Soot Oxidation in a Plasma-Catalytic Reactor: A Case Study of Zeolite-Supported Vanadium Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Textural Properties
2.2. Redox Properties
2.3. Effect of Catalyst Support on Plasma-Catalytic Soot Oxidation
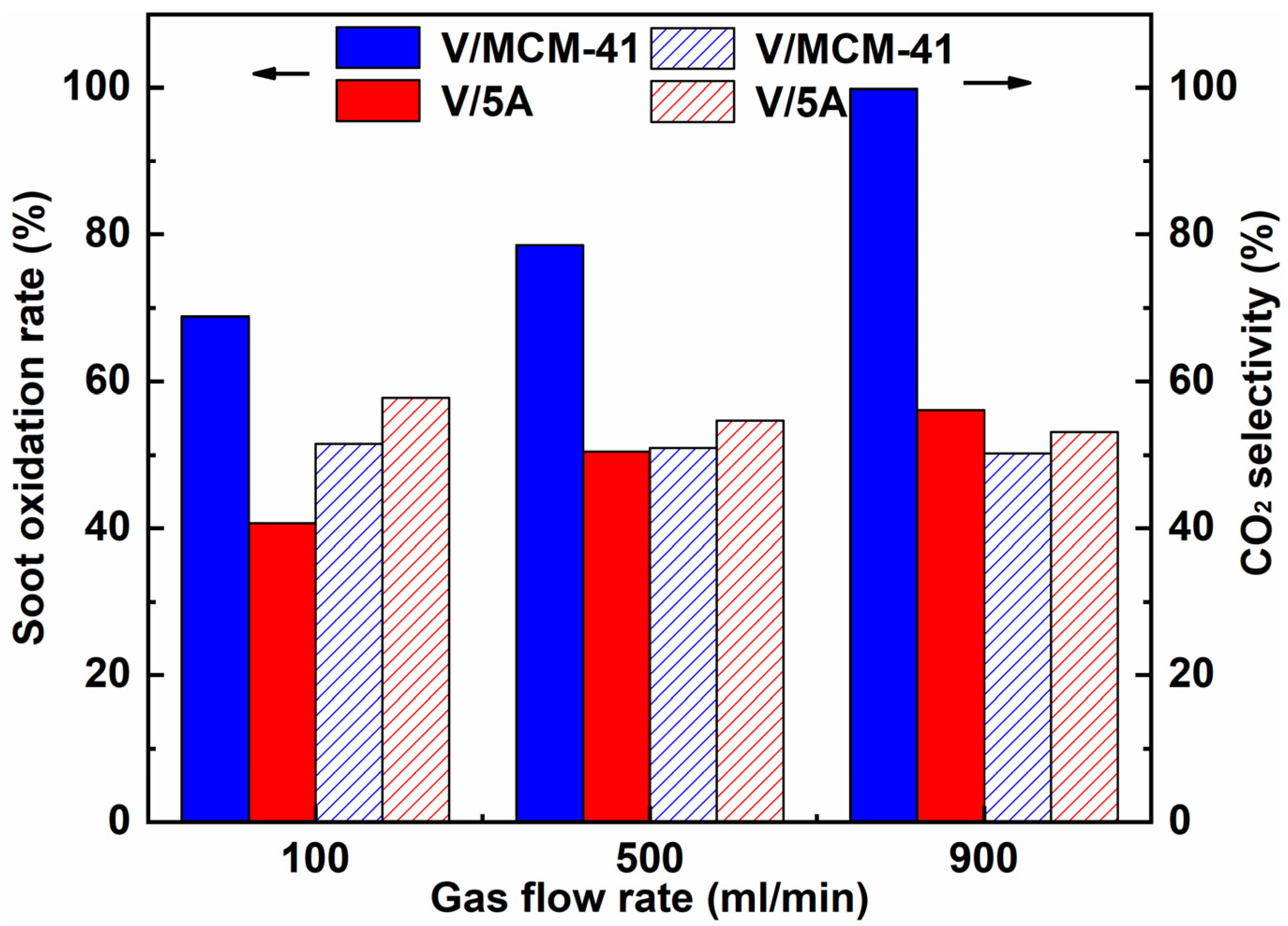
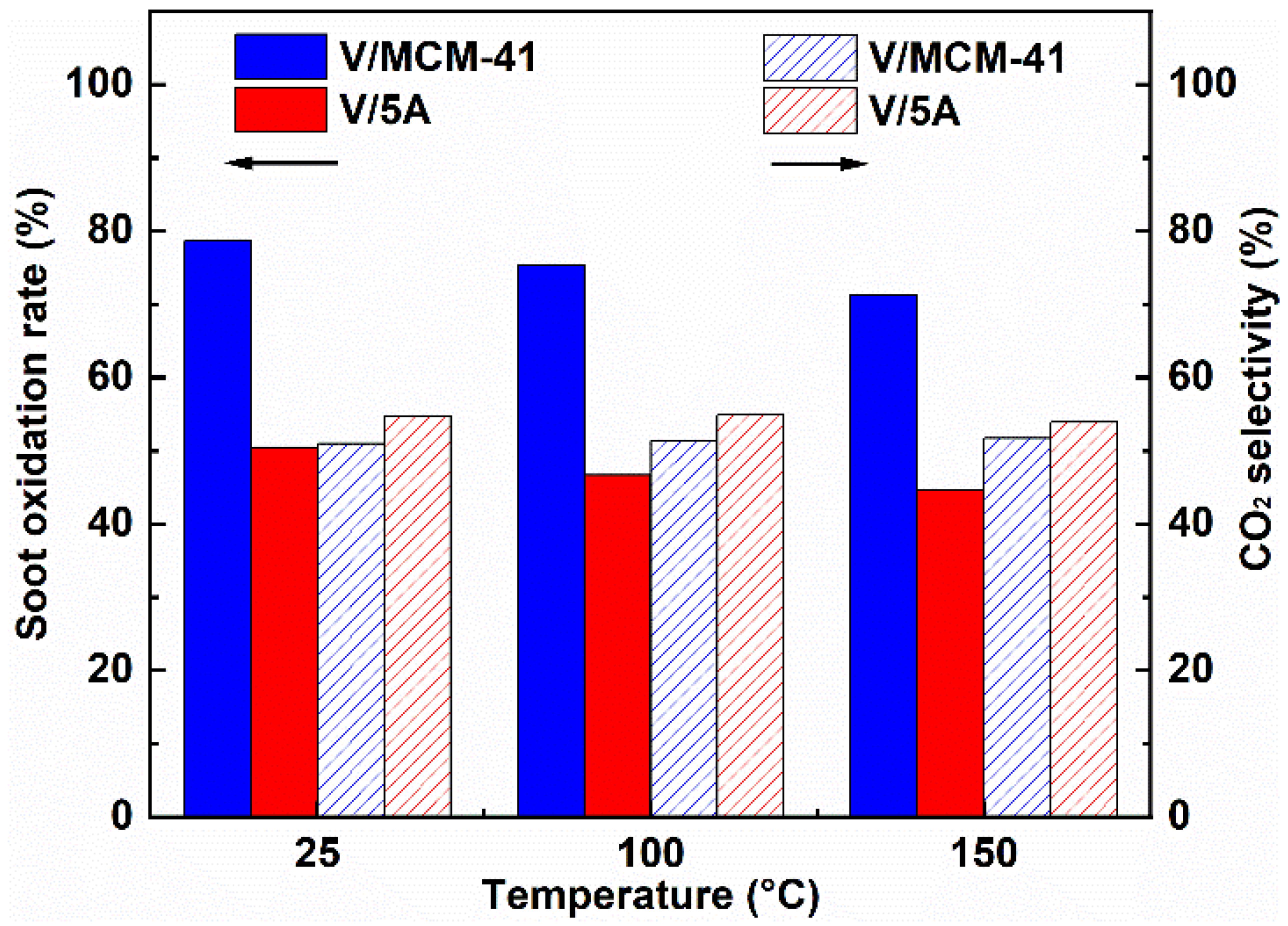
2.4. Effect of Operating Parameters
3. Materials and Methods
3.1. Catalyst Preparations
3.2. Catalyst Characterizations
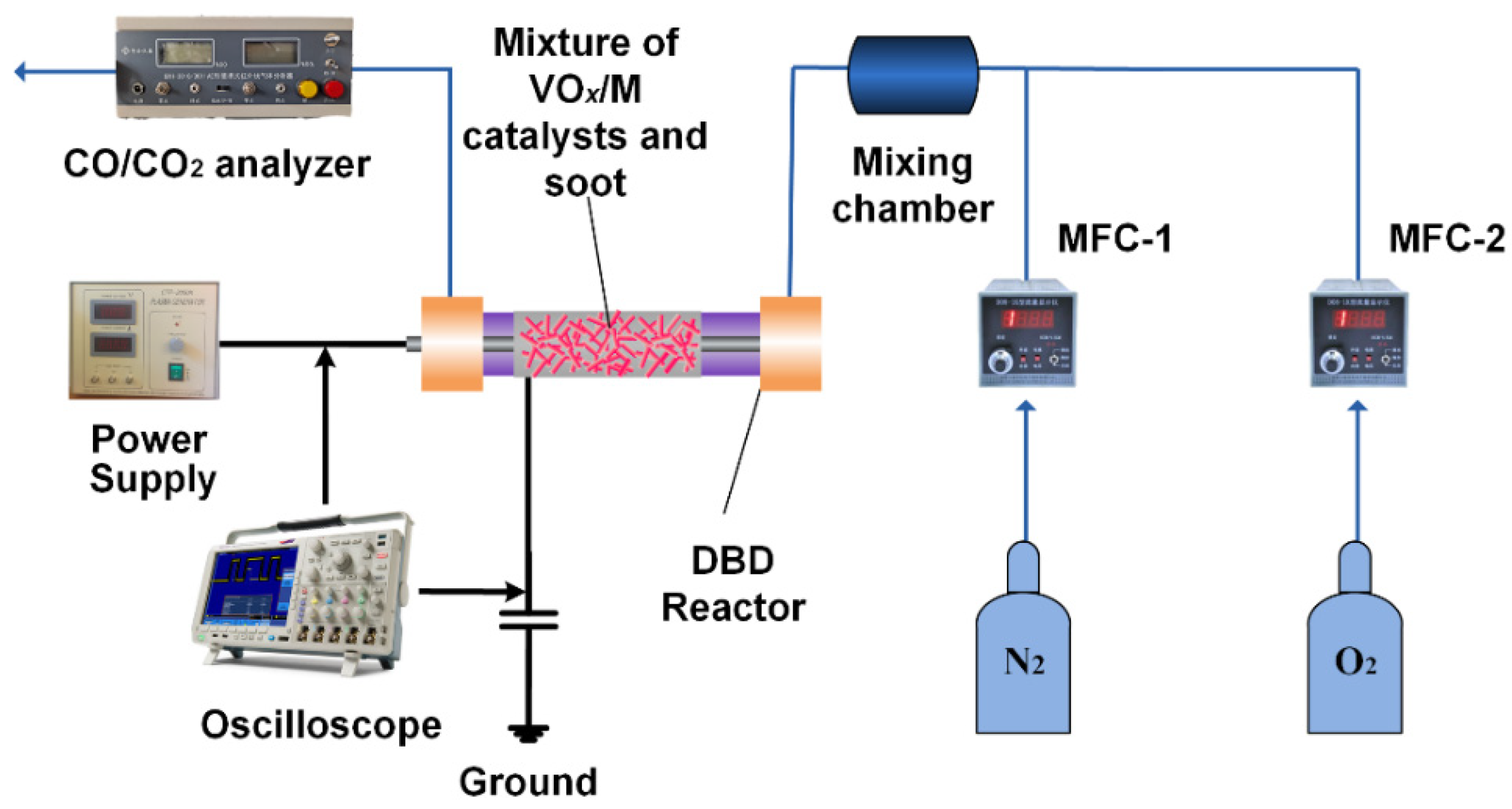
3.3. Plasma-Catalytic System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sawatmongkhon, B.; Theinnoi, K.; Wongchang, T.; Haoharn, C.; Wongkhorsub, C.; Sukjit, E.; Tsolakis, A. Catalytic oxidation of diesel particulate matter by using silver and ceria supported on alumina as the oxidation catalyst. Appl. Catal. A Gen. 2019, 574, 33–40. [Google Scholar] [CrossRef]
- Ou, J.; Meng, Z.; Hu, Y.; Du, Y. Experimental investigation on the variation characteristics of soot layer thickness and pressure drop during DPF/CDPF active regeneration. Chem. Eng. Sci. 2021, 241, 116682. [Google Scholar] [CrossRef]
- Liang, X.; Lv, X.; Wang, Y.; He, L.; Wang, Y.; Fu, K.; Liu, Q.; Wang, K. Experimental investigation of diesel soot oxidation reactivity along the exhaust after-treatment system components. Fuel 2021, 302, 121047. [Google Scholar] [CrossRef]
- Mishra, A.; Prasad, R. Preparation and application of perovskite catalysts for diesel soot emissions control: An overview. Catal. Rev. 2014, 56, 57–81. [Google Scholar] [CrossRef]
- Dhal, G.C.; Mohan, D.; Prasad, R. Preparation and application of effective different catalysts for simultaneous control of diesel soot and nox emissions: An overview. Catal. Sci. Technol. 2017, 7, 1803–1825. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Sun, P.; Ji, Q.; Meng, J.; Wang, P. Experimental investigation of in-cylinder soot distribution and exhaust particle oxidation characteristics of a diesel engine with nano-CeO2 catalytic fuel. Energy 2018, 161, 17–27. [Google Scholar] [CrossRef]
- Mori, K.; Miyauchi, Y.; Kuwahara, Y.; Yamashita, H. Shape effect of MnOx-decorated CeO2 catalyst in diesel soot oxidation. Bull. Chem. Soc. Jpn. 2017, 90, 556–564. [Google Scholar] [CrossRef]
- Shareei, M.; Taghvaei, H.; Azimi, A.; Shahbazi, A.; Mirzaei, M. Catalytic dbd plasma reactor for low temperature partial oxidation of methane: Maximization of synthesis gas and minimization of CO2. Int. J. Hydrogen Energy 2019, 44, 31873–31883. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Shangguan, W. Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Zheng, Y.; Grant, R.; Hu, W.; Marek, E.; Scott, S.A. H2 production from partial oxidation of CH4 by Fe2O3-supported Ni-based catalysts in a plasma-assisted packed bed reactor. Proc. Combust. Inst. 2019, 37, 5481–5488. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Yao, X.; Li, J.; Yao, S.; Wu, Z.; Zhang, H.; Lin, H.; Nozaki, T. Mechanism on the plasma-catalytic oxidation of graphitic carbon over Au/γ-Al2O3 by in situ plasma drifts-mass spectrometer. J. Hazard. Mater. 2020, 396, 122730. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Y.; Tang, D.; Miao, Y.; Cao, Z.; Zhao, Z. Synergy of NTP-La1−xAgxMn1−yCoyO3−δ hybrid for soot catalytic combustion at low temperature. Plasma Chem. Plasma Process. 2021, 41, 1009–1019. [Google Scholar] [CrossRef]
- Whitehead, J.C. Plasma–catalysis: The known knowns, the known unknowns and the unknown unknowns. J. Phys. D Appl. Phys. 2016, 49, 243001. [Google Scholar] [CrossRef]
- Bogaerts, A.; Tu, X.; Whitehead, J.C.; Centi, G.; Lefferts, L.; Guaitella, O.; Azzolina-Jury, F.; Kim, H.-H.; Murphy, A.B.; Schneider, W.F.; et al. The 2020 plasma catalysis roadmap. J. Phys. D Appl. Phys. 2020, 53, 443001. [Google Scholar] [CrossRef]
- Patil, B.S.; Cherkasov, N.; Srinath, N.V.; Lang, J.; Ibhadon, A.O.; Wang, Q.; Hessel, V. The role of heterogeneous catalysts in the plasma-catalytic ammonia synthesis. Catal. Today 2021, 362, 2–10. [Google Scholar] [CrossRef]
- Kim, H.H.; Teramoto, Y.; Sano, T.; Negishi, N.; Ogata, A. Effects of Si/Al ratio on the interaction of nonthermal plasma and ag/hy catalysts. Appl. Catal. B: Environ. 2015, 166, 9–17. [Google Scholar] [CrossRef]
- Xie, D.Y.; Sun, Y.; Zhu, T.L.; Hou, L.Y.; Hong, X.W. Nitric oxide oxidation and its removal in mist by nonthermal plasma: Effects of discharge conditions. Ind. Eng. Chem. Res. 2017, 56, 11336–11343. [Google Scholar] [CrossRef]
- Shi, Y.; Cai, Y.; Fan, R.; Cui, Y.; Chen, Y.; Ji, L. Characterization of soot inside a diesel particulate filter during a nonthermal plasma promoted regeneration step. Appl. Therm. Eng. 2019, 150, 612–619. [Google Scholar] [CrossRef]
- Pu, X.; Cai, Y.; Shi, Y.; Wang, J.; Gu, L.; Tian, J.; Li, W. Diesel particulate filter (DPF) regeneration using non-thermal plasma induced by dielectric barrier discharge. J. Energy Inst. 2018, 91, 655–667. [Google Scholar] [CrossRef]
- Ji, P.; Gao, X.; Du, X.; Zheng, C.; Luo, Z.; Cen, K. Relationship between the molecular structure of V2O5/TiO2 catalysts and the reactivity of SO2 oxidation. Catal. Sci. Technol. 2016, 6, 1187–1194. [Google Scholar] [CrossRef]
- Zhu, X.; Gao, X.; Yu, X.; Zheng, C.; Tu, X. Catalyst screening for acetone removal in a single-stage plasma-catalysis system. Catal. Today 2015, 256, 108–114. [Google Scholar] [CrossRef]
- Wei, L.; Kumar, N.; Haije, W.; Peltonen, J.; Peurla, M.; Grénman, H.; de Jong, W. Can bi-functional nickel modified 13X and 5A zeolite catalysts for CO2 methanation be improved by introducing ruthenium? Mol. Catal. 2020, 494, 111115. [Google Scholar] [CrossRef]
- Einaga, H.; Teraoka, Y.; Ogata, A. Catalytic oxidation of benzene by ozone over manganese oxides supported on usy zeolite. J. Catal. 2013, 305, 227–237. [Google Scholar] [CrossRef]
- de Oliveira, A.M.; Crizel, L.E.; da Silveira, R.S.; Pergher, S.B.C.; Baibich, I.M. NO decomposition on mordenite-supported pd and cu catalysts. Catal. Commun. 2007, 8, 1293–1297. [Google Scholar] [CrossRef]
- Liu, L.; Shen, B.; Si, M.; Yuan, P.; Lu, F.; Gao, H.; Yao, Y.; Liang, C.; Xu, H. Mn-based catalysts supported on γ-Al2O3, TiO2 and MCM-41: A comparison for low-temperature no oxidation with low ratio of O3/NO. RSC Adv. 2021, 11, 18945–18959. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhu, X.; Cai, Y.; Tu, X.; Gao, X. Coupling nonthermal plasma with V2O5/TiO2 nanofiber catalysts for enhanced oxidation of ethyl acetate. Ind. Eng. Chem. Res. 2019, 58, 2–10. [Google Scholar] [CrossRef]
- He, F.; Luo, J.; Liu, S. Novel metal loaded KIT-6 catalysts and their applications in the catalytic combustion of chlorobenzene. Chem. Eng. J. 2016, 294, 362–370. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, J.; Liang, X.; Long, C. Plasma-catalytic removal of toluene over the supported manganese oxides in DBD reactor: Effect of the structure of zeolites support. Chemosphere 2018, 208, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhao, Y.; Qiu, C.; Shang, K.; Lu, N.; Li, J.; Wu, Y.; Zhang, Y. Enhanced catalytic performance of CoOx-CeO2 for synergetic degradation of toluene in multistage sliding plasma system through response surface methodology (RSM). Appl. Catal. B Environ. 2019, 259, 118061. [Google Scholar] [CrossRef]
- Nagle, J. Oxidation of carbon between 1000–2000 °C. In Proceedings of the Fifth Conference on Carbon, London, UK; 1962. [Google Scholar]
- Turns, S.R. An Introduction to Combustion: Concepts and Applications, 2nd ed.; McGraw-Hill Education: New York, NY, USA, 1985. [Google Scholar]
- Li, Z.; Zhang, W.; Chen, Z.; Jiang, Q. Mechanism of accelerating soot oxidation by NO2 from diesel engine exhaust. Environ. Pollut. 2020, 264, 114708. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, X.; Hu, X.; Zhang, F.; Tu, X. Plasma-assisted ammonia synthesis in a packed-bed dielectric barrier discharge reactor: Effect of argon addition. Vacuum 2022, 197, 110786. [Google Scholar] [CrossRef]
- Roland, U.; Holzer, F.; Poppl, A.; Kopinke, F.D. Combination of non-thermal plasma and heterogeneous catalysis for oxidation of volatile organic compounds part 3. Electron paramagnetic resonance (EPR) studies of plasma-treated porous alumina. Appl. Catal. B: Environ. 2005, 58, 227–234. [Google Scholar] [CrossRef]
- Zheng, L.; Zimina, A.; Casapu, M.; Grunwaldt, J.D. Hydrocarbon and soot oxidation over cerium and iron doped vanadium SCR catalysts. ChemCatChem 2020, 12, 6272–6284. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, S.; Yang, Y.; Zheng, C.; Zhou, J.; Gao, X.; Tu, X. Enhanced performance for plasma-catalytic oxidation of ethyl acetate over La1−xCexCoO3+δ catalysts. Appl. Catal. B-Environ. 2017, 213, 97–105. [Google Scholar] [CrossRef]
- Ranji-Burachaloo, H.; Masoomi-Godarzi, S.; Khodadadi, A.A.; Mortazavi, Y. Synergetic effects of plasma and metal oxide catalysts on diesel soot oxidation. Appl. Catal. B: Environ. 2016, 182, 74–84. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, X.; Wu, X.; Tu, X.; Chen, G.; Yang, G. Plasma-catalytic reactions for soot oxidation on VOx/M (M = KIT-6, SBA-15 and SiO2) catalysts: Influence of pore structure. ChemistrySelect 2022, 7, e202103545. [Google Scholar] [CrossRef]
- Chang, T.; Ma, C.; Shen, Z.; Veerapandian, S.K.P.; Huang, Y.; De Geyter, N.; Morent, R. Mn-based catalysts for post non-thermal plasma catalytic abatement of vocs: A review on experiments, simulations and modeling. Plasma Chem. Plasma Process. 2021, 41, 1239–1278. [Google Scholar] [CrossRef]
- Országh, J.; Skalný, J.D.; Mason, N.J. Ozone formation in a coaxial DC corona discharge under carbon dioxide gas flow. Plasma Process. Polym. 2007, 4, 694–700. [Google Scholar] [CrossRef]
- Zhu, X.; Gao, X.; Zheng, C.; Wang, Z.; Ni, M.; Tu, X. Plasma-catalytic removal of a low concentration of acetone in humid conditions. RSC Adv. 2014, 4, 37796–37805. [Google Scholar] [CrossRef]
- Yao, S.L.; Shen, X.; Zhang, X.M.; Han, J.Y.; Wu, Z.L.; Tang, X.J.; Lu, H.; Jiang, B.Q. Sustainable removal of particulate matter from diesel engine exhaust at low temperature using a plasma-catalytic method. Chem. Eng. J. 2017, 327, 343–350. [Google Scholar] [CrossRef]






| Catalyst | Specific Surface Area (m2∙g−1) | Pore Volume (cm3∙g−1) | Pore Diameter (nm) | V4+/(V4+ + V5+) (%) | Oads/(Oads + Olatt) (%) | H2 Consumption (mmol∙g−1) | O2 Uptake (μmol∙g−1) |
|---|---|---|---|---|---|---|---|
| V/MCM-41 | 817 | 0.74 | 3.6 | 47.4 | 59.8 | 0.112 | 0.332 |
| V/mordenite | 383 | 0.24 | 2.5 | 45.8 | 57.2 | 0.109 | 0.327 |
| V/USY | 670 | 0.43 | 2.6 | 37.2 | 56.5 | 0.122 | 0.239 |
| V/5A | 354 | 0.33 | 3.7 | 33.7 | 54.6 | 0.118 | 0.169 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Wu, H.; Luo, J.; Liu, J.; Yan, J.; Zhou, Z.; Yang, Z.; Jiang, Y.; Chen, G.; Yang, G. Soot Oxidation in a Plasma-Catalytic Reactor: A Case Study of Zeolite-Supported Vanadium Catalysts. Catalysts 2022, 12, 677. https://doi.org/10.3390/catal12070677
Zhu X, Wu H, Luo J, Liu J, Yan J, Zhou Z, Yang Z, Jiang Y, Chen G, Yang G. Soot Oxidation in a Plasma-Catalytic Reactor: A Case Study of Zeolite-Supported Vanadium Catalysts. Catalysts. 2022; 12(7):677. https://doi.org/10.3390/catal12070677
Chicago/Turabian StyleZhu, Xinbo, Hanpeng Wu, Jianbin Luo, Jin Liu, Jiahao Yan, Zijian Zhou, Zhengda Yang, Ye Jiang, Geng Chen, and Guohua Yang. 2022. "Soot Oxidation in a Plasma-Catalytic Reactor: A Case Study of Zeolite-Supported Vanadium Catalysts" Catalysts 12, no. 7: 677. https://doi.org/10.3390/catal12070677
APA StyleZhu, X., Wu, H., Luo, J., Liu, J., Yan, J., Zhou, Z., Yang, Z., Jiang, Y., Chen, G., & Yang, G. (2022). Soot Oxidation in a Plasma-Catalytic Reactor: A Case Study of Zeolite-Supported Vanadium Catalysts. Catalysts, 12(7), 677. https://doi.org/10.3390/catal12070677






