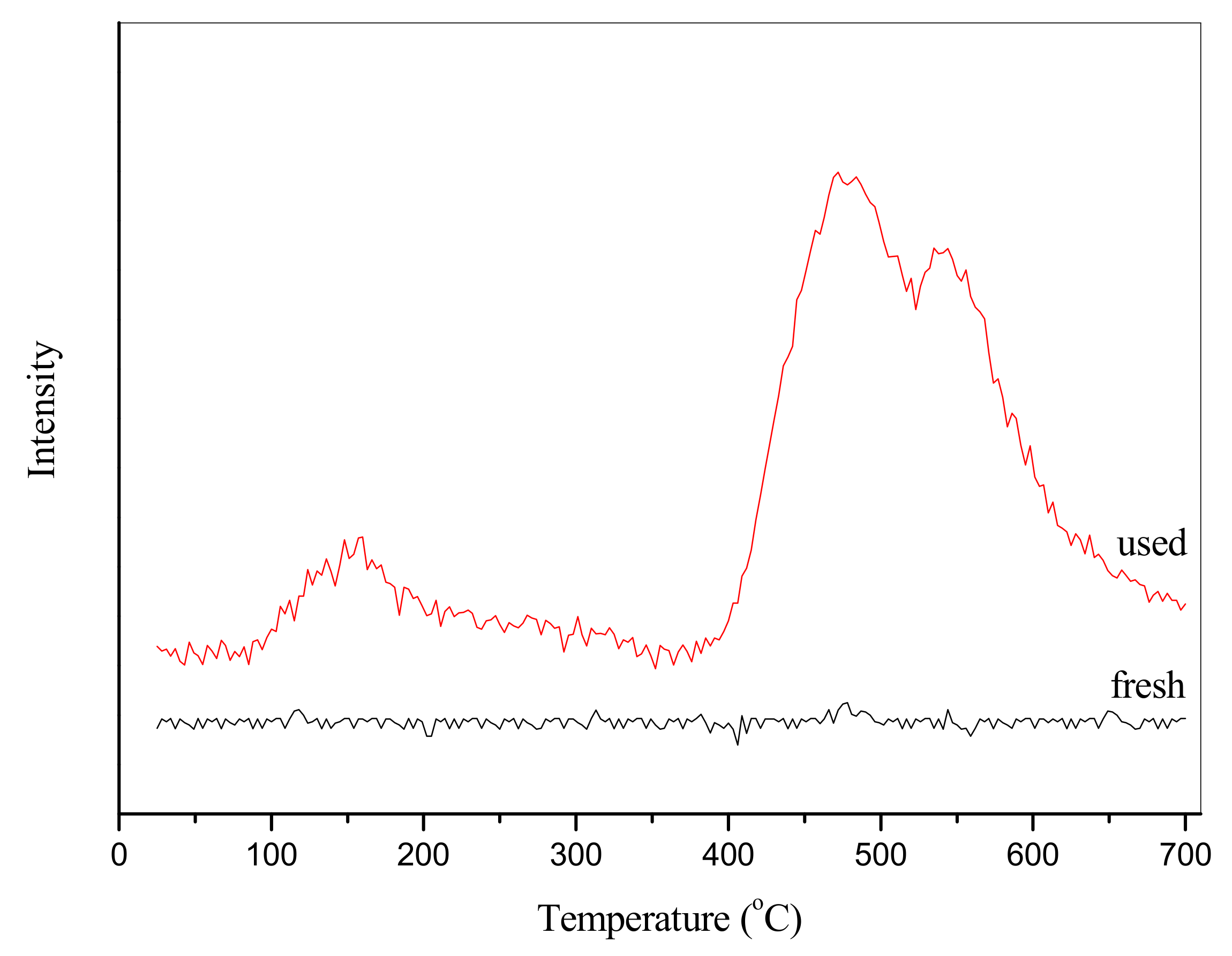
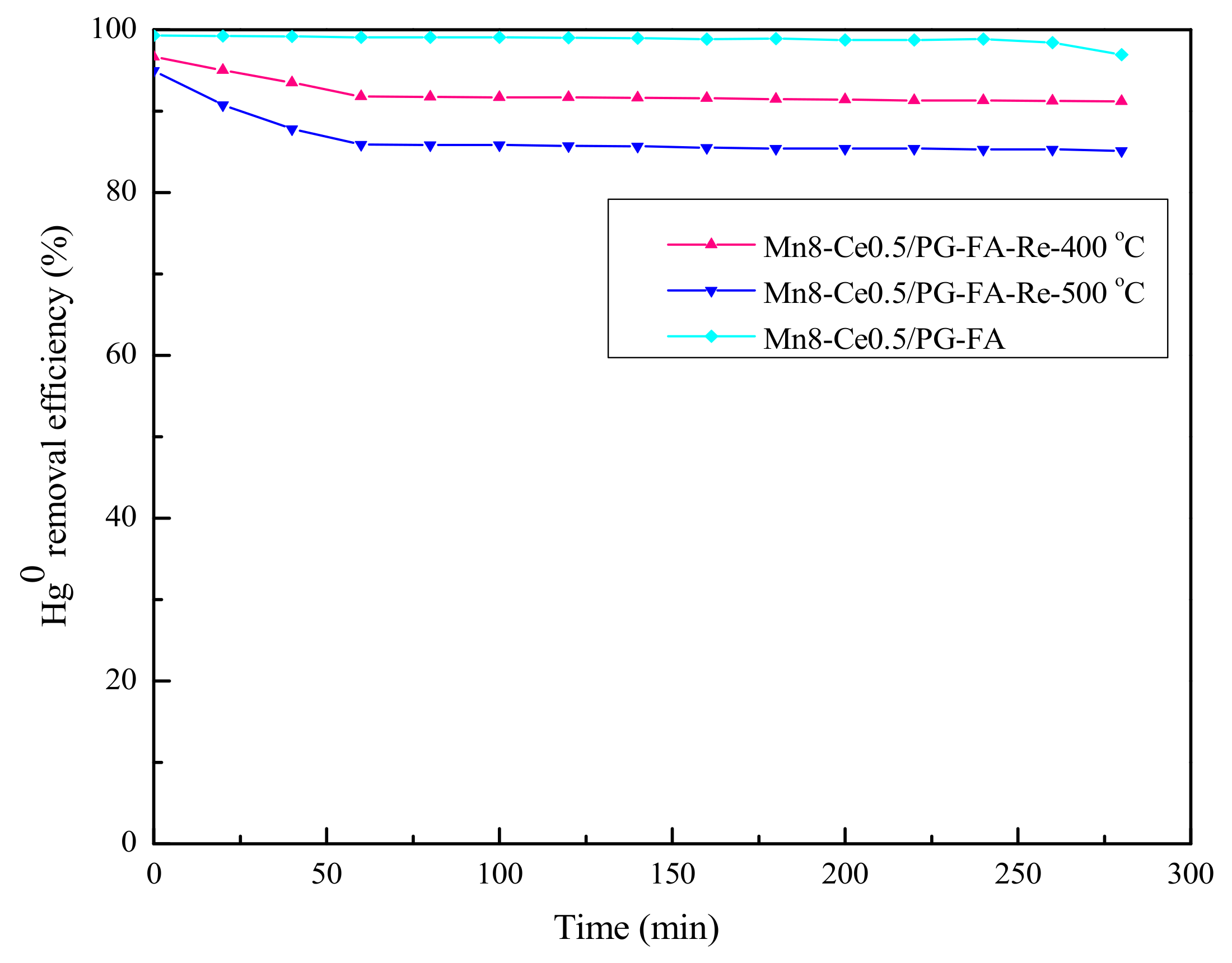
2.7. Regeneration of MnO2-CeO2/PG-FA Catalyst after Hg0 Removal
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Hg0 Removal by MnO2-CeO2/PG-FA Catalyst
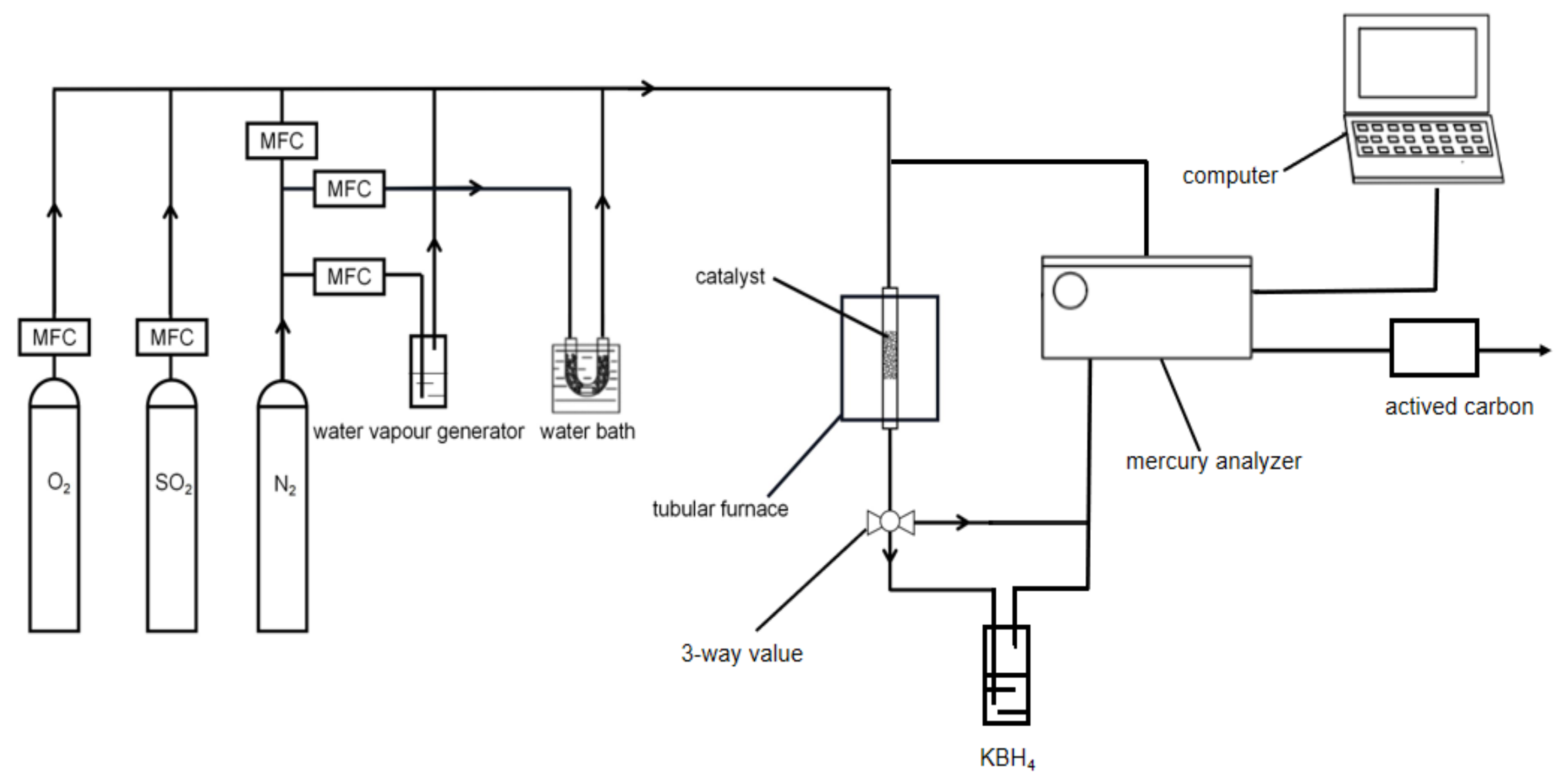
3.3. Catalyst Regeneration
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, L.X.; Zhang, Y.Y.; Wang, F.F.; Luo, Z.D.; Guo, S.J.; Strähle, U. Toxicity of mercury: Molecular evidence. Chemosphere 2020, 245, 125586. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Li, D.K.; Ju, F.L.; Han, L.N.; Chang, L.P.; Bao, W.R. Supercritical hydrothermal synthesis of zeolites from coal fly ash for mercury removal from coal derived gas. Fuel Process. Technol. 2015, 136, 96–105. [Google Scholar]
- Bank, M.S. The mercury science-policy interface: History, evolution and progress of the Minamata Convention. Sci. Total Environ. 2020, 722, 137832–137837. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, H.L. Effect of flue gas purification facilities of coal-fired power plant on mercury emission. Energy Rep. 2021, 7, 190–196. [Google Scholar] [CrossRef]
- Sjostrom, S.; Durham, M.; Bustard, C.J.; Martin, C. Activated carbon injection for mercury control: Overview. Fuel 2010, 89, 1320–1322. [Google Scholar] [CrossRef]
- Liu, H.; Chang, L.; Liu, W.J.; Xiong, Z.; Zhao, Y.C.; Zhang, J.Y. Advances in mercury removal from coal-fired flue gas by mineral adsorbents. Chem. Eng. J. 2020, 379, 122263. [Google Scholar]
- Takaoka, M.; Cheng, Y.C.; Oshit, K.; Watanabe, T.; Eguchi, S. Mercury removal from the flue gases of crematoria via pre-injection of lime and activated carbon into a fabric filter. Process Saf. Environ. 2021, 148, 323–332. [Google Scholar] [CrossRef]
- Wu, H.Y.; Li, C.T.; Zhao, L.K.; Zhang, J.; Zeng, G.M.; Xie, Y.E.; Zhang, X.N.; Wang, Y. Removal of gaseous elemental mercury by cylindrical activated coke loaded with CoOX-CeO2 from simulated coal combustion flue gas. Energy Fuels 2015, 29, 6747–6757. [Google Scholar]
- Zhao, H.T.; Ezeh, C.I.; Yin, S.F.; Xie, Z.L.; Pang, C.H.; Zheng, C.H.; Gao, X.; Wu, T. MoO3-adjusted δ-MnO2 nanosheet for catalytic oxidation of Hg0 to Hg2+. Appl. Catal. B 2020, 263, 117829. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Liu, X.W.; Zhao, B.; Shao, H.Z.; Xu, Y.S.; Xu, M.H. Elemental mercury oxidation over manganese-based perovskite-type catalyst at low temperature. Chem. Eng. J. 2016, 288, 701–710. [Google Scholar]
- Zhao, L.K.; Li, C.T.; Zhang, J.; Zhang, X.N.; Zhan, F.M.; Ma, J.F.; Xie, Y.E.; Zeng, G.M. Promotional effect of CeO2 modified support on V2O5-WO3/TiO2 catalyst for elemental mercury oxidation in simulated coal-fired flue gas. Fuel 2015, 153, 361–369. [Google Scholar] [CrossRef]
- Ye, D.; Wang, X.X.; Wang, R.X.; Wang, S.Y.; Liu, H.; Wang, H.N. Recent advances in MnO2-based adsorbents for mercury removal from coal-fired flue gas. J. Environ. Chem. Eng. 2021, 9, 105993. [Google Scholar] [CrossRef]
- Zhou, Q.; Tao, X.; Di, G.; Shang, Y.; Lu, P.; Xu, G.; Liu, M.; Zheng, Y.; Dong, L. Elemental mercury capture from flue gas by magnetic recyclable Fe6Mn1-xCexOy sorbent. Part 1. Performance evaluation and regeneration. Fuel 2021, 304, 120723. [Google Scholar] [CrossRef]
- Zhang, S.B.; Zhao, Y.C.; Yang, J.P.; Zhang, Y.; Sun, P.; Yu, X.H.; Zhang, J.Y.; Zheng, C.G. Simultaneous NO and mercury removal over MnOx/TiO2 catalyst in different atmospheres. Fuel Process. Technol. 2017, 166, 282–290. [Google Scholar] [CrossRef]
- Yang, Y.J.; Liu, J.; Zhang, B.K.; Zhao, Y.C.; Chen, X.; Shen, F.H. Experimental and theoretical studies of mercury oxidation over CeO2-WO3/TiO2 catalysts in coal-fired flue gas. Chem. Eng. J. 2017, 317, 758–765. [Google Scholar] [CrossRef]
- Li, H.L.; Zhang, W.L.; Wang, J.; Yang, Z.Q.; Li, L.Q.; Shih, K. Coexistence of enhanced Hg0 oxidation and induced Hg2+ reduction on CuO/TiO2 catalyst in the presence of NO and NH3. Chem. Eng. J. 2017, 330, 1248–1254. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, X.W.; Zhou, Z.J.; Shao, H.Z.; Xu, M.H. Catalytic oxidation of elemental mercury by Mn-Co/CNT at low temperature. Chem. Eng. J. 2016, 284, 1233–1241. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Yang, S.; Lai, Y.K.; Liu, H.; Fan, Y.; Liu, C.; Wang, H.Y.; Chai, L.Y. In-situ synthesis of monodispersed CuxO heterostructure on porous carbon monolith for exceptional removal of gaseous Hg0. Appl. Catal. B 2020, 265, 118556. [Google Scholar] [CrossRef]
- Zhang, X.L.; Wu, Q.; Diao, Q.C.; Wang, J.W.; Xiao, K.S.; Yang, B.J.; Wu, X.P. Performance study for NH3-SCR at low temperature based on different methods of Mnx/SEP catalyst. Chem. Eng. J. 2019, 370, 364–371. [Google Scholar] [CrossRef]
- Wang, J.W.; Xu, C.; Qin, W.; Zhang, J.L.; Zhang, X.L.; Cui, X.F. Hg0 removal by palygorskite (PG) supported MnOx catalyst. J. Fuel Chem. Technol. 2020, 48, 1442–1451. [Google Scholar]
- Wang, J.W.; Shen, Y.Y.; Dong, Y.J.; Qin, W.; Zhang, Q.P.; Lu, L.; Zhang, Y.G. Oxidation and adsorption of gas-phase Hg0 over a V2O5/AC catalyst. RSC Adv. 2016, 6, 77553–77557. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Hou, Y.Q.; Wang, J.W.; Guo, Y.P.; Huang, Z.G.; Han, X.J. Effect of SCR atmosphere on the removal of Hg0 by a V2O5-CeO2/AC catalyst at low temperature. Environ. Sci. Technol. 2019, 53, 5521–5527. [Google Scholar]
- Rumayor, M.; Diaz-Somoano, M.; López-Antón, M.A. Temperature programmed desorption as a tool for the identification of mercury fate in wet-desulphurization systems. Fuel 2015, 148, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.J.; Uddin, M.; Nagano, S. Fundamental study on decomposition characteristics of mercury compounds over solid powder by temperature-programmed decomposition desorption mass spectrometry. Energy Fuels 2011, 25, 144–153. [Google Scholar] [CrossRef]
- Uddin, M.A.; Ozaki, M.; Sasaoka, E.J.; Wu, S.J. Temperature-programmed decomposition desorption of mercury species over activated carbon sorbents for mercury removal from coal-derived fuel gas. Energy Fuels 2009, 23, 4710–4716. [Google Scholar] [CrossRef]
- Liu, M.; Li, C.T.; Zeng, Q.; Du, X.Y.; Gao, L.; Li, S.H.; Zhai, Y.B. Study on removal of elemental mercury over MoO3-CeO2/cylindrical activated coke in the presence of SO2 by Hg-temperature-programmed desorption. Chem. Eng. J. 2019, 371, 666–678. [Google Scholar] [CrossRef]
- Wang, J.W.; Yang, J.L.; Liu, Z.Y. Gas-phase elemental mercury capture by a V2O5/AC catalyst. Fuel Process. Technol. 2010, 91, 676–680. [Google Scholar] [CrossRef]
| Sample | Chemical Compositions | |||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | TiO2 | K2O | MnO | |
| PG | 69.45 | 11.84 | 5.14 | 0.21 | 12.17 | 0.44 | 0.51 | 0.05 |
| FA | 48.29 | 21.64 | 3.05 | 20.33 | 1.74 | 0.98 | 1.66 | 0.04 |
| Sample | BET Area (m2/g) | Micropore Volume (cm3/g) | Average Diameter (nm) |
|---|---|---|---|
| PG-FA | 100.99 | 0.35 | 13.86 |
| Mn8/PG-FA | 91.22 | 0.31 | 13.59 |
| Mn8-Ce0.5/PG-FA | 87.63 | 0.28 | 11.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Jiang, C.; Shi, L.; Xue, Z.; Wang, X.; Xu, C.; Zhang, X.; Zhang, J. Hg0 Removal by a Palygorskite and Fly Ash Supported MnO2-CeO2 Catalyst at Low Temperature. Catalysts 2022, 12, 662. https://doi.org/10.3390/catal12060662
Wang J, Jiang C, Shi L, Xue Z, Wang X, Xu C, Zhang X, Zhang J. Hg0 Removal by a Palygorskite and Fly Ash Supported MnO2-CeO2 Catalyst at Low Temperature. Catalysts. 2022; 12(6):662. https://doi.org/10.3390/catal12060662
Chicago/Turabian StyleWang, Junwei, Caihong Jiang, Liming Shi, Zhifeng Xue, Xie Wang, Can Xu, Xianlong Zhang, and Jianli Zhang. 2022. "Hg0 Removal by a Palygorskite and Fly Ash Supported MnO2-CeO2 Catalyst at Low Temperature" Catalysts 12, no. 6: 662. https://doi.org/10.3390/catal12060662
APA StyleWang, J., Jiang, C., Shi, L., Xue, Z., Wang, X., Xu, C., Zhang, X., & Zhang, J. (2022). Hg0 Removal by a Palygorskite and Fly Ash Supported MnO2-CeO2 Catalyst at Low Temperature. Catalysts, 12(6), 662. https://doi.org/10.3390/catal12060662





