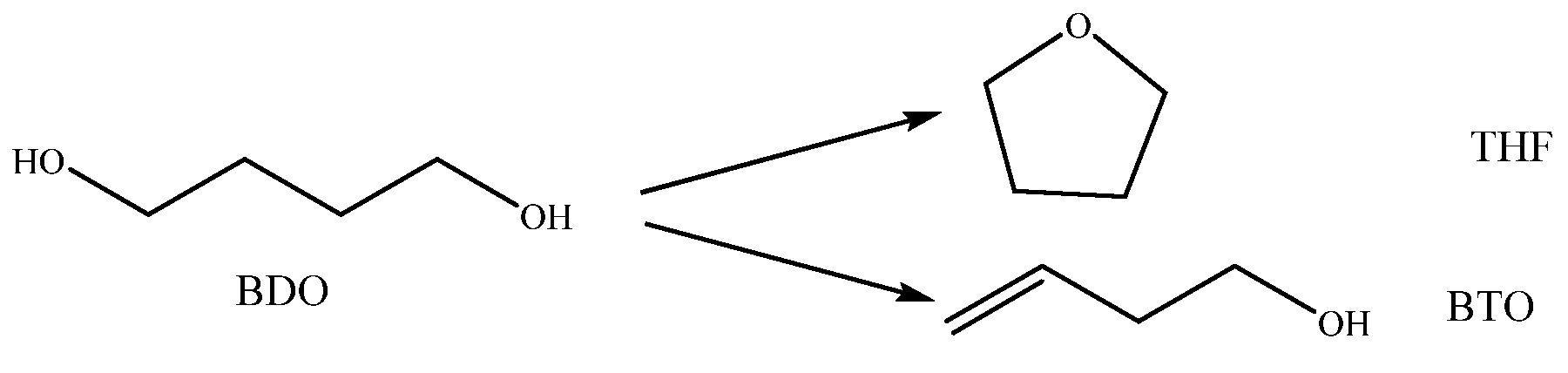
1,4-Butanediol Selective Dehydration to 3-Butene-1-ol over Ca–Zr–Sn Composite Oxide Catalysts
Abstract
:1. Introduction
2. Results and Discussion
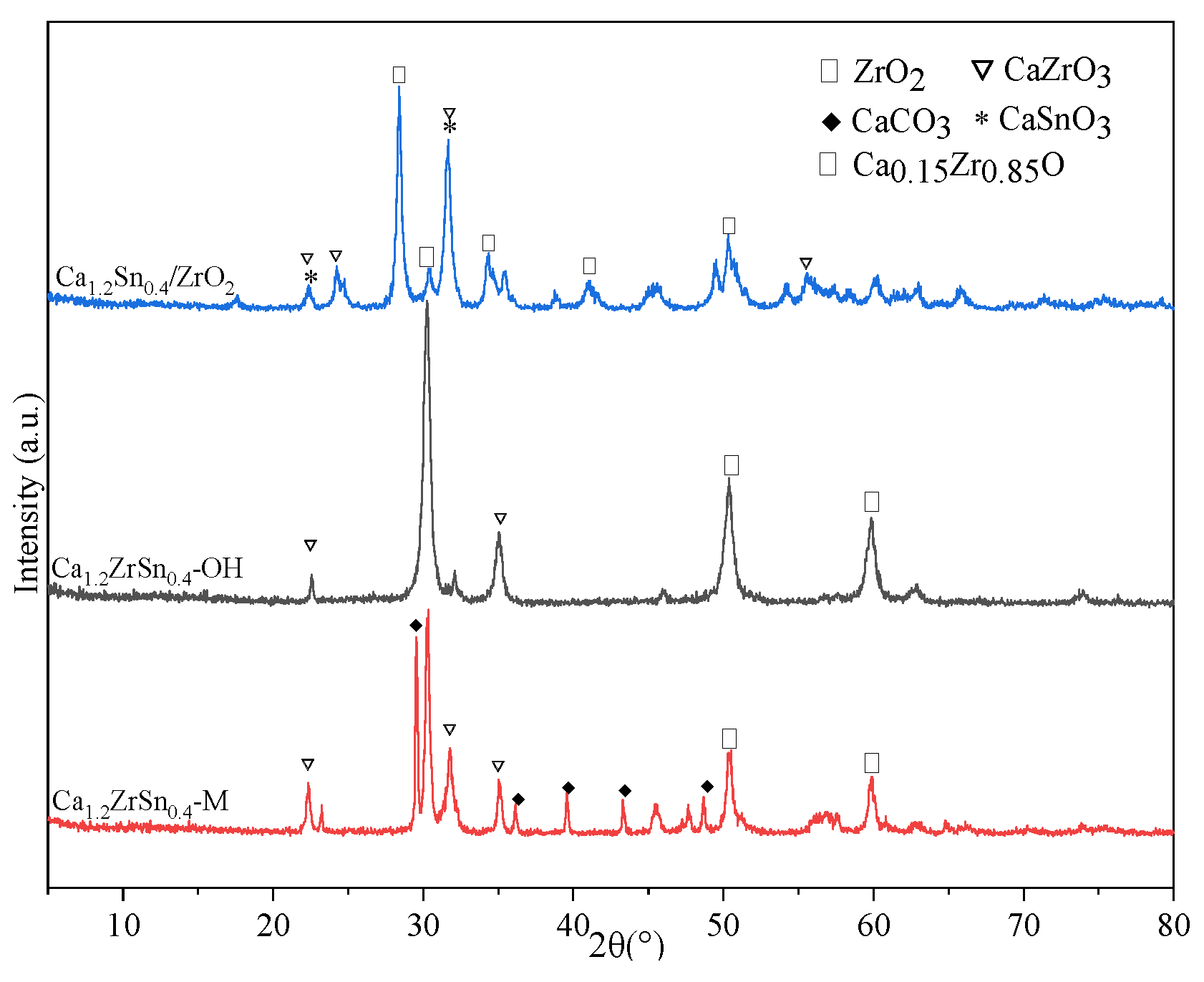
2.1. Selection of Preparation Method
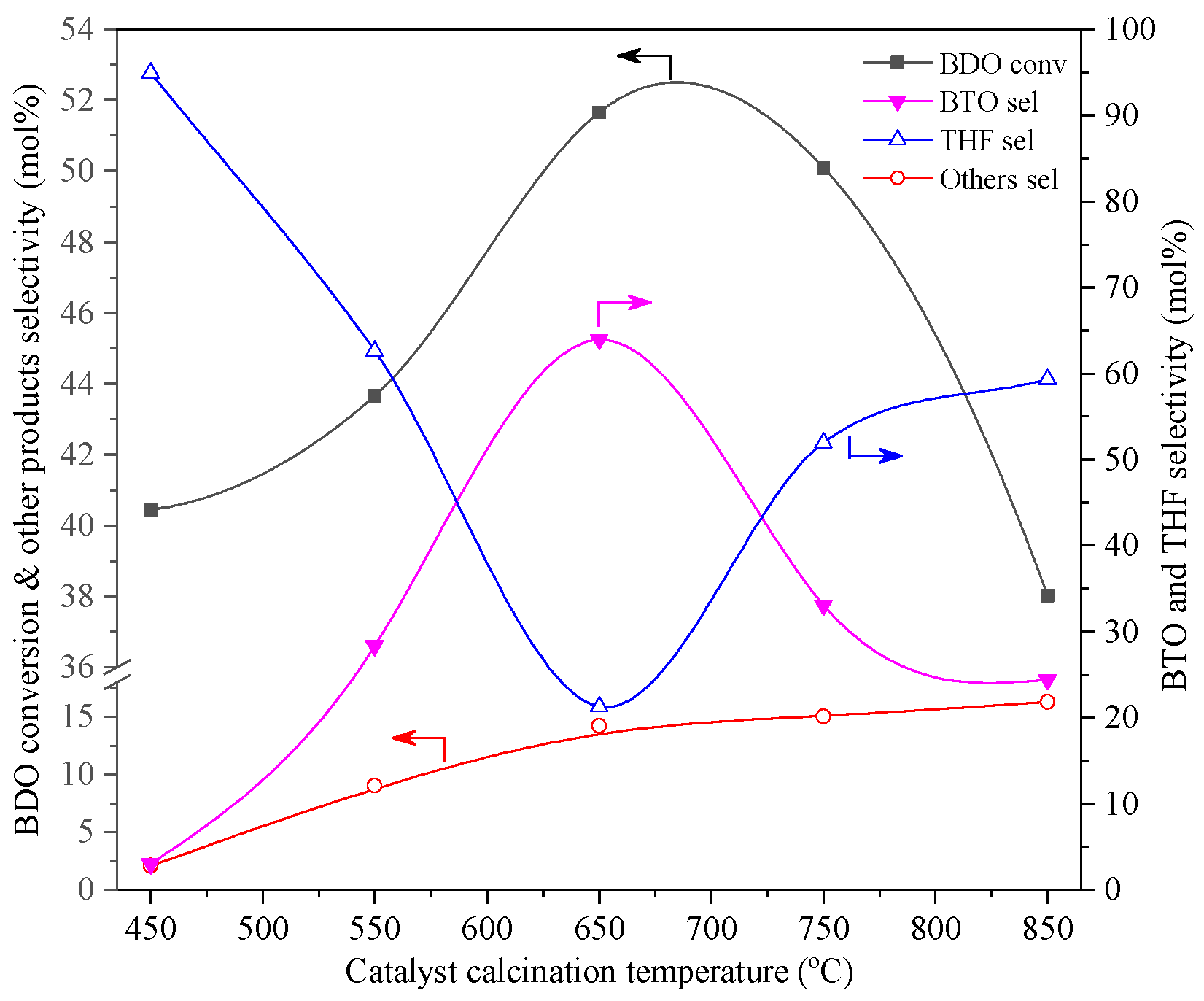
2.2. Calcination Temperature
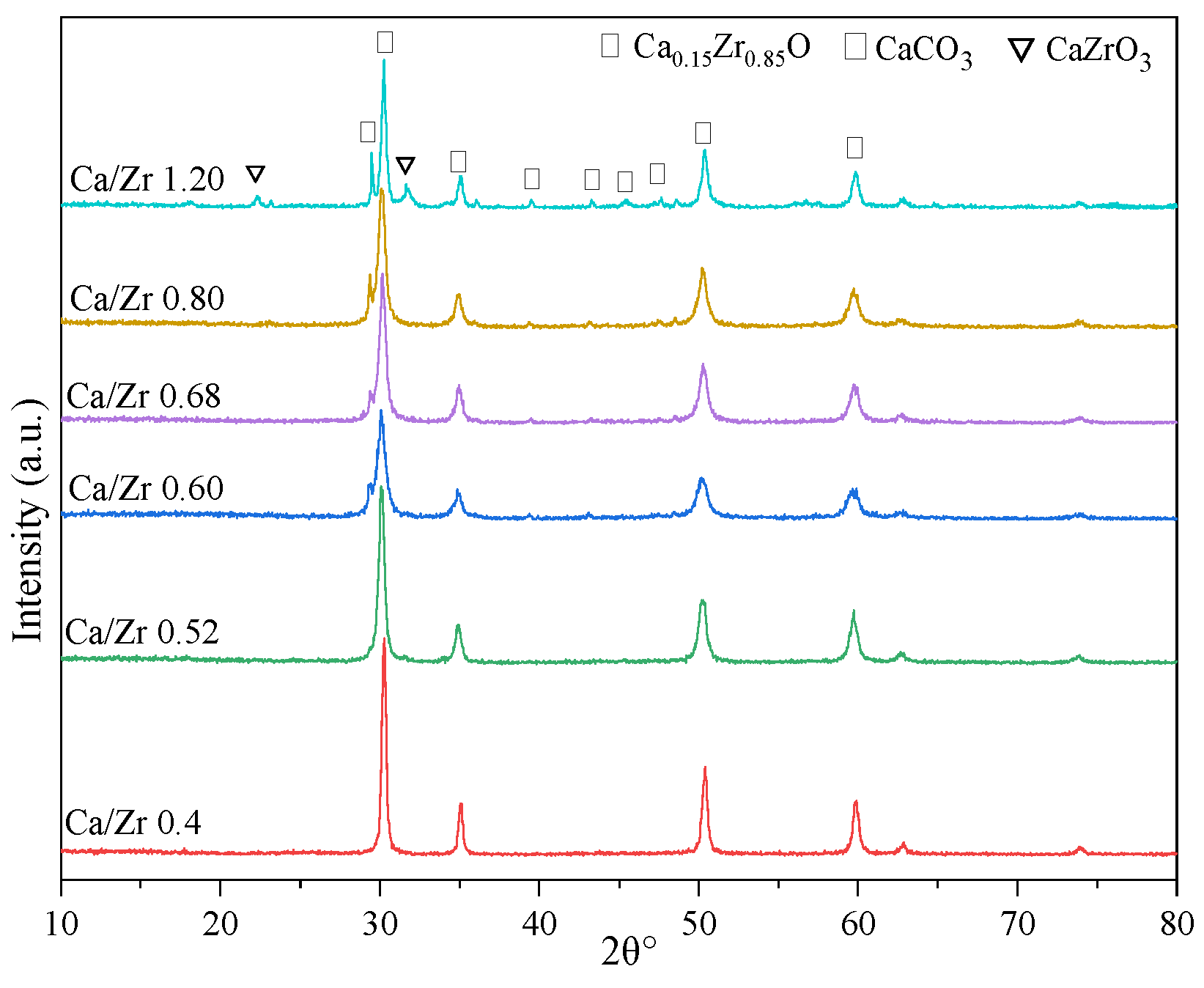
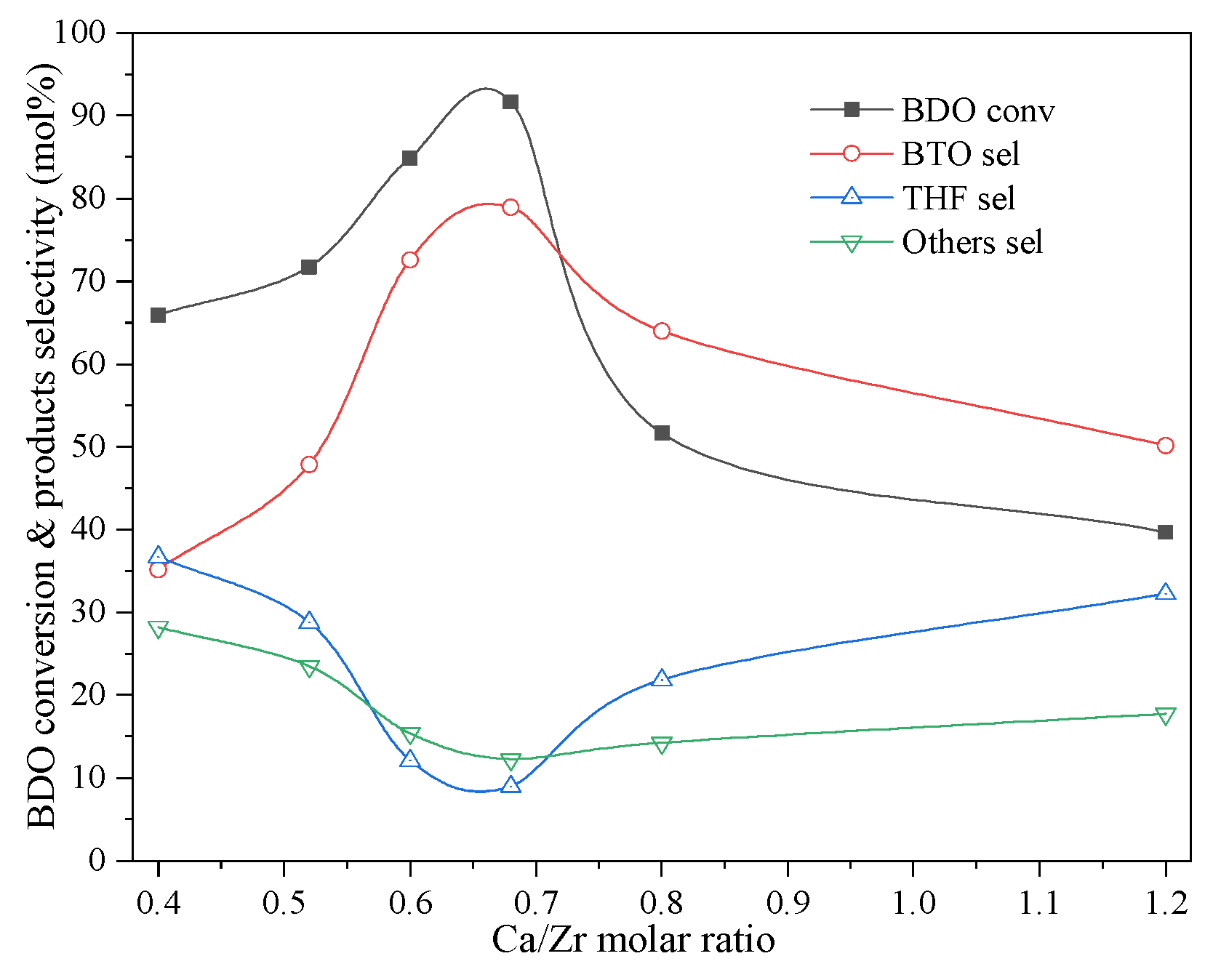
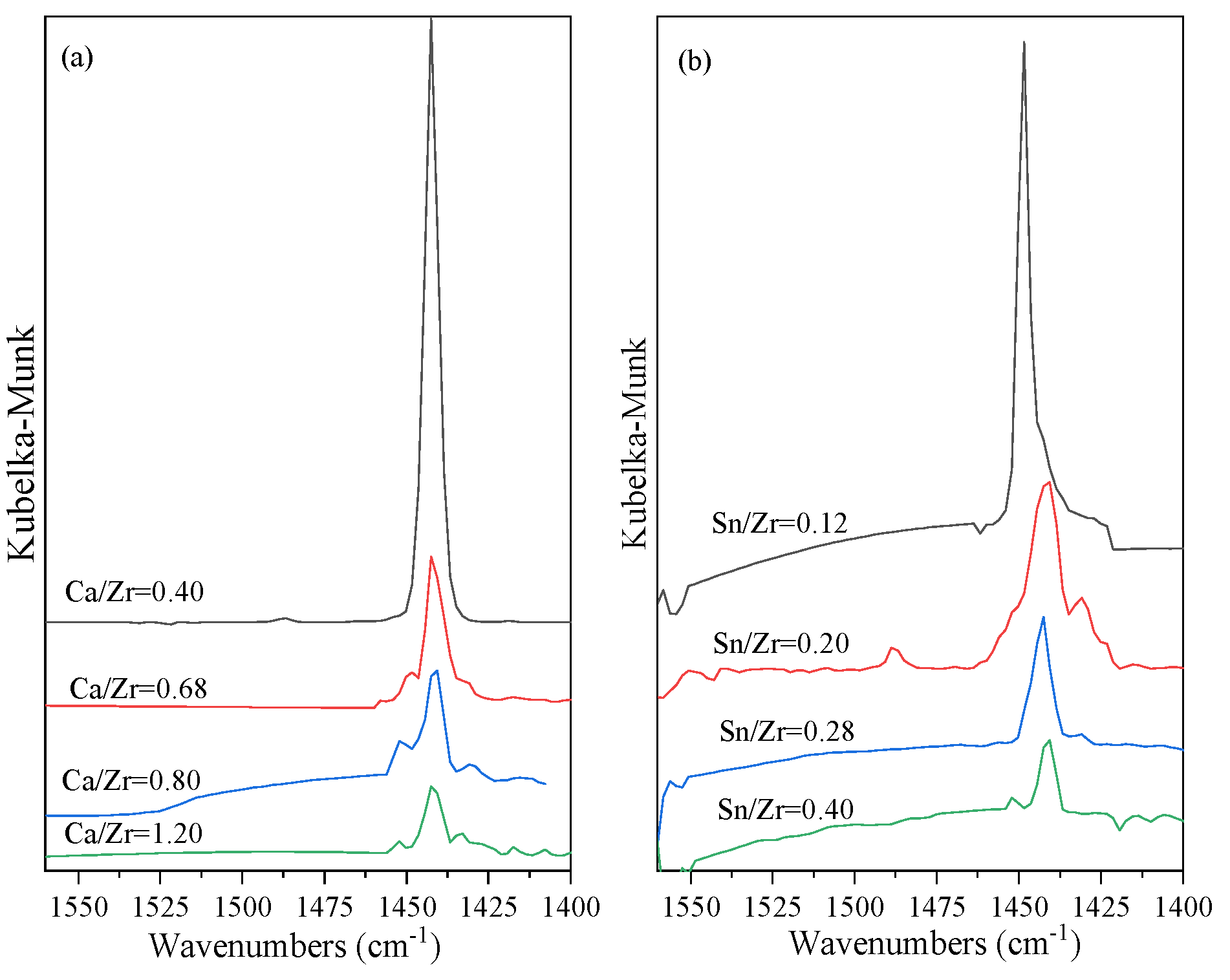
2.3. Ca Function
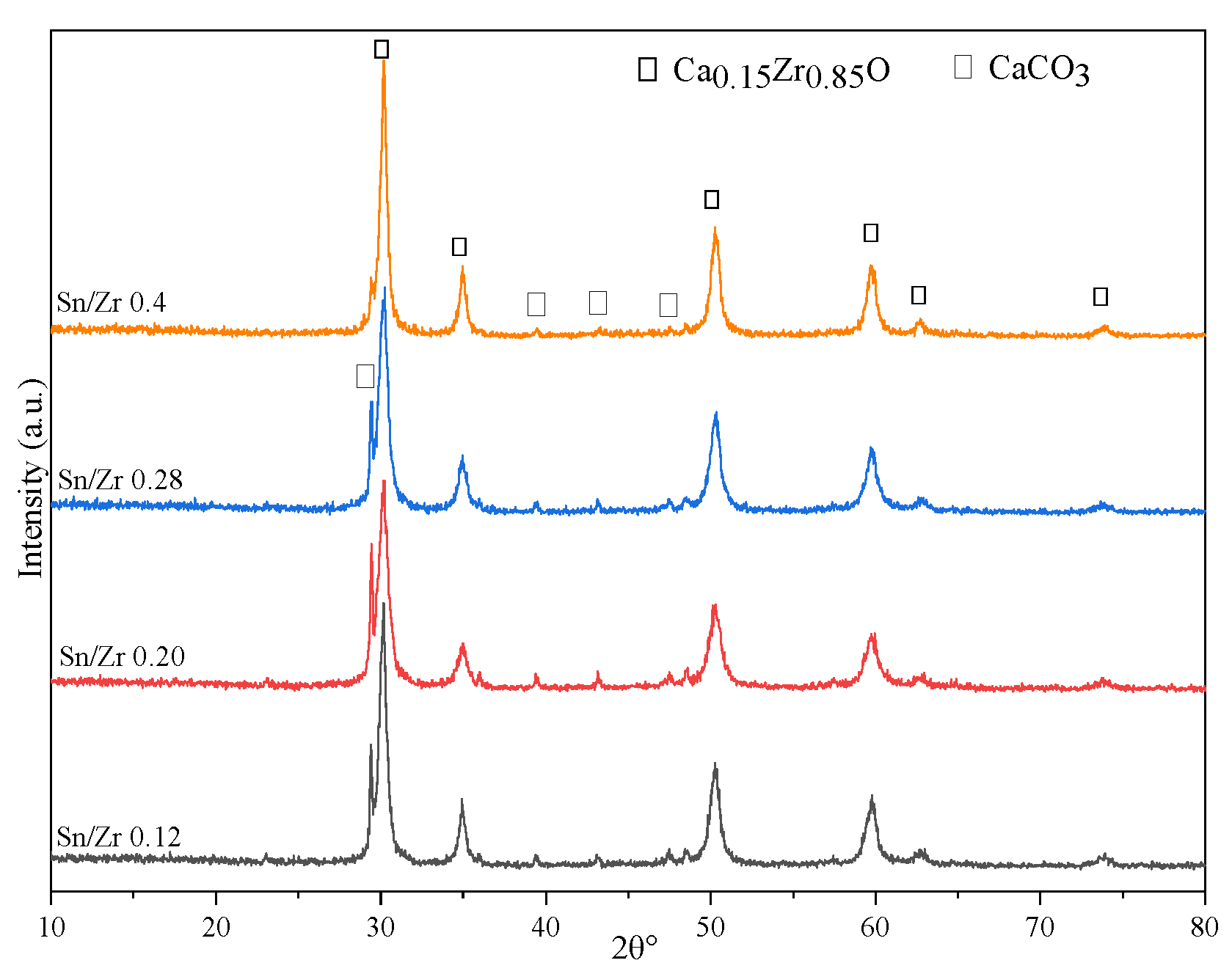
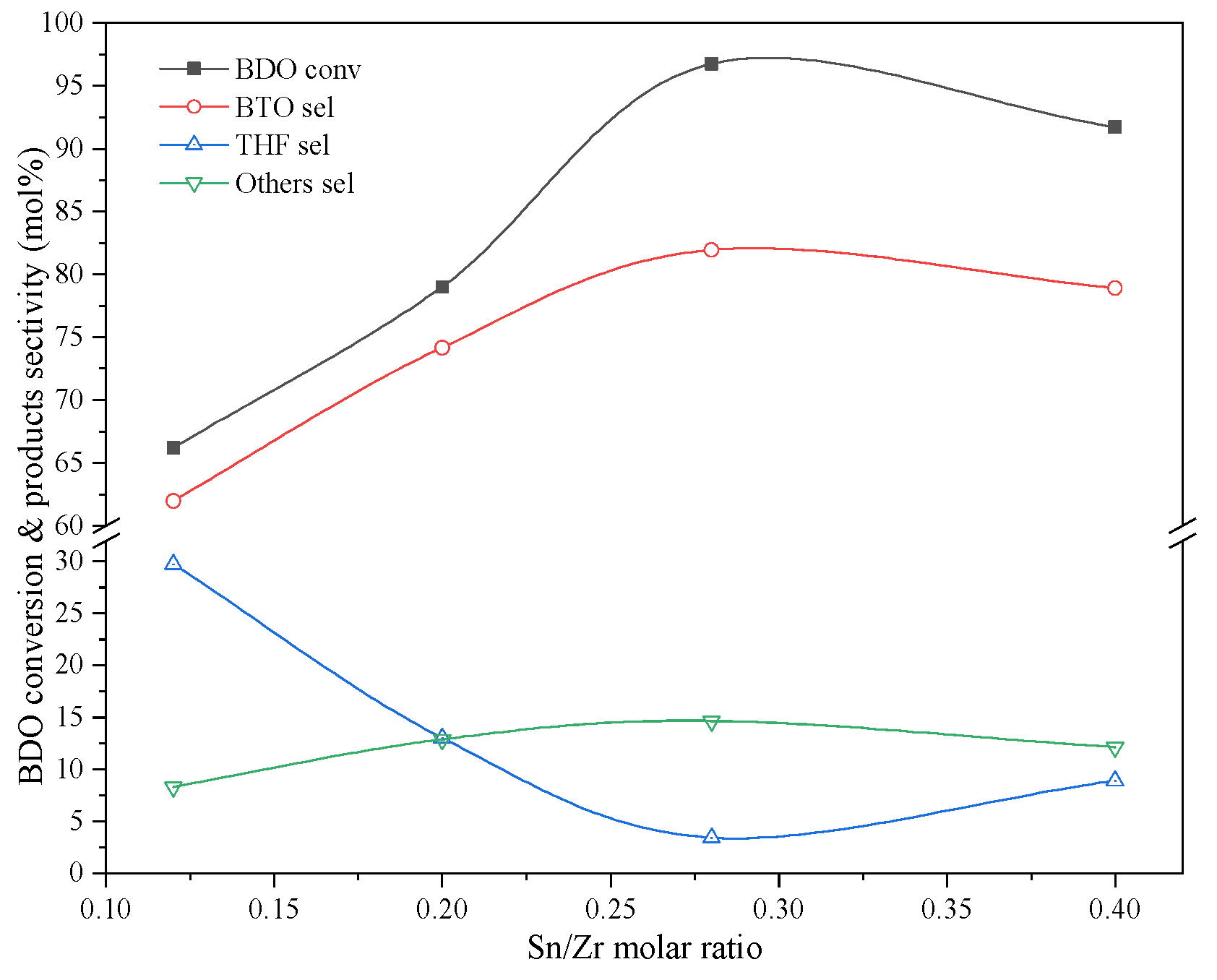
2.4. Sn Function
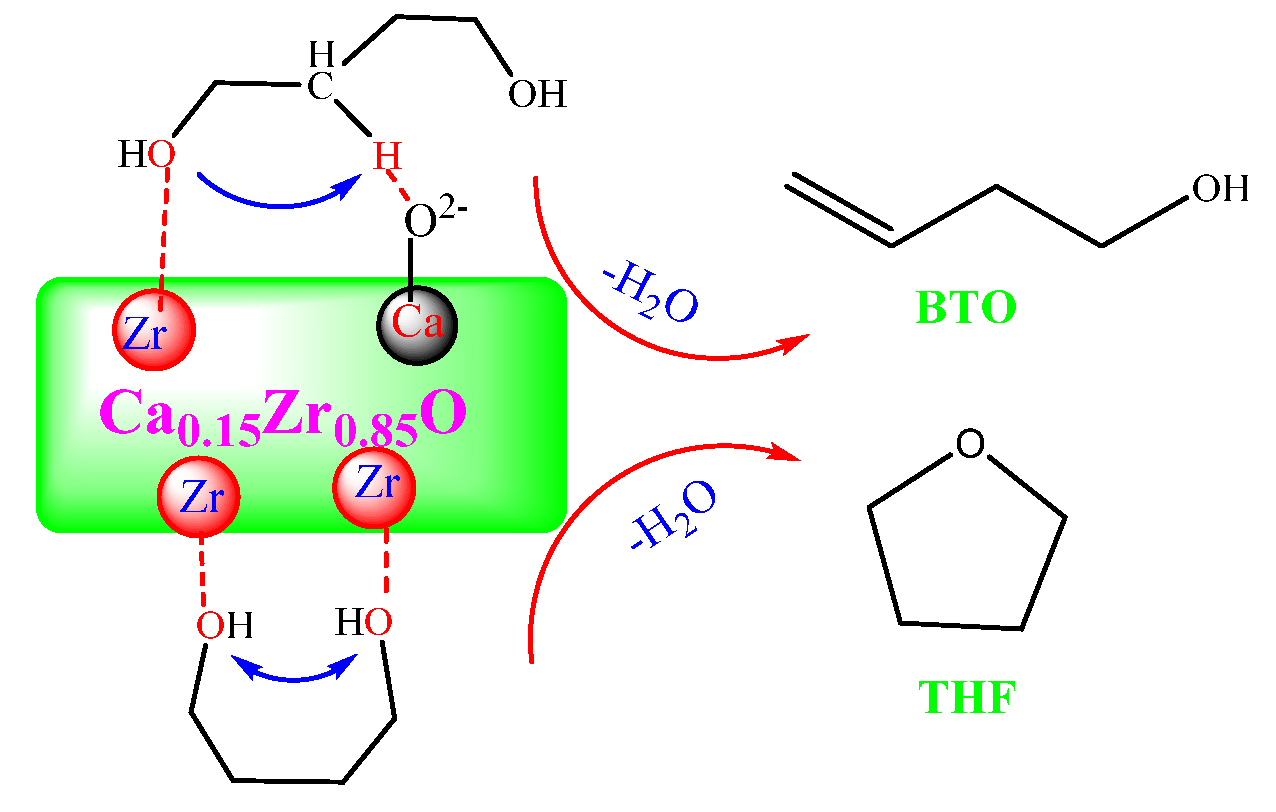
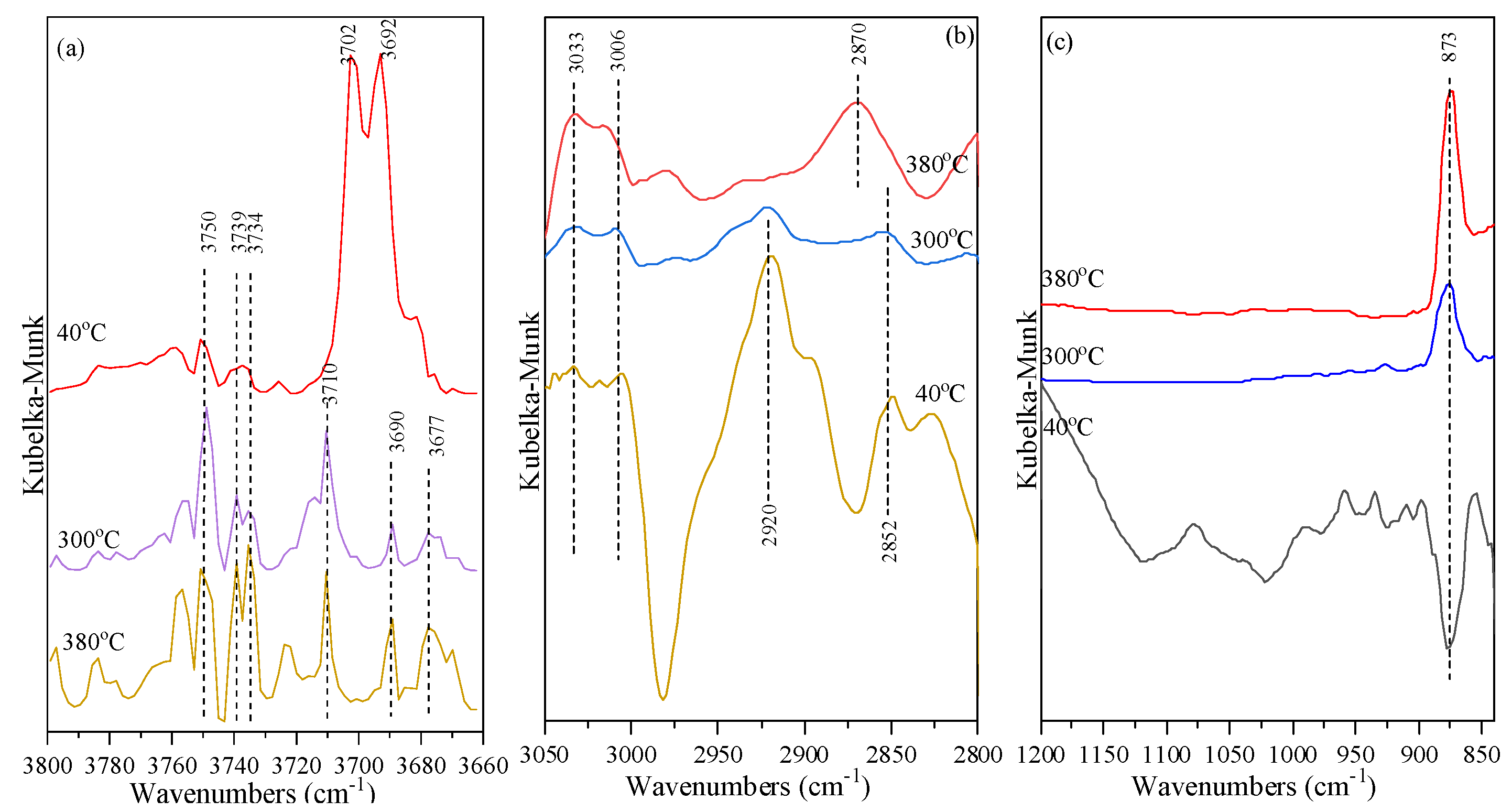
2.5. Mechanism of BDO Dehydration to BTO
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalysts Characterization
3.3. Catalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takagaki, A. Kinetic analysis of aqueous-phase cyclodehydration of 1,4-butanediol and erythritol over a layered niobium molybdate solid acid. Catal. Sci. Technol. 2016, 6, 791–799. [Google Scholar] [CrossRef]
- Bhanushali, J.T.; Prasad, D.; Patil, K.N.; Reddy, K.S.; Rao, K.S.R.; Jadhav, A.H.; Nagaraja, B.M. Simultaneous dehydrogenation of 1,4-butanediol to γ-butyrolactone and hydrogenation of benzaldehyde to benzyl alcohol mediated over competent CeO2-Al2O3 supported Cu as catalyst. Int. J. Hydrogen Energy 2020, 45, 12874–12888. [Google Scholar] [CrossRef]
- Duan, H.L.; Hirota, T.; Ohtsuka, S.; Yamada, Y.; Sato, S. Vapor-phase catalytic dehydration of 1,4-butanediol to 3-buten-1-ol over modified ZrO2 catalysts. Appl. Catal. A 2017, 535, 9–16. [Google Scholar] [CrossRef]
- Bhanushalia, J.T.; Prasada, D.; Patil, K.N.; Reddy, K.S.; Kainthla, I.; Rao, K.S.R.; Jadhav, A.H.; Nagaraja, B.M. Tailoring the catalytic activity of basic mesoporous Cu/CeO2 catalyst by Al2O3 for selective lactonization and dehydrogenation of 1,4-butanediol to γ-butyrolactone. Catal. Commun. 2020, 143, 106049. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Sodesawa, T.; Yamamoto, N. Dehydration of 1,4-butanediol into 3-buten-1-ol catalyzed by ceria. Catal. Commun. 2004, 5, 397–400. [Google Scholar] [CrossRef]
- Igarashi, A.; Ichikawa, N.; Sato, S.; Takahashi, R.; Sodesawa, T. Dehydration of butanediols over CeO2 catalysts with different particle sizes. Appl. Catal. A 2006, 300, 50–57. [Google Scholar] [CrossRef]
- Yamamoto, N.; Sato, S.; Takahashi, R.; Inui, K. Synthesis of homoallyl alcohol from 1,4-butanediol over ZrO2 catalyst. Catal. Commun. 2005, 6, 480–484. [Google Scholar] [CrossRef]
- Yamamoto, N.; Sato, S.; Takahashi, R.; Inui, K. Synthesis of 3-buten-1-ol from 1,4-butanediol over ZrO2 catalyst. J. Mol. Catal. A 2006, 243, 52–59. [Google Scholar] [CrossRef]
- Magnin-Lachaux, M.; Tan, Z.; Liang, B.; Negishi, E.I. Efficient and selective synthesis of siphonarienolone and related reduced polypropionates via Zr-catalyzed asymmetric carboalumination. Org. Lett. 2004, 6, 1425–1427. [Google Scholar] [CrossRef]
- Min, T.; Yi, B.X.; Zhang, P.; Liu, J.; Zhang, C.; Zhou, H.P. Novel furoxan NO-donor pemetrexed derivatives: Design, synthesis, and preliminary biological evaluation. Med. Chem. Res. 2009, 18, 495–510. [Google Scholar] [CrossRef]
- Michalak, O.; Gruza, M.M.; Witkowska, A.; Bujak, I.; Cmoch, P. Synthesis and physicochemical characterization of the impurities of pemetrexed disodium, an anticancer drug. Molecules 2015, 20, 10004–10031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, H.; Wen, J.; Li, L.; Bai, R.; Chen, L.; Wang, D. An efficient synthesis of pemetrexed disodium. J. Heterocycl. Chem. 2014, 52, 1565–1569. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Li, H.T.; Gao, C.G.; Zhao, Y.X. Heterogeneous CaO-ZrO2 acid-base bifunctional catalysts for vapor-phase selective dehydration of 1,4-butanediol to 3-buten-1-ol. Appl. Catal. A 2013, 466, 233–239. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Yamamoto, N.; Kaneko, E.; Inoue, H. Vapor-phase dehydration of 1,5-pentanediol into 4-penten-1-ol. Appl. Catal. A 2008, 334, 84–91. [Google Scholar] [CrossRef]
- He, Y.Y.; Li, Q.B.; Wang, Y.Z.; Zhao, Y.X. Selective catalytic dehydration of 1,4-butanediol to 3-buten-1-ol over CeO2 with different morphology. Chin. J. Catal. 2010, 31, 619–622. [Google Scholar] [CrossRef]
- Mi, R.L.; Hu, Z.; Yang, B.L. In situ DRIFTS for the mechanistic studies of 1,4-butanediol dehydration over Yb/Zr catalysts. J. Catal. 2019, 370, 138–151. [Google Scholar] [CrossRef]
- Vaidya, S.H.; Bhandari, V.M.; Chaudhari, R.V. Reaction kinetics studies on catalytic dehydration of 1,4-butanediol using cation exchange resin. Appl. Catal. A 2003, 242, 321–328. [Google Scholar] [CrossRef]
- Baba, T.; Ono, Y. Kinetic studies in liquid phase dehydration-cyclization of 1,4-butanediol to tetrahydrofuran with heteropoly acids. J. Mol. Catal. 1986, 37, 317–326. [Google Scholar] [CrossRef]
- Igarashi, A.; Sato, S.; Takahashi, R.; Sodesawa, T.; Kobune, M. Dehydration of 1,4-butanediol over lanthanide oxides. Catal. Commun. 2007, 8, 807–810. [Google Scholar] [CrossRef]
- Inoue, H.; Sato, S.; Takahashi, R.; Izawa, Y.; Ohno, H.; Takahashi, K. Dehydration of 1,4-butanediol over supported rare earth oxide catalysts. Appl. Catal. A 2009, 352, 66–73. [Google Scholar] [CrossRef]
- Zhao, C.J.; Zhou, Z.M.; Cheng, Z.M.; Fang, X.C. Sol-gel-derived, CaZrO3-stabilized Ni/CaO-CaZrO3 bifunctional catalyst for sorption-enhanced steam methane reforming. Appl. Catal. B. 2016, 196, 16–26. [Google Scholar] [CrossRef]
- Moshtaghi, S.; Gholamrezaei, S.; Niasari, M.S. Nano cube of CaSnO3: Facile and green co-precipitation synthesis, characterization and photocatalytic degradation of dye. J. Mol. Struct. 2017, 1134, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.; Thorimbert, S.; Maier, W.F. Amorphous microporous titania-silica mixed oxides: Preparation, characterization, and catalytic redox properties. J. Catal. 1996, 163, 476–488. [Google Scholar] [CrossRef]
- Nakabayashi, H. Properties of acid sites on TiO2-SiO2 and TiO2-Al2O3 mixed oxides measured by infrared spectroscopy. Bull. Chem. Soc. Jpn. 1992, 65, 914–916. [Google Scholar] [CrossRef]
- Miller, J.B.; Ko, E.I. Acidic properties of silica-containing mixed oxide aerogels: Preparation and characterization of zirconia-silica and comparison to titania-silica. J. Catal. 1996, 159, 58–68. [Google Scholar] [CrossRef]
- Sato, S.; Sato, F.; Gotoh, H.; Yamada, Y. Selective dehydration of alkanediols into unsaturated alcohols over rare earth oxide catalysts. ACS Catal. 2013, 3, 721–734. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Kobune, M.; Inoue, H.; Izawa, Y.; Ohno, H.; Takahashi, K. Dehydration of 1,4-butanediol over rare earth oxides. Appl. Catal. A 2009, 356, 64–71. [Google Scholar] [CrossRef]
- Yang, X.M.; Wei, Y.; Su, Y.L.; Zhou, L.P. Characterization of fused Fe-Cu based catalyst for higher alcohols synthesis and DRIFTS investigation of TPSR. Fuel Process. Technol. 2010, 91, 1168–1173. [Google Scholar] [CrossRef]
- Plyler, E.K. Infrared spectra of methanol, ethanol, and n-propanol. J. Res. Nat. Bur. Stand. 1952, 48, 281–286. [Google Scholar] [CrossRef]
- Bensitel, M.; Moravek, V.; Lamotte, J.; Saur, O.; Lavalley, J.C. Infrared study of alcohols adsorption on zirconium oxide: Reactivity of alkoxy species towards CO2. Spectrochim. Acta 1987, 43, 1487–1491. [Google Scholar] [CrossRef]



| Ca/Zr Molar Ratio | SBET (m2·g−1) | VBJH (cm3·g−1) | Dpore (nm) | Acid Amount (μmol·g−1) a | Base Amount (μmol·g−1) a | ||||
|---|---|---|---|---|---|---|---|---|---|
| Weak | Medium | Strong | Weak | Medium | Strong | ||||
| 0.4 | 3.77 | 0.07 | 23.51 | 187.2 | - | - | 294.3 | - | - |
| 0.52 | 11.13 | 0.11 | 20.16 | 210.5 | - | - | 313.2 | - | - |
| 0.6 | 29.73 | 0.25 | 16.85 | 321.3 | 238.5 | - | 342.1 | 284.4 | - |
| 0.68 | 49.23 | 0.26 | 10.21 | 845.3 | 934.7 | 253.5 | 367.8 | 224.9 | 1718.3 |
| 0.8 | 57.24 | 0.32 | 9.44 | 795.6 | 999.5 | 163.1 | 381.2 | 325.7 | 4306.6 |
| 1.2 | 69.24 | 0.28 | 7.97 | 58.6 | 210.8 | 357.7 | 923.2 | 203.3 | 5040.6 |
| Sn/Zr Molar Ratio | SBET (m2·g−1) | VBJH (cm3·g−1) | Dpore (nm) | Acid Amount (μmol·g−1) a | Basic Amount (μmol·g−1) a | ||||
|---|---|---|---|---|---|---|---|---|---|
| Weak | Medium | Strong | Weak | Medium | Strong | ||||
| 0.12 | 24.27 | 0.21 | 15.68 | 354.3 | 163.5 | - | 298.4 | - | 39.8 |
| 0.2 | 49.23 | 0.25 | 11.22 | 1213.4 | 795.5 | - | 334.2 | - | 808.9 |
| 0.28 | 49.59 | 0.28 | 10.91 | 1352.3 | 813.3 | 318.8 | 310.6 | 120.5 | 1266.9 |
| 0.4 | 56.07 | 0.31 | 10.21 | 1398.3 | 934.7 | 323.5 | 273.5 | 124.9 | 1718.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Xu, C.-H.; Yang, F.-L.; Du, L.; Liu, C.-L.; Chen, W.-J.; Wang, L. 1,4-Butanediol Selective Dehydration to 3-Butene-1-ol over Ca–Zr–Sn Composite Oxide Catalysts. Catalysts 2022, 12, 685. https://doi.org/10.3390/catal12070685
Dong H, Xu C-H, Yang F-L, Du L, Liu C-L, Chen W-J, Wang L. 1,4-Butanediol Selective Dehydration to 3-Butene-1-ol over Ca–Zr–Sn Composite Oxide Catalysts. Catalysts. 2022; 12(7):685. https://doi.org/10.3390/catal12070685
Chicago/Turabian StyleDong, Hao, Cheng-Hua Xu, Fang-Lu Yang, Lei Du, Chen-Long Liu, Wen-Jing Chen, and Lin Wang. 2022. "1,4-Butanediol Selective Dehydration to 3-Butene-1-ol over Ca–Zr–Sn Composite Oxide Catalysts" Catalysts 12, no. 7: 685. https://doi.org/10.3390/catal12070685
APA StyleDong, H., Xu, C.-H., Yang, F.-L., Du, L., Liu, C.-L., Chen, W.-J., & Wang, L. (2022). 1,4-Butanediol Selective Dehydration to 3-Butene-1-ol over Ca–Zr–Sn Composite Oxide Catalysts. Catalysts, 12(7), 685. https://doi.org/10.3390/catal12070685






