Cobalt-Based Metal-Organic Framework Nanoparticles with Peroxidase-like Catalytic Activity for Sensitive Colorimetric Detection of Phosphate
Abstract
:1. Introduction
2. Results and Discussion
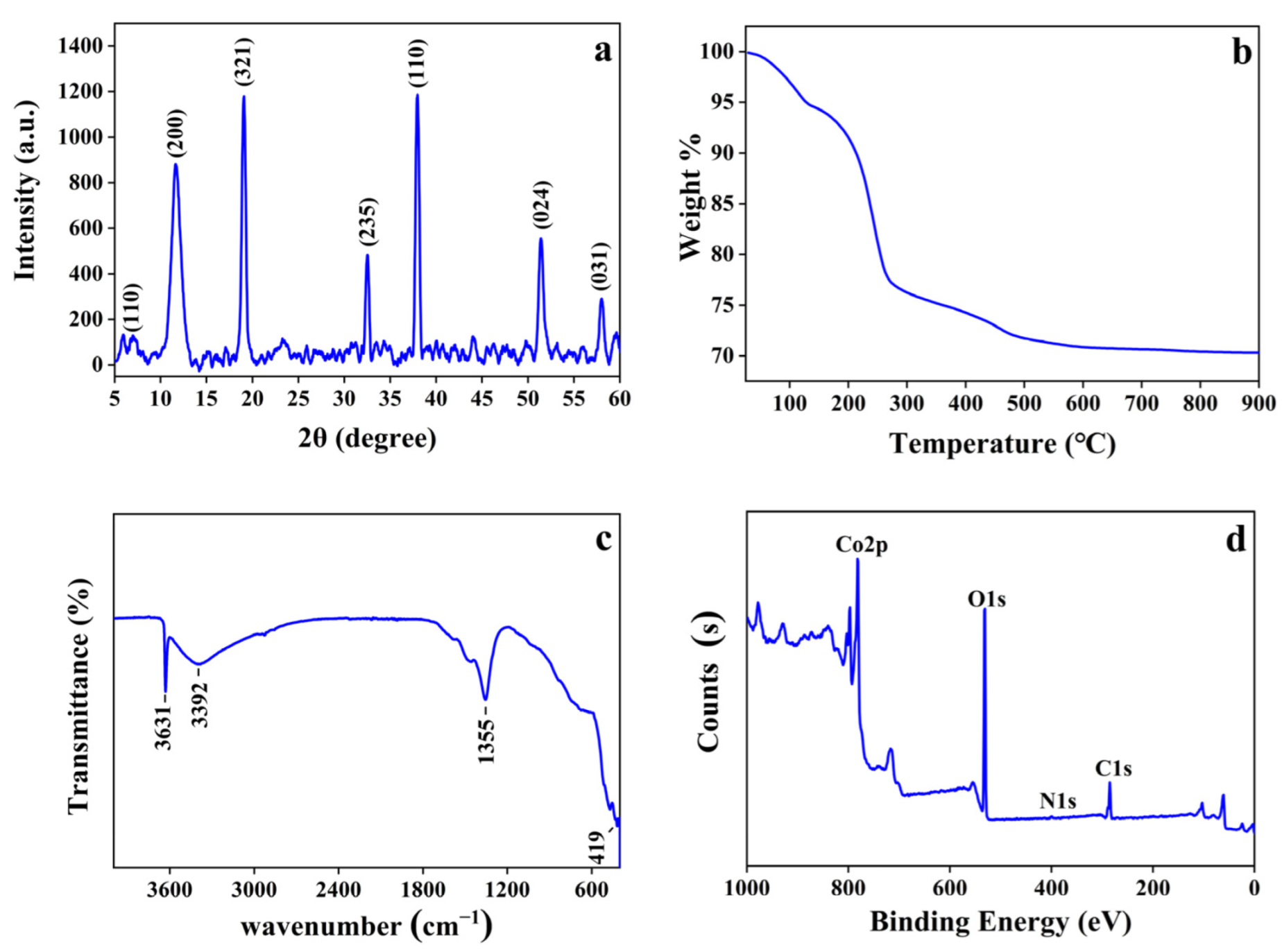
2.1. Preparation and Characterization of Co-MOF
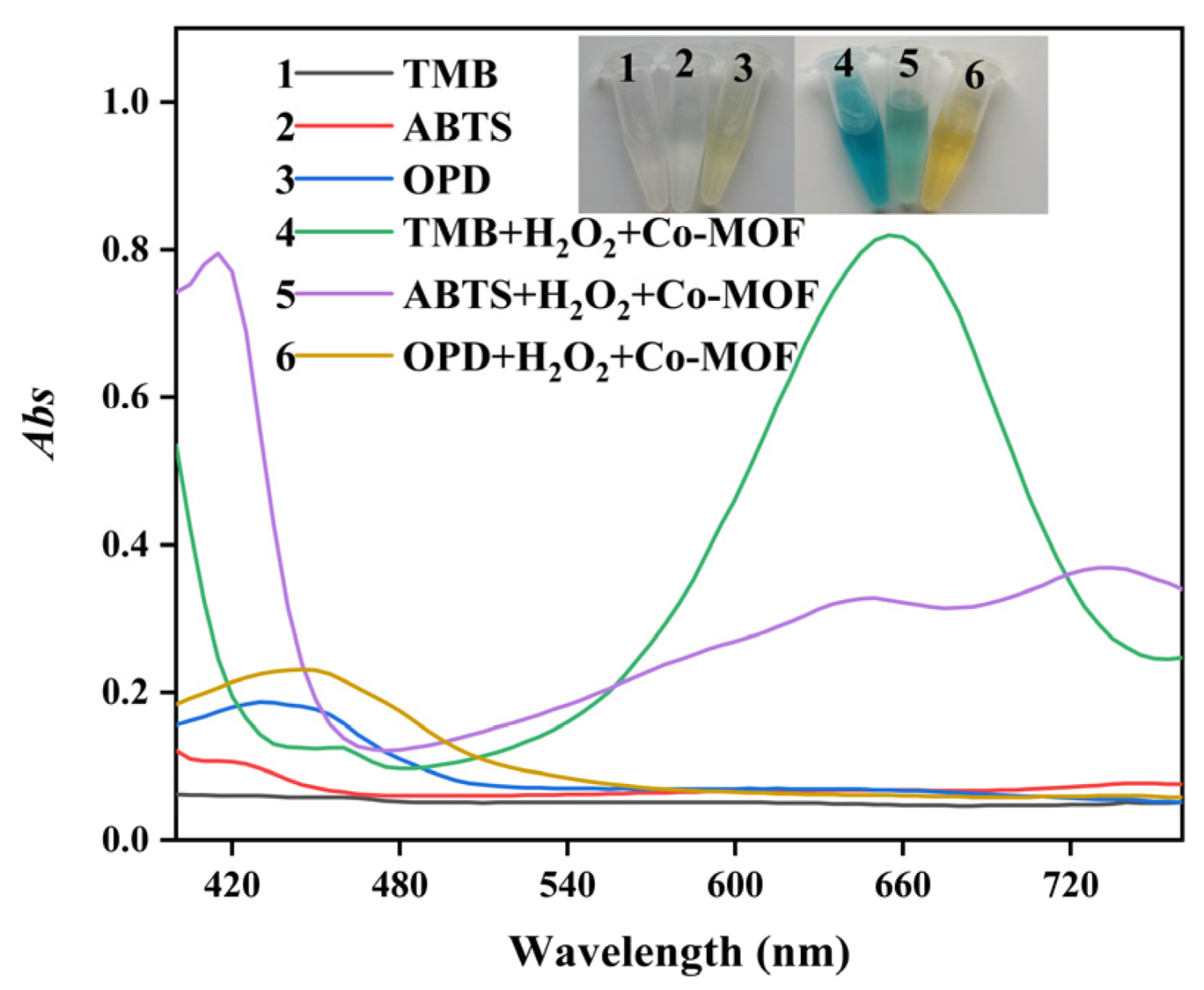
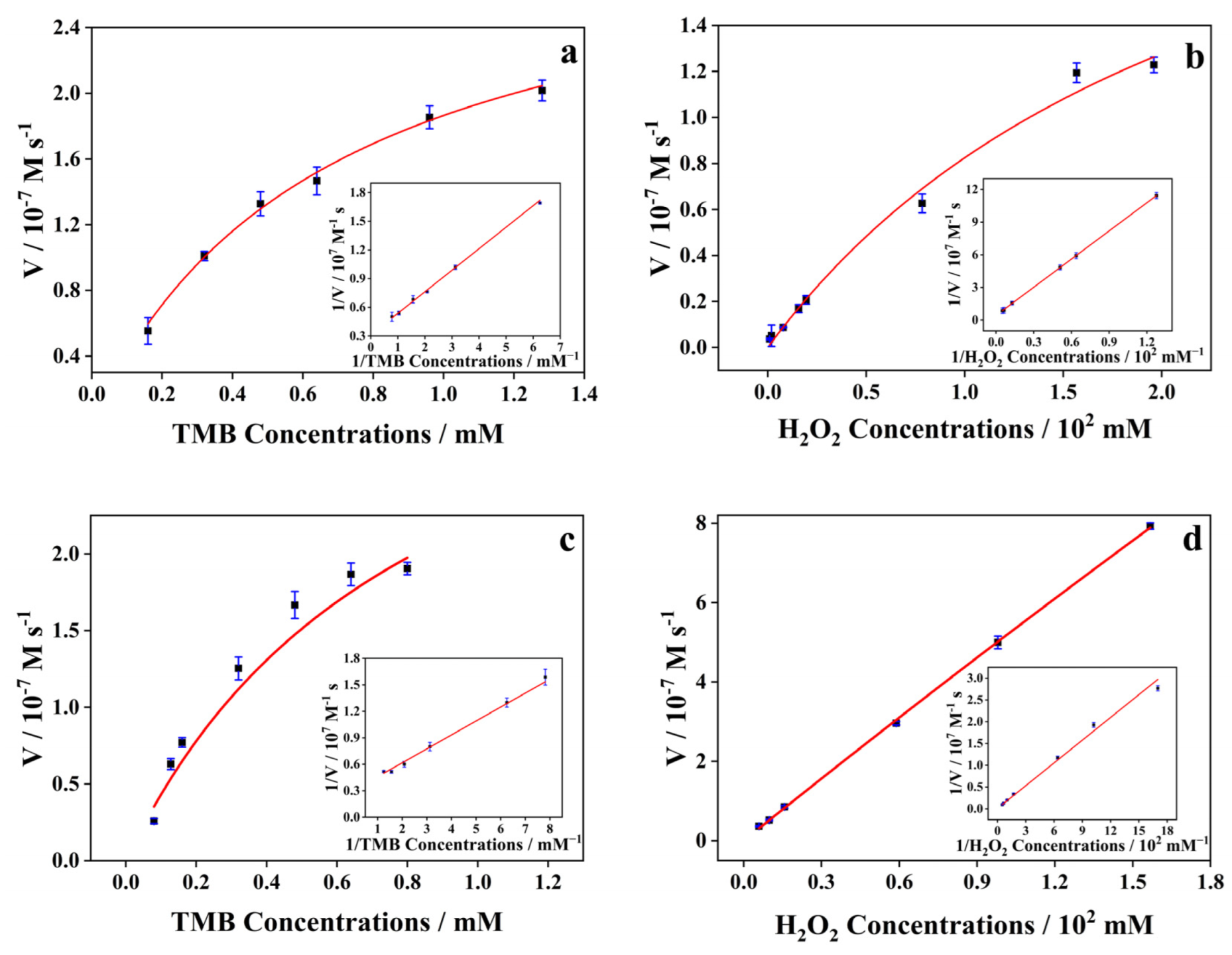
2.2. Peroxidase-like Activity of Co-MOF
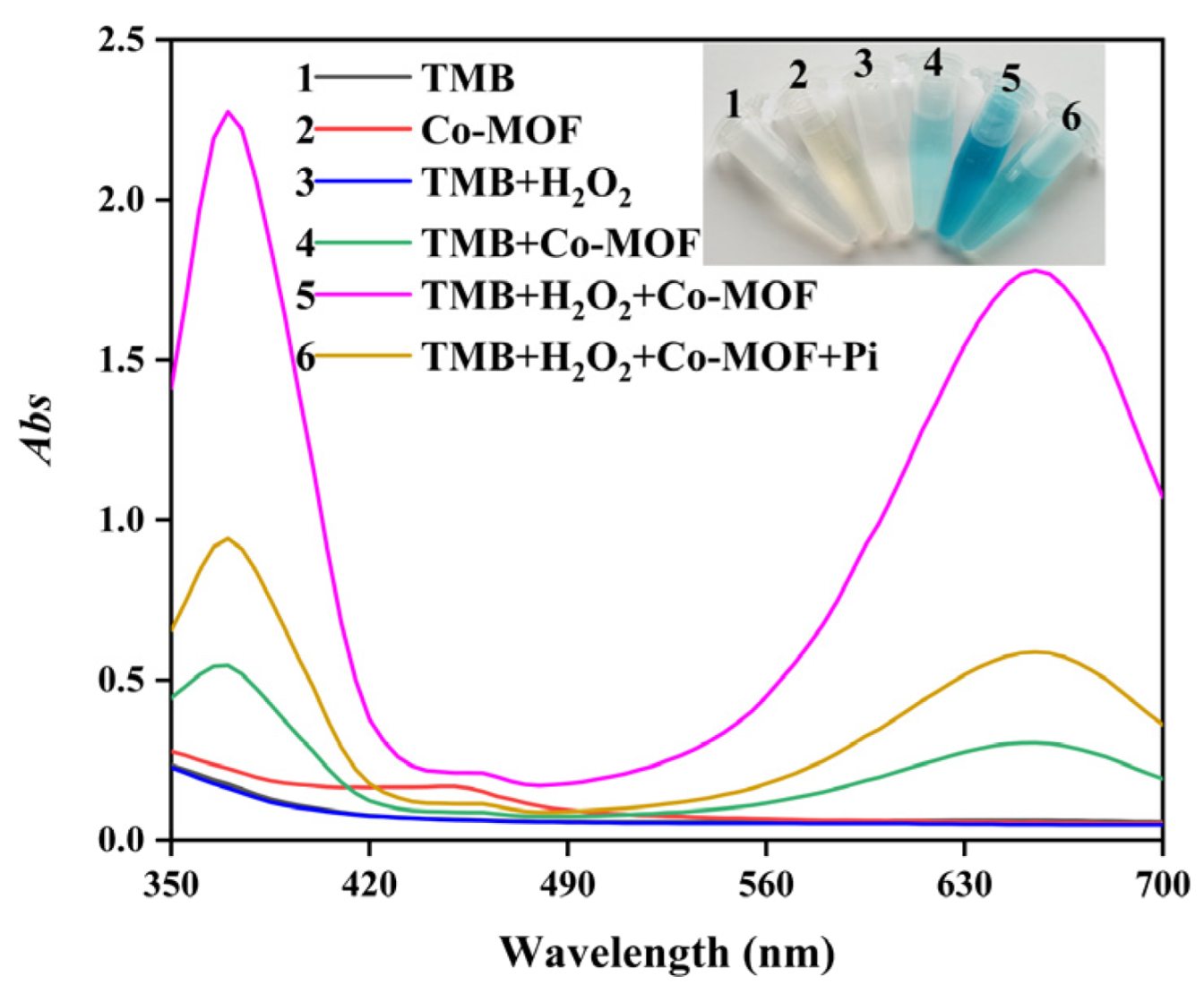
2.3. Inhibition of Pi on Co-MOF Catalyzed System
2.4. Colorimetric Assay for Detection of Pi
2.5. Selectivity
2.6. Determination of Pi in Actual Samples
3. Experimental Part
3.1. Chemical and Materials
3.2. Apparatus
3.3. Synthesis of Co-MOF
3.4. Study of Catalytic Activity
3.5. Analytical Procedures
3.6. Determination of Pi in Actual Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalantar-Zadeh, K.; Gutekunst, L.; Mehrotra, R.; Kovesdy, C.P.; Bross, R.; Shinaberger, C.S.; Noori, N.; Hirschberg, R.; Benner, D.; Nissenson, A.R.; et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin. J. Am. Soc. Nephro. 2010, 5, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, P.; Niu, X.H.; Ye, K.; Ni, L.; Du, D.; Pan, J.M.; Lin, Y.H. Tri-functional Fe-Zr bi-metal–organic frameworks enable high-performance phosphate ion ratiometric fluorescent detection. Nanoscale 2020, 12, 19383–19389. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Gupta, R.C.; Srivastava, P.; Singh, P.; Koch, B.; Maiti, B.; Misra, A. Dual fluorophore containing efficient photoinduced electron transfer based molecular probe for selective detection of Cr3+ and Pi ions through fluorescence “turn-on-off” response in partial aqueous and biological medium: Live cell imaging and logic application. Anal. Chem. 2018, 90, 10974–10981. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.P.; Mandal, S. Europium-based metal–organic framework as a dual luminescence sensor for the selective detection of the phosphate anion and Fe3+ ion in aqueous media. Inorg. Chem. 2018, 57, 11855–11858. [Google Scholar] [CrossRef]
- Asha, K.S.; Bhattacharjee, R.; Mandal, S. Complete transmetalation in a metal-organic framework by metal ion metathesis in a single crystal for selective sensing of phosphate ions in aqueous media. Angew. Chem. Int. Edit. 2016, 55, 11528–11532. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Song, P.; Liu, X.; Maruo, M.; Ban, S. Differences in dissolved phosphate in shallow-lake waters as determined by spectrophotometry and ion chromatography. Limnology 2020, 21, 329–339. [Google Scholar] [CrossRef]
- Iammarino, M.; Haouet, N.; Taranto, A.D.; Berardi, G.; Benedetti, F.; Bella, S.D.; Chiaravalle, A.E. The analytical determination of polyphosphates in food: A point-to-point comparison between direct ion chromatography and indirect photometry. Food. Chem. 2020, 325, 126937. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Pan, D.; Zhou, Z.W.; Han, H.T.; Zhu, R.L. On-site electrochemical determination of phosphate with high sensitivity and anti-interference ability in turbid coastal waters. Ecotox. Environ. Safe. 2021, 221, 112444. [Google Scholar] [CrossRef] [PubMed]
- Kalayci, S. A New phosphate selective electrode and its application in some foods. Int. J. Electrochem. Sci. 2021, 16, 210949. [Google Scholar] [CrossRef]
- Wu, T.; Xia, D.; Xu, J.; Ye, C.Z.; Zhang, D.; Deng, D.W.; Zhang, J.S. Sequential injection-square wave voltammetric sensor for phosphate detection in freshwater using silanized multi-walled carbon nanotubes and gold nanoparticles. Microchem. J. 2021, 167, 106311. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, M.; Yu, J.; Zhang, X.M.; Li, Z.M.; Li, H. Using the interfacial barrier effects of p-n junction on electrochemistry for detection of phosphate. Analyst 2020, 145, 3217–3221. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.X.; Hu, Z.; Liu, P.; Ye, K.; Pan, J.M.; Niu, X.H. Smartphone-assisted off-on photometric determination of phosphate ion based on target-promoted peroxidase-mimetic activity of porous CexZr1−xO2 (x ≥ 0.5) nanocomposites. Environ. Res. 2020, 189, 109921. [Google Scholar] [CrossRef]
- Pinyorospathum, C.; Rattanarat, P.; Chaiyo, S.; Siangproh, W.; Chailapakul, O. Colorimetric sensor for determination of phosphate ions using anti-aggregation of 2-mercaptoethanesulfonate-modified silver nanoplates and europium ions. Sens. Actuat. B Chem. 2019, 290, 226–232. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Ye, K.; Ni, L.; Xu, X.C.; Qiu, F.X.; Pan, J.M.; Niu, X.H. Highly sensitive and specific colorimetric detection of phosphate by using Zr (ΙV) to synergistically suppress the peroxidase-mimicking activity of hydrophilic Fe3O4 nanocubes. Sens. Actuat. B Chem. 2019, 297, 126822. [Google Scholar] [CrossRef]
- Nagul, E.A.; McKelvie, I.D.; Kolev, S.D. The use of on-line UV photoreduction in the flow analysis determination of dissolved reactive phosphate in natural waters. Talanta 2015, 133, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.X.; Wang, X.Y.; Wang, Q.; Lou, Z.P.; Li, S.R.; Zhu, Y.Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Shanahan, J.; Rizaldo, S.; Kissel, D.S.; Stone, K.L. Co-immobilization of an enzyme system on a metal-organic framework to produce a more effective biocatalyst. Catalysts 2020, 10, 499. [Google Scholar] [CrossRef]
- Liang, M.M.; Yan, X.Y. Nanozymes: From new concepts, mechanisms, and standards to applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef]
- Li, X.; Zhou, H.; Qi, F.; Niu, X.H.; Xu, X.C.; Qiu, F.X.; He, Y.F.; Pan, J.M.; Ni, L. Three hidden talents in one framework: A terephthalic acid-coordinated cupric metal-organic framework with cascade cysteine oxidase- and peroxidase-mimicking activities and stimulus-responsive fluorescence for cysteine sensing. J. Mater. Chem. B 2018, 6, 6207–6211. [Google Scholar] [CrossRef]
- Li, X.; Niu, X.; Liu, P.; Xu, X.C.; Du, D.; Lin, Y.H. High-performance dual-channel ratiometric colorimetric sensing of phosphate ion based on target-induced differential oxidase-like activity changes of Ce-Zr bimetal-organic frameworks. Sens. Actuat. B Chem. 2020, 321, 128546. [Google Scholar] [CrossRef]
- Qiu, P.P.; Yuan, P.; Deng, Z.C.; Su, Z.Q.; Bai, Y.; He, J.C. One-pot facile synthesis of enzyme-encapsulated Zn/Co-infinite coordination polymer nanospheres as a biocatalytic cascade platform for colorimetric monitoring of bacteria viability. Microchim. Acta 2021, 188, 322. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Li, W.Y.; Zheng, Y.Q. Colorimetric assay for sensitive detection of phosphate in water based on metal-organic framework nanospheres possessing catalytic activity. New J. Chem. 2020, 44, 19683–19689. [Google Scholar] [CrossRef]
- Ai, L.H.; Li, L.L.; Zhang, C.H.; Fu, J.; Jiang, J. MIL-53(Fe): A metal-organic framework with intrinsic peroxidase-like catalytic activity for colorimetric biosensing. Chem.—Eur. J. 2013, 19, 15105–15108. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Cao, H.Y.; Shi, W.B.; Liu, H.; Huang, Y.M. Fe-Co bimetallic alloy nanoparticles as a highly active peroxidase mimetic and its application in biosensing. Chem. Commun. 2013, 49, 5013. [Google Scholar] [CrossRef]
- Tang, X.Q.; Zhang, Y.D.; Jiang, Z.W.; Wang, D.M.; Huang, C.Z. Fe3O4 and metal–organic framework MIL-101(Fe) composites catalyze luminol chemiluminescence for sensitively sensing hydrogen peroxide and glucose. Talanta 2018, 179, 43–50. [Google Scholar] [CrossRef]
- Yang, J.; Dai, Y.; Zhu, X.Y.; Wang, Z.; Li, Y.S.; Zhuang, Q.X.; Shi, J.L.; Gu, J.L. Metal-organic frameworks with inherent recognition sites for selective phosphate sensing through their coordination-induced fluorescence enhancement effect. J. Mater. Chem. A 2015, 3, 7445–7452. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Li, B.; Yang, J.; Li, Y.S.; Zhao, W.R.; Shi, J.L.; Gu, J.L. Effective adsorption and enhanced removal of organophosphorus pesticides from aqueous solution by Zr-based MOFs of UiO-67. ACS Appl. Mater. Inter. 2015, 7, 223–231. [Google Scholar] [CrossRef]
- Yang, H.G.; Yang, R.T.; Zhang, P.; Qin, Y.M.; Chen, T.; Ye, F.G. A bimetallic (Co/2Fe) metal-organic framework with oxidase and peroxidase mimicking activity for colorimetric detection of hydrogen peroxide. Microchim. Acta 2017, 184, 4629–4635. [Google Scholar] [CrossRef]
- Johir, M.A.H.; Pradhan, M.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Phosphate adsorption from wastewater using zirconium (IV) hydroxide: Kinetics, thermodynamics and membrane filtration adsorption hybrid system studies. J. Environ. Manag. 2016, 167, 167–174. [Google Scholar] [CrossRef]
- Fadhel, A.A.; Johnson, M.; Trieu, K.; Koculi, E.; Campiglia, A.D. Selective nano-sensing approach for the determination of inorganic phosphate in human urine samples. Talanta 2017, 164, 209–215. [Google Scholar] [CrossRef]
- Song, X.Y.; Ma, Y.; Ge, X.; Zhou, H.J.; Wang, G.Z.; Zhang, H.M.; Tang, X.X.; Zhang, Y.X. Europium-based infinite coordination polymer nanospheres as an effective fluorescence probe for phosphate sensing. RSC Adv. 2017, 7, 8661–8669. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Zhang, Y.Q.; Li, X.Y.; Yang, P.; Yue, J.Y.; Jiang, Y.; Tang, B. Linker-eliminated nano metal–organic framework fluorescent probe for highly selective and sensitive phosphate ratiometric detection in water and body fluids. Anal. Chem. 2020, 92, 3722–3727. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.K.; Draz, M.A. Selective colorimetric nano-sensing solution for the determination of phosphate ion in drinking water samples. Int. J. Environ. Anal. Chem. 2020, 101, 2329–2338. [Google Scholar] [CrossRef]
- Liu, W.Q.; Du, Z.F.; Qian, Y.; Li, F. A specific colorimetric probe for phosphate detection based on anti-aggregation of gold nanoparticles. Sens. Actuat. B Chem. 2013, 176, 927–931. [Google Scholar] [CrossRef]
- Pourreza, N.; Sharifi, H.; Golmohammadi, H. A green chemosensor for colorimetric determination of phosphate ion in soil, bone and water samples using curcumin nanoparticles. Anal. Sci. 2020, 36, 1297–1302. [Google Scholar] [CrossRef]
- GB5009.87-2016; National Food Safety Standard Determination of Food Phosphate. China Food and Drug Administration: Beijing, China, 2008.



| Coexistent Ions | Tolerance Ratio | Relative Error (%) | Coexistent Ions | Tolerance Ratio | Relative Error (%) |
|---|---|---|---|---|---|
| Na+ | 400 | −5.8 | Al3+ | 50 | −6.9 |
| Mg2+ | 50 | −6.7 | CH3COO− | 700 | −5.4 |
| Cu2+ | 50 | 3.8 | F− | 250 | −4.1 |
| Zn2+ | 100 | −7.4 | arginine | 80 | 7.7 |
| Fe3+ | 100 | 7.6 | glutamate | 60 | 0.5 |
| SO42− | 100 | −5.4 | aspartate | 20 | 4.1 |
| CO32− | 200 | −3.2 | cysteine | 50 | −4.3 |
| HCO3− | 200 | −4.0 | glycine | 40 | −6.6 |
| K+ | 200 | 4.7 | glucose | 50 | −5.9 |
| Sample | Added (g/kg)/(g/L) | Detcted (g/kg)/(g/L) | Recovery (%) | RSD (n = 3, %) | Standard Method [36] (g/kg)/(g/L) | t-Test |
|---|---|---|---|---|---|---|
| Biscuits | 0 | 3.83 ± 0.14 | - | - | 3.38 ± 0.17 | p = 0.083 > 0.05 |
| 0.5 | 4.35 ± 0.038 | 103.8 | 7.3 | - | - | |
| 2 | 5.97 ± 0.20 | 107.4 | 6.8 | - | - | |
| Milk | 0 | 0.73 ± 0.025 | - | - | 0.69 ± 0.02 | p = 0.097 > 0.05 |
| 0.025 | 0.96 ± 0.019 | 92.2 | 5.4 | - | - | |
| 1 | 1.71 ± 0.026 | 98.0 | 2.7 | - | - | |
| Drinking water | 0 | ND | - | - | ND | - |
| 0.009 | 0.0097 ± 0.00068 | 107.8 | 7.1 | - | - | |
| 0.036 | 0.033 ± 0.00094 | 92.6 | 2.8 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Z.; Zhang, H.; Yuan, P.; Su, Z.; Bai, Y.; Yin, Z.; He, J. Cobalt-Based Metal-Organic Framework Nanoparticles with Peroxidase-like Catalytic Activity for Sensitive Colorimetric Detection of Phosphate. Catalysts 2022, 12, 679. https://doi.org/10.3390/catal12070679
Deng Z, Zhang H, Yuan P, Su Z, Bai Y, Yin Z, He J. Cobalt-Based Metal-Organic Framework Nanoparticles with Peroxidase-like Catalytic Activity for Sensitive Colorimetric Detection of Phosphate. Catalysts. 2022; 12(7):679. https://doi.org/10.3390/catal12070679
Chicago/Turabian StyleDeng, Zhichen, Huifeng Zhang, Ping Yuan, Zhengquan Su, Yan Bai, Zhina Yin, and Jincan He. 2022. "Cobalt-Based Metal-Organic Framework Nanoparticles with Peroxidase-like Catalytic Activity for Sensitive Colorimetric Detection of Phosphate" Catalysts 12, no. 7: 679. https://doi.org/10.3390/catal12070679
APA StyleDeng, Z., Zhang, H., Yuan, P., Su, Z., Bai, Y., Yin, Z., & He, J. (2022). Cobalt-Based Metal-Organic Framework Nanoparticles with Peroxidase-like Catalytic Activity for Sensitive Colorimetric Detection of Phosphate. Catalysts, 12(7), 679. https://doi.org/10.3390/catal12070679






