Abstract
Developing highly efficient and stable electrocatalysts for hydrogen evolution reaction (HER) is regarded as a crucial way to reduce energy loss in water splitting. Herein, NiSe2/MoSe2 heterostructures grown on graphene nanosheets (NiSe2-MoSe2 HTs/G) have been in situ synthesized by a simple hydrothermal reaction. As an electrocatalyst for HER, NiSe2-MoSe2 HTs/G delivers superior performance with a low Tafel slope of 65 mV dec−1, a small overpotential of 144 mV at 10 mA cm−2, and long-term stability up to 24 h. The superior performance for HER can be mainly ascribed to the synergistic effects of NiSe2-MoSe2 heterostructures, which can facilitate the rapid electron transfer from the electrode to the exposed MoSe2 edges to take part in the HER reaction, thus boosting the HER kinetics. Moreover, the graphene matrix with high conductivity can not only improve the overall conductivity of the composite but also greatly increase the exposed active sites, therefore further promoting the HER performance. This study provides a simple route for fabricating bimetallic selenides-based heterostructures on graphene as an efficient and stable electrocatalyst for HER.
1. Introduction
The excessive combustion of fossil fuels in our daily life has brought about severe environmental problems and energy crises [1]. As we all know, hydrogen, a renewable energy source, has been considered a promising candidate to replace natural fossil fuels due to its clean combustion products and high energy density [2,3,4]. Electrochemical water splitting has been regarded as an efficient strategy to produce hydrogen [5]. However, the high overpotentials on the electrodes severely increase the power consumption and cost in the real electrocatalytic process [6]. Platinum (Pt), a commercial catalyst, has been identified as the most efficient electrocatalyst for the HER [7]. However, its extreme scarcity results in high costs, which limits the commercialization. Hence, highly efficient non-noble metal catalysts must be developed to reduce the overpotential in the HER, aiming to cut costs [8].
In recent years, two-dimensional (2D) transition-metal dichalcogenides (TMDs) including MoS2 [9], WS2 [10], WSe2 [11], MoSe2 [12], etc., have emerged as competitive alternatives for HER to Pt-based electrocatalysts due to their numerous advantages, including excellent catalytic activity, high stability, and low costs [13]. Generally, the monolayer TMDs consist of a transition-metal atomic plane (Mo, W, Re, Hf, etc.) sandwiched between two chalcogen (S or Se) planes [14]. The adjacent layers of 2D TMDs are coupled by van der Waals force to construct bulk crystals [15]. Among them, nonprecious 2D MoSe2 has received widespread attention as a promising HER catalyst due to its fascinating structures and low Gibbs free energy [16]. However, it is well-known that the HER performance of 2D MoSe2 is strongly limited by its weak conductivity and the lack of exposed active edges, which play key roles in the HER process [17,18,19]. At present, the two parameters are still challenging to simultaneously optimize. Furthermore, MoSe2-based catalysts exhibit higher HER activity in acidic media, but often show slower HER kinetics in alkaline media because the HER activity under alkaline conditions is strongly limited by the lack of H+. Hence, these above shortcomings result in its severely limited potential application in the HER.
To address these issues, combining 2D MoSe2 with metallic TMDs such as CoSe2 [20], Ni0.85Se [21], NiSe [22], NiSe2 [23], etc., has been demonstrated as an effective strategy to promote its catalytic HER performance under alkaline conditions. For instance, Wang et al. reported that MoSe2–CoSe2 nanotubes present highly efficient performance for HER in the alkaline medium because their unique hierarchical architecture can suppress the restacking of MoSe2 layers to gain more active sites for HER [24]. Zhou et al. found that the electrons in MoSe2-NiSe nanohybrids can be easily transferred from the metallic NiSe nanocrystallite to the exposed MoSe2 edges, thus enhancing HER performance [22]. Similarly, Zhang et al. demonstrated that 3D MoSe2@Ni0.85Se nanowire exhibits remarkably improved alkaline HER performance because the conductive Ni0.85Se can facilitate fast electron transfers from the electrode to the exposed MoSe2 edges [16]. Despite such great achievements that have been made, it is urgent to design and synthesize novel MoSe2-based bimetallic selenides for further improving HER performance compared with noble metal-based catalysts.
In addition to this strategy, carbon-based materials such as MOF-derived carbon [25], carbon nanotube [26], graphene [27], etc., are broadly applied in enhancing the catalytic performance of transition-metal selenides. In particular, graphene has been demonstrated as an ideal carbon matrix for the transition-metal selenide catalysts, owing to its large surface area, adjustable porosity, high conductivity, and uniform catalyst distribution [28]. Considering the above, it is expected that a simple method can be developed to synthesize Mo-based bimetallic selenides incorporated with graphene as efficient hybrid electrocatalysts for HER under alkaline conditions.
In this work, we proposed a simple hydrothermal reaction to construct a NiSe2-MoSe2 heterostructures grown on graphene nanosheets (NiSe2-MoSe2 HTs/G). It demonstrated that the NiSe2-MoSe2 HTs constructed by metallic NiSe2 nanocrystallites embedded in few-layer MoSe2 nanosheets show a remarkably enhanced HER performance in alkaline media compared to pure MoSe2 and NiSe2. When supported on graphene, its HER performance can be further improved. The enhanced HER activity of NiSe2-MoSe2 HTs/G can be mainly attributed to the synergistic effects between metallic NiSe2 nanocrystallites and MoSe2 nanosheets, which can efficiently promote the rapid electron transfer from metallic NiSe2 to the exposed MoSe2 edges. Furthermore, the unique nanostructure of NiSe2-MoSe2 heterostructures supported on highly conductive graphene can not only efficiently expose the active sites on the heterostructures but also greatly enhance the overall conductivity of the composite, thus resulting in superior HER performance. This work might shed new light on the rational synthesis of graphene-supported bimetallic selenide heterostructures of the tunable HER activities.
2. Results and Discussion
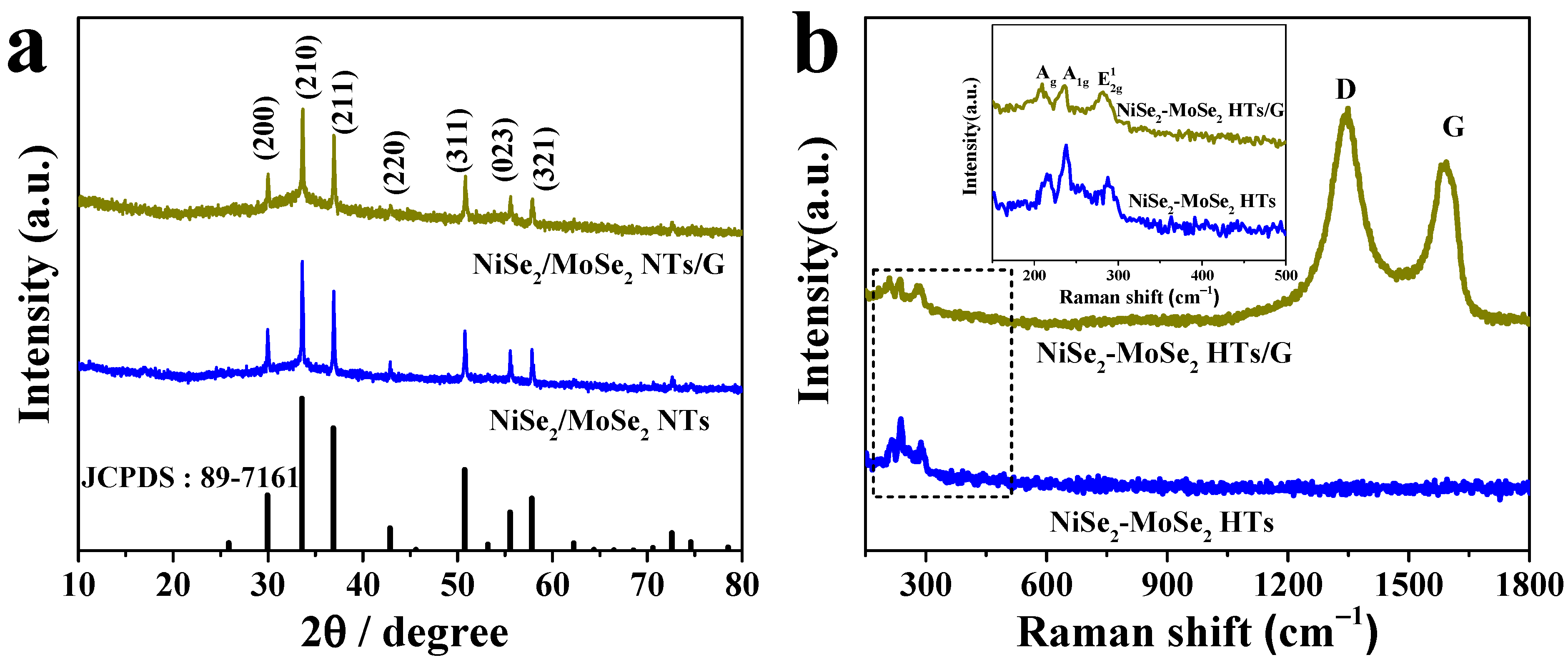
NiSe2-MoSe2 heterostructures grown on graphene nanosheets (NiSe2-MoSe2 HTs/G) were synthesized by a one-step hydrothermal method. In the hydrothermal process, the graphene oxides (GO) were reduced to graphene nanosheets by hydrazine hydrate. Meanwhile, the Ni2+ and Mo4+ cations reacted with Se2- anions to form NiSe2-MoSe2 heterostructures, which were in situ grown on reduced graphene nanosheets to obtain NiSe2-MoSe2 HTs/G composite. The crystalline structures of NiSe2, MoSe2, NiSe2-MoSe2 HTs, and NiSe2-MoSe2 HTs/G were determined by X-ray diffraction (XRD). The diffraction peaks at 13.25°, 31.78°, 37.98°, and 56.01° in the XRD pattern of MoSe2 (Figure S1a) correspond to the (002), (100), (103), and (110) planes of hexagonal 2H-MoSe2, respectively (JCPDS No. 87-2419). In the XRD patterns of NiSe2 (Figure S1b), NiSe2-MoSe2 HTs, and NiSe2-MoSe2 HTs/G (Figure 1a), the diffraction peaks at 29.95°, 33.58°, 36.89°, 42.86°, 50.74°, 55.52°, and 57.81° are ascribed to the (200), (210), (211), (220), (311), (023), and (321) planes of cubic NiSe2, respectively (JCPDS No. 89-7161). It is worth noting that there are no obvious diffraction peaks of MoSe2 appear in both NiSe2-MoSe2 HTs and NiSe2-MoSe2 HTs/G samples, suggesting the MoSe2 in the composites with few layers or low crystallinity. This demonstrates that the formation of NiSe2-MoSe2 heterostructures can prevent the restacking of the adjacent layers for 2D MoSe2. As shown in Raman profiles (Figure 1b), both NiSe2-MoSe2 HTs and NiSe2-MoSe2 HTs/G samples present bands that are identical with the characteristic peaks observed on pure NiSe2 and MoSe2 counterparts (Figure S2), implying the coexistence of NiSe2 and 2H-MoSe2 in these two composites. In detail, the band at 215.2 cm−1 is corresponded to the Ag signal of NiSe2, while the bands at 238.1 and 287.6 cm−1 are associated with the A1g and E12g vibration modes of 2H-MoSe2 [16]. Moreover, the D (1348.8 cm−1) and G (1592.1 cm−1) bands of graphene are observed in the Raman spectrum of NiSe2-MoSe2 HTs/G, which suggests the formation of the NiSe2-MoSe2 HTs/G hybrid [29]. The high ID/IG value (1.14) indicated that the reduced graphene has abundant carbon defects, which can efficiently promote the growth of NiSe2-MoSe2 heterostructures on the surfaces of graphene nanosheets [30].
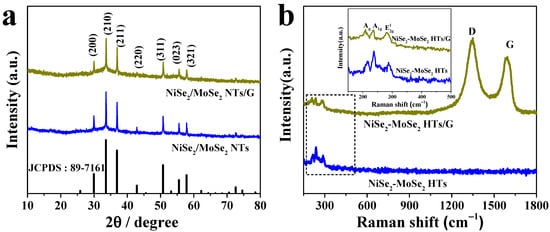
Figure 1.
(a) XRD patterns of NiSe2-MoSe2 HTs and NiSe2-MoSe2 HTs/G composite. (b) Raman spectra of NiSe2-MoSe2 HTs and NiSe2-MoSe2 HTs/G composite. The inset is the enlarged Raman spectra.
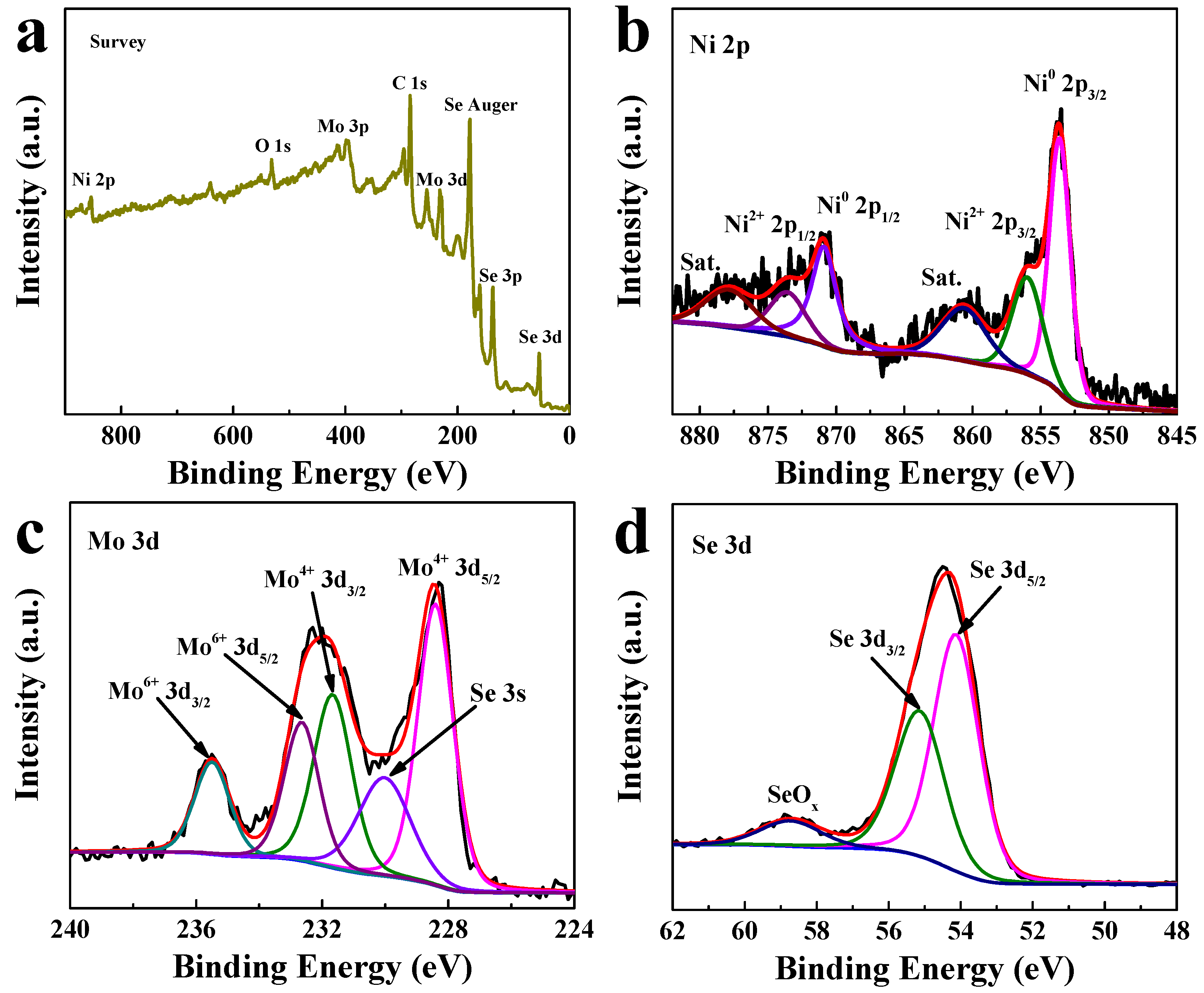
The chemical states of as-prepared NiSe2/MoSe2 Hs/G composite were measured by X-ray photoelectron spectroscopy (XPS). As shown in Figure 2a, the predominant elements of Ni, Mo, Se, and C are observed in the XPS survey spectrum of the NiSe2/MoSe2 Hs/G composite. The Ni 2p spectrum (Figure 2b) demonstrates that peaks located at 853.6 and 870.9 eV are assigned to the Ni0 2p3/2 and Ni0 2p1/2 of metallic NiSe2, and a couple of peaks located at 856.1 and 873.7 eV belong to the Ni2+ 2p3/2 and Ni2+ 2p1/2 of Ni oxidation state [31]. Moreover, the remaining two peaks located at 860.8 and 877.9 eV are ascribed to the satellite peaks of Ni 2p [32]. In the Mo 3d spectrum (Figure 2c), the Mo4+ 3d5/2 and Mo4+ 3d3/2 peaks are observed at 228.4 and 231.7 eV, which are ascribed to Mo4+ in MoSe2 [33]. Furthermore, the relatively smaller peak at 230.1 eV is attributed to the Se 3s, while the high binding-energy peaks of Mo6+ 3d5/2 (232.7) and Mo6+ 3d3/2 (235.5 eV) correspond to MoO3 species, which may result from the oxidation of the catalyst sample in air [24]. In the Se 3d spectrum (Figure 2d), the Se 3d5/2 and Se 3d3/2 peaks appear at 54.1 and 55.2 eV, respectively, revealing the existence of Se2- [34]. In addition, another peak at 58.9 eV is originated from the oxidation states of the exposed Se edge [35]. The XPS data of Ni 2p, Mo 3d and Se 3d including peak positions and amounts for the composite material is summarized in Table S1. The ratio of Ni to Mo in the hybrid material is ~1.16:1, which is close to the raw materials. In the C 1s spectrum (Figure S3), the peaks located at 284.6, 286.7, and 288.9 eV are attributed to the C-C, C-O, and COOH bonds of graphene, respectively [36]. These XPS results further confirm that the NiSe2-MoSe2 HTs/graphene composite was successfully formed in the one-step hydrothermal process.
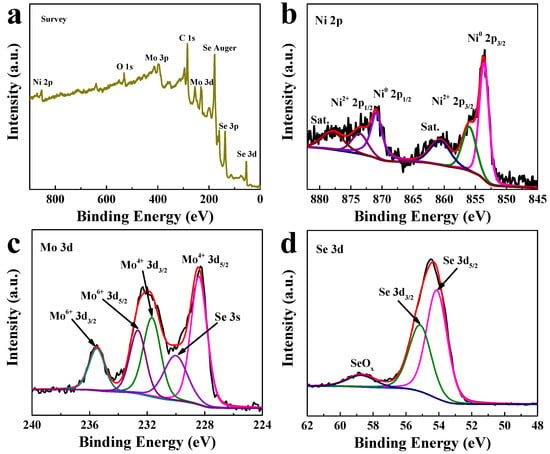
Figure 2.
XPS spectra of NiSe2-MoSe2 HTs/G composite: (a) survey, (b) Ni 2p, (c) Mo 3d, and (d) Se 3d.
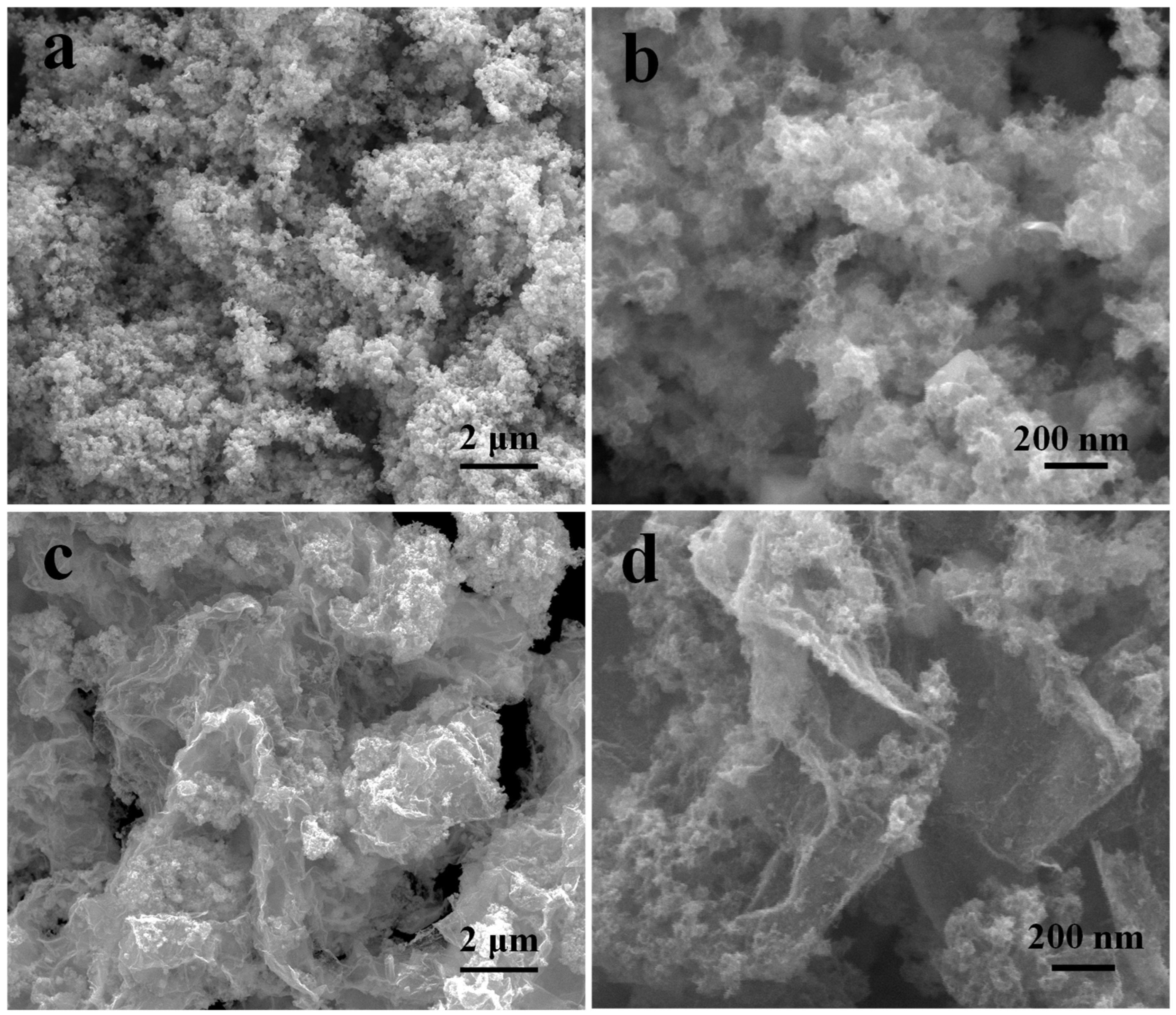
The morphologies of MoSe2, NiSe2, NiSe2-MoSe2 HTs, and NiSe2-MoSe2 HTs/G were first characterized by SEM. The SEM images shown in Figure S4 indicate that pure MoSe2 has a flower-like morphology with many nanosheets. In sharp contrast, pure NiSe2 presents large globular nanostructures with an average size of 100 nm (Figure S5). Notably, the NiSe2-MoSe2 HTs show a similar morphology with no obvious stack compared to pure MoSe2 (Figure 3a). More importantly, there are no large globular NiSe2 nanostructures that appear in the NiSe2-MoSe2 HTs (Figure 3b), which suggests that the NiSe2-MoSe2 heterostructures prevented the growing up of pure NiSe2. As shown in Figure 3c, the NiSe2-MoSe2 HTs have been uniformly grown on the graphene matrix in the hydrothermal process because of the rich oxidizing functional groups on graphene oxide are beneficial to the growth of the catalysts [37]. Undoubtedly, the porous graphene matrixes with high conductivity can remarkably enhance the electron transfer from the electrode to the active sites on NiSe2-MoSe2 HTs and further improve the HER performance [38]. Furthermore, the high-resolution SEM image (Figure 3d) indicates that the graphene matrixes efficiently prevent the aggregation of NiSe2-MoSe2 HTs, thus promoting the maximumly exposed reactive sites.
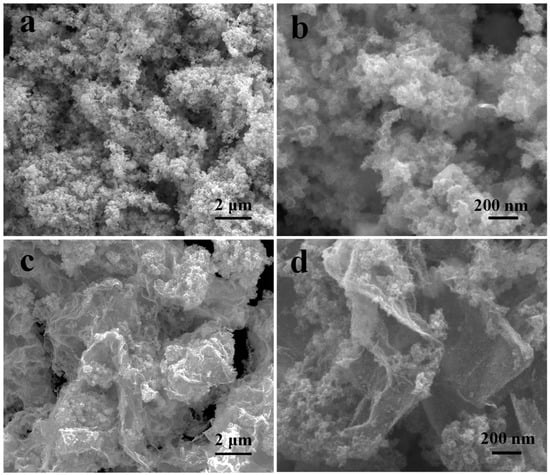
Figure 3.
(a,b) SEM images of NiSe2-MoSe2 HTs. (c,d) SEM images of NiSe2-MoSe2 HTs/G composite.
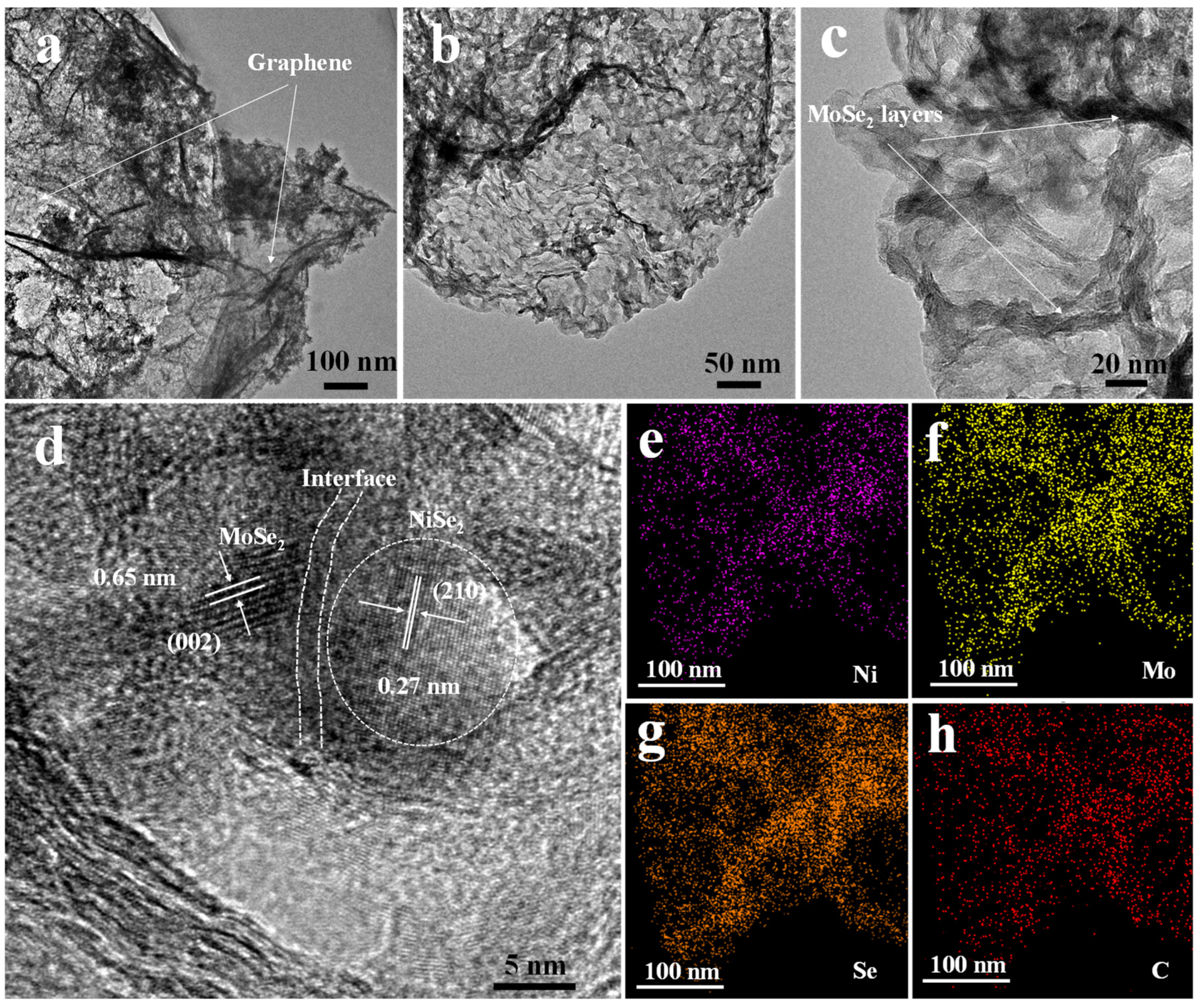
The TEM analysis was further used to characterize the nanostructures of the NiSe2-MoSe2 HTs/G composite. The TEM images (Figure 4a,b) show that the uniform NiSe2-MoSe2 HTs nanostructures are tightly supported on the surfaces of graphene, which is consistent with SEM images. The high-resolution TEM image (Figure 4c) further indicates that many nanosheet-like MoSe2 nanostructures present abundant exposed few-layered edges, implying that combining MoSe2 with NiSe2 and graphene can be advantageous to prevent the stack of MoSe2 layers and maximally expose more active MoSe2 edges. Moreover, the HRTEM images (Figure 4d and Figure S6) demonstrate the formation of the NiSe2-MoSe2 heterostructures, which are constructed by NiSe2 nanocrystallites uniformly embedded in few-layered MoSe2 nanosheets (<10 layer). Compared to pure NiSe2, the NiSe2 nanocrystallites show a smaller particle size of ~10 nm. The discontinuous and distortion lattice fringes reveal that the NiSe2 and MoSe2 in the heterostructures possess rich defects, which can be employed as active sites in HER reaction [39]. Compared with bulk MoSe2 (0.65 nm), the highly expanded lattice fringe of about 0.68 nm corresponds to the (002) planes of 2H-MoSe2 [40]. The clear lattice fringes of 2H-MoSe2 indicate that the MoSe2 in the NiSe2-MoSe2 hybrid is not amorphous. In addition, the lattice fringes of 0.27 nm on ultra-small NiSe2 nanocrystallites correspond to the (210) planes of cubic NiSe2 [41]. The relevant element-mapping images of NiSe2-MoSe2 heterostructure-grown graphene (Figure 4e–h) display that the Ni, Mo, and Se elements are evenly distributed across the nanosheet-like nanostructure (Figure S7), suggesting that the ultra-small NiSe2 nanoparticles are uniformly distributed in the MoSe2 nanosheets.
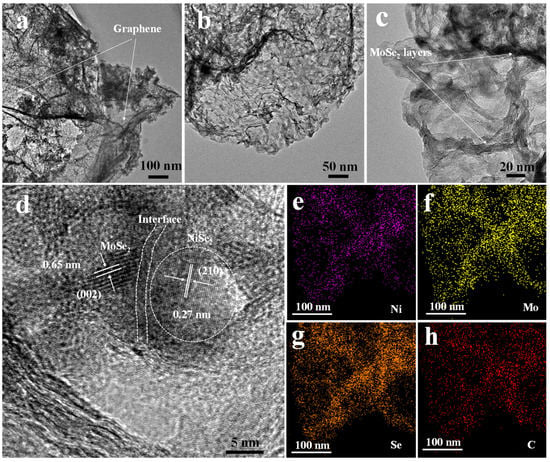
Figure 4.
(a–c) TEM images of NiSe2-MoSe2 HTs/G composite. (d) High-resolution TEM (HRTEM) images of NiSe2-MoSe2 HTs/G. (e–h) EDS elemental mapping images of NiSe2-MoSe2 HTs/G.
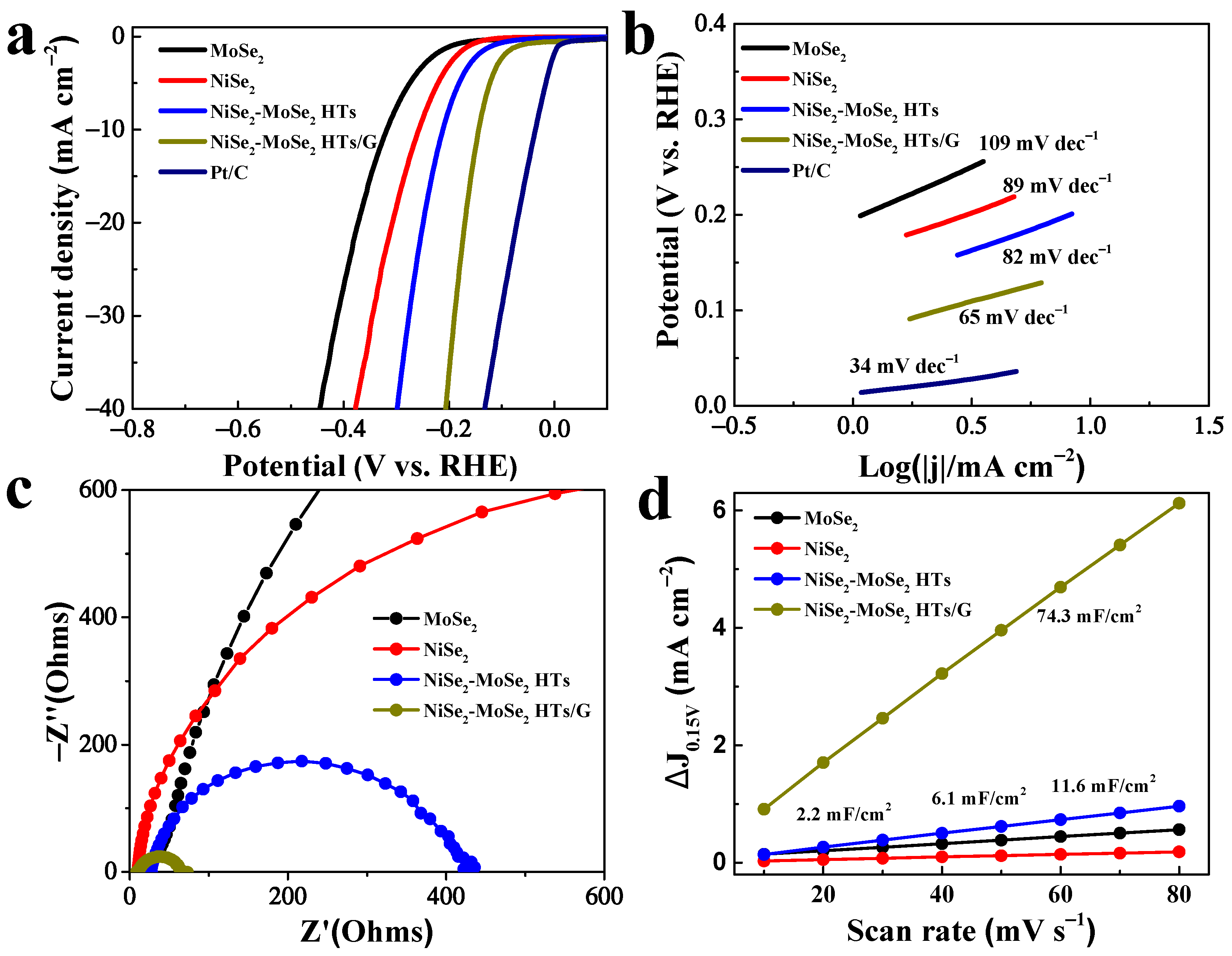
The NiSe2, MoSe2, NiSe2-MoSe2 HTs, NiSe2-MoSe2 HTs/G, and Pt-C samples were used as HER catalysts to assess their electrocatalytic performance in 1.0 M KOH solution using the linear-sweep voltammetry measurement. Before tests, the electrolyte was bubbled with N2 saturation for 30 min. As displayed in Figure 5a, the LSV curves indicate that the 20% Pt-C catalyst delivers the highest HER activity in 1.0 M KOH, which is consistent with the previous report [42]. Compared to pure NiSe2 and MoSe2, the NiSe2-MoSe2 HTs composite exhibits a remarkedly improved electrocatalytic performance. Since the pure NiSe2 and MoSe2 in our work show inferior electrocatalytic activity, the much-enhanced HER performance of NiSe2-MoSe2 HTs can be attributed to the synergistic effect of NiSe2 nanocrystallites and MoSe2 nanosheets. The metallic NiSe2 nanocrystallites embedded in MoSe2 nanosheets can boost the electrons’ rapid transfer from the graphene matrix to the exposed MoSe2 edges to take part in the HER reaction, and thus promoting the HER kinetics [43]. More importantly, the electrocatalytic HER performance of NiSe2-MoSe2 HTs/G is remarkably improved compared with NiSe2-MoSe2 HTs, which suggests that the adding of graphene further enhances their HER performance. It can be expected that the highly conductive graphene nanosheets can accelerate the electron transfers from the electrode to NiSe2-MoSe2 HTs, and therefore further promoting the HER kinetics. As a result, NiSe2-MoSe2 HTs/G delivers a much lower onset overpotential of 69 mV, compared with NiSe2-MoSe2 HTs (119 mV), NiSe2 (164 mV), and MoSe2 (196 mV). More importantly, a current density of 10 mA cm−2 was achieved at a relatively small overpotential of 144 mV, which is lower than that of NiSe2-MoSe2 HTs (212 mV), NiSe2 (258 mV), and MoSe2 (319 mV). In addition, NiSe2-MoSe2 HTs/G possesses favorable catalytic HER performance compared to most of the TMDs-based HER electrocatalysts reported in recent years (Table S2), such as CoSe2-MoSe2/rGO-C (215 mV) [44], MoSe2-NiSe@C heterostructures (154 mV) [33], 10% Ni–WSe2 (215 mV) [45], MoSe2–CoSe2 NTs (206 mV) [24], NiSe2/MoS2 heterostructures (188 mV) [46], and so on, suggesting the potential practical application of NiSe2-MoSe2 HTs/G in alkaline electrolyte.
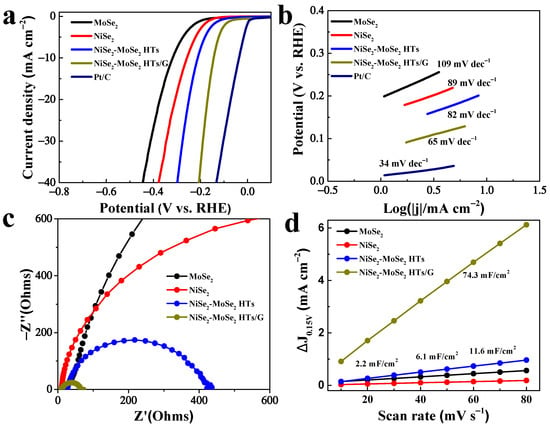
Figure 5.
(a) Polarization curves of MoSe2, NiSe2, NiSe2-MoSe2 HTs, NiSe2-MoSe2 HTs/G, and Pt/C. (b) Tafel plots of MoSe2, NiSe2, NiSe2-MoSe2 HTs, NiSe2-MoSe2 HTs/G, and Pt/C. (c) EIS spectra of MoSe2, NiSe2, NiSe2-MoSe2 HTs, and NiSe2-MoSe2 HTs/G. (d) Estimated Cdl of MoSe2, NiSe2, NiSe2-MoSe2 HTs, and NiSe2-MoSe2 HTs/G.
As shown in Figure 5b, the HER kinetics of as-prepared catalysts were first investigated by Tafel plots. It is noted that the Tafel slope of NiSe2-MoSe2 HTs/G is 65 mV dec−1, which is lower than those of 82 mV dec−1 for NiSe2-MoSe2 HTs hybrid, pure NiSe2 for 89 mV dec−1, pure MoSe2 for 109 mV dec−1, and close to the commercial Pt-C of 34 mV dec−1, indicating the faster kinetics of NiSe2-MoSe2 HTs/G for HER in alkaline solution [47]. A smaller Tafel slope of 65 mV dec−1 for NiSe2-MoSe2 HTs/G suggests that the HER kinetics on this composite abides by the Volmer-Heyrovsky mechanism with the Heyrovsky step being rate-limiting in the alkaline HER process [48]. Furthermore, EIS measurement was employed to study the HER kinetics. The calculated charge-transfer resistance (Rct) of NiSe2-MoSe2 HTs/G (Figure 5c) is 58.7 Ω, which is much lower than that of NiSe2-MoSe2 HTs (392.4 Ω), NiSe2 (1462 Ω), and MoSe2 (3764 Ω), demonstrating more rapid charge transfers within the composite [49]. According to these results, we can surmise that the faster HER kinetic in alkaline solution is attributed to the promoted charge transfer by metallic NiSe2 and conductive graphene. In addition, the electrochemical active surface areas (ECSA) of NiSe2-MoSe2 HTs/G, NiSe2-MoSe2 HTs, pure NiSe2, and pure MoSe2 were assessed by extracting the electrochemical double-layer capacitances (Cdl) from their corresponding voltammograms (Figure S8) to obtain insight into catalytic active sites for HER. As shown in Figure 5d, a very large Cdl value (74.3 mF/cm2) for NiSe2-MoSe2 HTs/G was achieved, which is much higher than those of 11.6 mF/cm2 for NiSe2-MoSe2 HTs, 2.2 mF/cm2 for pure NiSe2, and 6.1 mF/cm2 for pure MoSe2, demonstrating the rich electrocatalytic active sites in the composite. These abundant active sites can be attributed to the unique heterostructures supported on graphene that efficiently prevent the stack of the MoSe2 layers and the aggregation of NiSe2 [21].
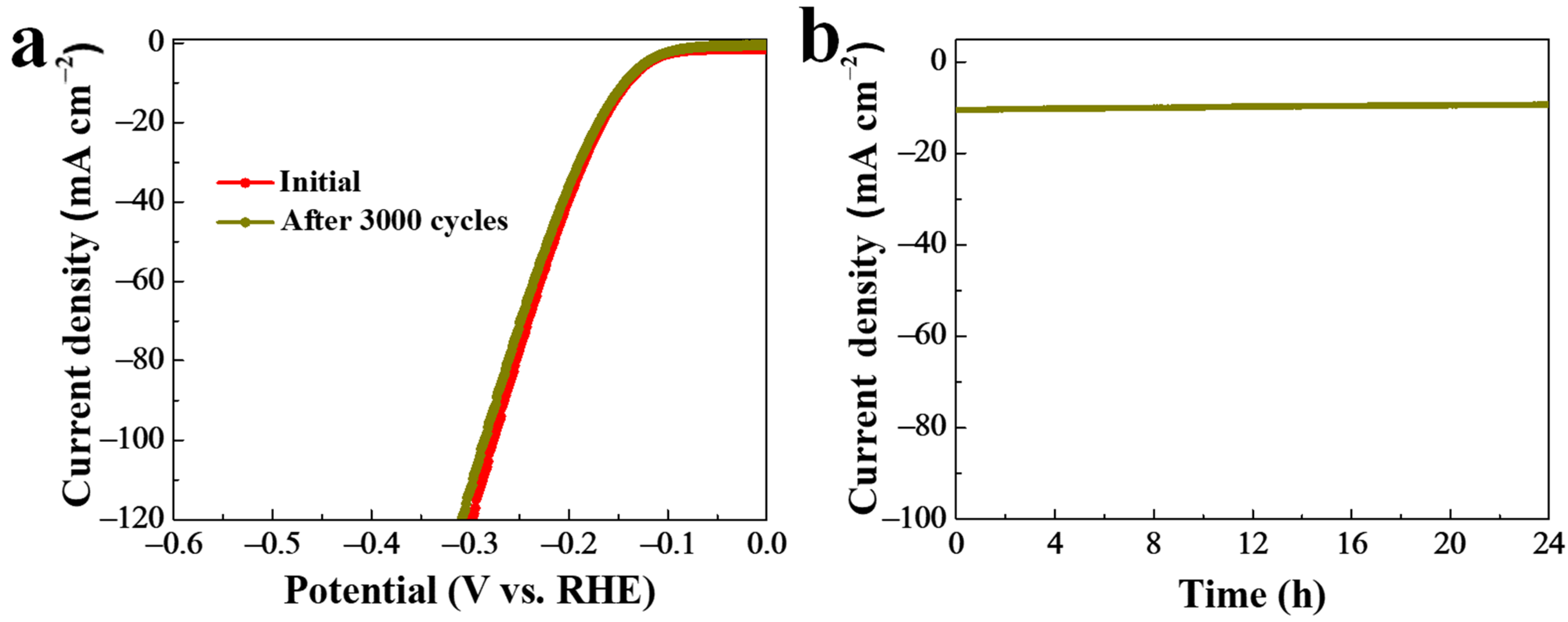
To measure the stability of the as-prepared NiSe2-MoSe2 HTs/G composite during HER testing under alkaline conditions, a successive 3000 CV measurement was performed (Figure 6a). It indicated that the LSV curve shape of NiSe2-MoSe2 HTs/G after 3000 CV cycles is very close to the initial one, revealing the excellent stability under alkaline condition. Moreover, the HER stability of NiSe2-MoSe2 HTs/G was also evaluated by using a time dependence of the current density (i-t) test at a constant overpotential of 144 mV. As shown in Figure 6b, the current density at 10 mA cm−2 displays no significant variation from 0 to 24 h, suggesting the long-term stability in the alkaline HER.
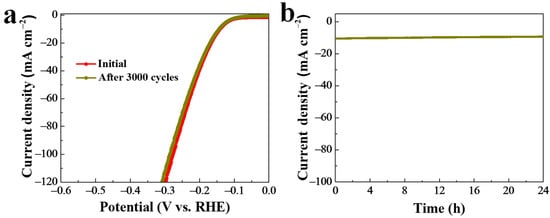
Figure 6.
(a) Stability of NiSe2-MoSe2 HTs/G after 3000 CV tests. (b) I-t test of H-NiSe2/MoSe2/G under a constant overpotential of 144 mV.
3. Experimental Section
3.1. Synthesis of NiSe2-MoSe2 HTs/G
NiSe2-MoSe2 heterostructures grown on graphene nanosheets (NiSe2-MoSe2 HTs/G) were synthesized by a simple hydrothermal method. In a typical procedure, 8 mmol of Se powder was dispersed and slowly dissolved in 8 mL of hydrazine hydrate (HHA) to form a red solution A. Subsequently, 120 mg of GO powder was dispersed in 52 mL of distilled water, and then 2 mmol of nickel chloride hexahydrate (NiCl2·6 H2O) and 2 mmol of sodium molybdate tetrahydrate (NaMoO4·4 H2O) were successively dissolved in above dispersion to obtain solution B. Finally, solution A was slowly poured into the solution B under continuous stirring for 20 min and was carefully transferred into 100 mL of autoclave and then heated to the reaction temperature of 200 °C for 20 h. The as-obtained black precipitate was filtered from the solution and washed using distilled water and ethanol repeatedly. After drying at 60 °C, the above product was collected.
3.2. Synthesis of NiSe2/MoSe2 HTs
The synthetic procedure of NiSe2/MoSe2 HTs was the same as that of NiSe2-MoSe2 HTs/G without adding GO.
3.3. Synthesis of NiSe2 or MoSe2
The synthetic procedures of NiSe2 or MoSe2 were the same as that of NiSe2/MoSe2 HTs just using 4 mmol of NiCl2·6 H2O or 4 mmol of NaMoO4·4 H2O as metal sources, respectively.
3.4. Characterizations
X-ray diffraction (XRD) measurement (Rigaku D/MAX-rA, Rigaku, Tokyo, Japan) was conducted to obtain the crystallography of the as-synthesized samples. Raman spectroscopy (Horiba, Lille, France) with a 532 nm Argon laser, was used to determine the bonding nature of as-synthesized materials. The morphology and microstructure of as-obtained products were observed by a scanning electron microscope (SEM, FEI Inspect F50, Thermo Fisher Scientific, Waltham, USA) and transmission electron microscopy (TEM, FEIG2F20, Thermo Fisher Scientific, Waltham, USA). The surface chemistries and information of NiSe2-MoSe2 HTs/G were collected by X-ray photoelectron spectroscopy (XPS) equipped with Al Kα source (AXI Sultra DLD, Kratos, Manchester, UK).
3.5. Electrochemical Measurements
Electrochemical measurements were conducted using a CHI670E workstation. The HER catalytic activities of as-synthesized catalysts were evaluated by a three-electrode system in 1.0 M KOH solution. In this system, a Hg/HgO electrode was used as the reference electrode, and a graphite rod was used as the counter electrode. In this work, the working electrodes were prepared as follows. Firstly, 4 mg of the 20% Pt/C or as-prepared catalysts were mixed with 600 μL deionized water and 350 μL of absolute ethanol by sonication for 30 min. Subsequently, 50 μL of Nafion solution (5 wt%) was added into the former aqueous dispersion under continuing sonication for 10 min to form the catalyst ink. A droplet of the ink (10 μL) was dripped onto a working electrode (glassy carbon electrode, 3 mm diameter) and left to dry in natural conditions. For the activation, 30 cyclic voltammetry (CV) cycles should be performed at 100 mV s−1 from −0.7 to −1.4 V (vs. Hg/HgO) before all the tests. The polarization curves were carried out at a scan rate of 5 mV s−1. We applied the EIS measurement to study the HER catalytic kinetics, which is measured at an overpotential of 150 mV vs. RHE with an AC voltage (5 mV amplitude, 0.1 Hz–100 kHz). The electrochemical double-layer capacitance (Cdl) was assessed by the CV tests in a potential range of 0.1–0.2 V vs. RHE with different scan rates (10–80 mV s−1). The electrochemical stability in the HER process was investigated at a constant overpotential of 144 mV vs. RHE. All potentials in our work need to be switched to a reversible hydrogen electrode (RHE) by this equation (E(RHE) = EHg/HgO + 0.099 + 0.059 × pH).
4. Conclusions
In summary, NiSe2-MoSe2 heterostructures constructed by embedding metallic NiSe2 nanocrystallites in few-layer MoSe2 nanosheets have been supported on graphene nanosheets by a simple hydrothermal reaction. The metallic NiSe2 and conductive graphene play key roles to enhance HER performance. The metallic NiSe2 can prevent the restacking of MoSe2 layers in the hydrothermal process to expose much more active edges. Furthermore, the graphene provides a conductive matrix for the growth of NiSe2-MoSe2 heterostructures, thus facilitating the fast electron transfers and HER kinetics. As a result, NiSe2-MoSe2 HTs/G composite exhibits a small overpotential of 144 mV at 10 mA cm−2, a low Tafel slope of 65 mV dec−1, and long-term stability for 24 h in alkaline media, which are comparable with most TMD-based catalysts (Table S2). The superior HER performance is ascribed to the unique NiSe2-MoSe2 heterostructures supported on graphene that can synergistically modulate the conductivity as well as exposed active sites. Our work provides a simple vision to synthesize graphene-supported bimetallic selenide heterostructures as highly efficient and stable electrocatalysts for HER.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal12070701/s1, Figure S1: XRD patterns of pure MoSe2 and NiSe2; Figure S2: Raman spectra of pure MoSe2 and NiSe2; Figure S3: XPS spectrum of C 1s for NiSe2-MoSe2 HTs/G; Figure S4: SEM images of pure MoSe2; Figure S5: SEM images of pure NiSe2; Figure S6: TEM image of NiSe2-MoSe2 HTs/G; Figure S7: STEM image and corresponding EDS elemental mapping area of NiSe2-MoSe2 HTs/G; Figure S8: Voltammogram of the different catalysts; Table S1: XPS data including peak positions and amounts of NiSe2-MoSe2 HTs/G; Table S2: Comparison of electrochemical performances. References [50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, X.W. and Z.Z.; methodology, H.X.; software, T.D.; validation, X.W. and T.D.; formal analysis, Z.Z.; investigation, T.D.; resources, Z.Z. and Y.L.; data curation, H.X.; writing—original draft preparation, T.D.; writing—review and editing, X.W. and Z.Z.; visualization, Y.X.; supervision, X.W. and Z.Z.; project administration, Z.Z.; funding acquisition, Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Discipline and Master’s Site Construction Project of Guiyang University by Guiyang City Financial Support Guiyang University [2021-xk13], the Key field Foundation of GuiZhou Provincial Department of Education (No KY[2020]046), the Natural Science Research Project of the Education Department of Guizhou Province (Grant No. QJHKYZ[2020]089).
Data Availability Statement
All the relevant data used in this study have been provided in the form of figures and tables in the published article, and all data provided in the present manuscript are available to whom it may concern.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Men, Y.; Tan, Y.; Li, P.; Cao, X.; Jia, S.; Wang, J.; Chen, S.; Luo, W. Tailoring the 3d-orbital electron filling degree of metal center to boost alkaline hydrogen evolution electrocatalysis. Appl. Catal. B Environ. 2021, 284, 119718. [Google Scholar] [CrossRef]
- Yang, L.; Huang, L.; Yao, Y.; Jiao, L. In-situ construction of lattice-matching NiP2/NiSe2 heterointerfaces with electron redistribution for boosting overall water splitting. Appl. Catal. B Environ. 2021, 282, 119584. [Google Scholar] [CrossRef]
- Mao, B.; Sun, P.; Jiang, Y.; Meng, T.; Guo, D.; Qin, J.; Cao, M. Identifying the Transfer Kinetics of Adsorbed Hydroxyl as a Descriptor of Alkaline Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2020, 59, 15232–15237. [Google Scholar] [CrossRef]
- Suen, N.; Hung, S.; Quan, Q.; Zhang, N.; Xu, Y.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
- Zhang, H.; Maijenburg, A.W.; Li, X.; Schweizer, S.L.; Wehrspohn, R.B. Bifunctional Heterostructured Transition Metal Phosphides for Efficient Electrochemical Water Splitting. Adv. Funct. Mater. 2020, 30, 2003261. [Google Scholar] [CrossRef]
- Xie, C.; Chen, W.; Du, S.; Yan, D.; Zhang, Y.; Chen, J.; Liu, B.; Wang, S. In-situ phase transition of WO3 boosting electron and hydrogen transfer for enhancing hydrogen evolution on Pt. Nano Energy 2020, 71, 104653. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, B.; Xu, J.; Chen, S.; Rawat, R.S.; Fan, H.J. 3D Porous Hierarchical Nickel-Molybdenum Nitrides Synthesized by RF Plasma as Highly Active and Stable Hydrogen-Evolution-Reaction Electrocatalysts. Adv. Energy Mater. 2016, 6, 1600221. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Zhang, Z.; Hu, S.; Ma, M.; Zhang, Z.; Zhou, W.; Liu, H. Addressable surface engineering for N-doped WS2 nanosheet arrays with abundant active sites and the optimal local electronic structure for enhanced hydrogen evolution reaction. Nanoscale 2020, 12, 22541–22550. [Google Scholar] [CrossRef]
- Wang, H.; Kong, D.; Johanes, P.; Cha, J.J.; Zheng, G.; Yan, K.; Liu, N.; Cui, Y. MoSe2 and WSe2 Nanofilms with Vertically Aligned Molecular Layers on Curved and Rough Surfaces. Nano Lett. 2013, 13, 3426–3433. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.S.; Kwak, I.H.; Debela, T.T.; Abbas, H.G.; Park, Y.C.; Ahn, J.-P.; Park, J.; Kang, H.S. Se-Rich MoSe2 Nanosheets and Their Superior Electrocatalytic Performance for Hydrogen Evolution Reaction. ACS Nano 2020, 14, 6295–6304. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Sherrell, P.; Liu, Y.; Lei, W.; Zhang, S.; Zhang, H.; Wallace, G.G.; Chen, J. Engineered 2D Transition Metal Dichalcogenides-A Vision of Viable Hydrogen Evolution Reaction Catalysis. Adv. Energy Mater. 2020, 10, 1903870. [Google Scholar] [CrossRef]
- Zhang, X.; Lai, Z.; Ma, Q.; Zhang, H. Novel structured transition metal dichalcogenide nanosheets. Chem. Soc. Rev. 2018, 47, 3301–3338. [Google Scholar] [CrossRef]
- Kwon, I.S.; Kwak, I.H.; Ju, S.; Kang, S.; Han, S.; Park, Y.C.; Park, J.; Park, J. Adatom Doping of Transition Metals in ReSe2 Nanosheets for Enhanced Electrocatalytic Hydrogen Evolution Reaction. ACS Nano 2020, 14, 12184–12194. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, T.; Sun, L.; Sun, Y.; Hu, T.; Xu, K.; Ma, F. Hydrothermal synthesis of 3D hierarchical MoSe2/NiSe2 composite nanowires on carbon fiber paper and their enhanced electrocatalytic activity for the hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 19752–19759. [Google Scholar] [CrossRef]
- Najafi, L.; Bellani, S.; Oropesa-Nuñez, R.; Prato, M.; Martín-García, B.; Brescia, R.; Bonaccorso, F. Carbon Nanotube-Supported MoSe2 Holey Flake:Mo2C Ball Hybrids for Bifunctional pH-Universal Water Splitting. ACS Nano 2019, 13, 3162–3176. [Google Scholar] [CrossRef]
- Tan, C.; Luo, Z.; Chaturvedi, A.; Cai, Y.; Du, Y.; Gong, Y.; Huang, Y.; Lai, Z.; Zhang, X.; Zheng, L.; et al. Preparation of High-Percentage 1T-Phase Transition Metal Dichalcogenide Nanodots for Electrochemical Hydrogen Evolution. Adv. Mater. 2018, 30, 1705509. [Google Scholar] [CrossRef]
- Jian, C.; Hong, W.; Cai, Q.; Liu, W. The local electronic structure modulation of the molybdenum selenide–nitride heterojunction for efficient hydrogen evolution reaction. J. Mater. Chem. A 2021, 9, 26113–26118. [Google Scholar] [CrossRef]
- Zhao, G.; Li, P.; Rui, K.; Chen, Y.; Dou, S.X.; Sun, W. CoSe2/MoSe2 Heterostructures with Enriched Water Adsorption/Dissociation Sites towards Enhanced Alkaline Hydrogen Evolution Reaction. Chem. Eur. J. 2018, 24, 11158–11165. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhang, P.; Lei, J.; Dong, W.; Wang, J. Integrated 3D MoSe2@Ni0.85Se Nanowire Network with Synergistic Cooperation as Highly Efficient Electrocatalysts for Hydrogen Evolution Reaction in Alkaline Medium. Electrochim. Acta 2017, 246, 712–719. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Ju, H.; Pan, B.; Zhu, J.; Ding, T.; Wang, C.; Yang, Q. Design and Epitaxial Growth of MoSe2–NiSe Vertical Heteronanostructures with Electronic Modulation for Enhanced Hydrogen Evolution Reaction. Chem. Mater. 2016, 28, 1838–1846. [Google Scholar] [CrossRef]
- Qin, R.; Wang, P.; Li, Z.; Zhu, J.; Cao, F.; Xu, H.; Ma, Q.; Zhang, J.; Yu, J.; Mu, S. Ru-Incorporated Nickel Diselenide Nanosheet Arrays with Accelerated Adsorption Kinetics toward Overall Water Splitting. Small 2022, 18, 2105305. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, B.; Yu, B.; Wang, B.; Hou, W.; Zhang, W.; Chen, Y. In situ synthesis of hierarchical MoSe2-CoSe2 nanotubes as an efficient electrocatalyst for the hydrogen evolution reaction in both acidic and alkaline media. J. Mater. Chem. A 2018, 6, 7842–7850. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, Y.; Huang, Y.; Zhang, C.; Liu, T. Reaction Packaging CoSe2 Nanoparticles in N-Doped Carbon Polyhedra with Bifunctionality for Overall Water Splitting. ACS Appl. Mater. Interfaces 2018, 11, 3372–3381. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, A.; Liu, Z.; Xu, Y.; Fan, B.; Tang, Q.; Zhang, S.; Deng, W.; Chen, X. Synergistic effect of three-dimensional cobalt diselenide/carbon nanotube arrays composites for enhanced hydrogen evolution reaction. Electrochim. Acta 2018, 285, 254–261. [Google Scholar] [CrossRef]
- Mao, S.; Wen, Z.; Ci, S.; Guo, X.; Ostrikov, K.K.; Chen, J. Perpendicularly Oriented MoSe2/Graphene Nanosheets as Advanced Electrocatalysts for Hydrogen Evolution. Small 2015, 11, 414–419. [Google Scholar] [CrossRef]
- Chia, X.; Pumera, M. Characteristics and performance of two-dimensional materials for electrocatalysis. Nat. Catal. 2018, 1, 909–921. [Google Scholar] [CrossRef]
- Yi, Y.; Sun, Z.; Li, C.; Tian, Z.; Lu, C.; Shao, Y.; Li, J.; Sun, J.; Liu, Z. Designing 3D Biomorphic Nitrogen-Doped MoSe2/Graphene Composites toward High-Performance Potassium-Ion Capacitors. Adv. Funct. Mater. 2019, 30, 1903878. [Google Scholar] [CrossRef]
- Wang, X.; He, J.; Yu, B.; Sun, B.; Yang, D.; Zhang, X.; Zhang, Q.; Zhang, W.; Gu, L.; Chen, Y. CoSe2 nanoparticles embedded MOF-derived Co-N-C nanoflake arrays as efficient and stable electrocatalyst for hydrogen evolution reaction. Appl. Catal. B Environ. 2019, 258, 117996. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, W.; Xiao, Y.; Shi, Z.; Cao, X.; Tang, Y.; Gao, Q. CoNiSe2 heteronanorods decorated with lay-ered-double-hydroxides for efficient hydrogen evolution. Appl. Catal. B Environ. 2019, 242, 132–139. [Google Scholar] [CrossRef]
- Hao, T.; Liu, Y.; Liu, G.; Peng, C.; Chen, B.; Feng, Y.; Ru, J.; Yang, J. Insight into faradaic mechanism of polyaniline@NiSe2 core-shell nanotubes in high-performance supercapacitors. Energy Storage Mater. 2019, 23, 225–232. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Zheng, X.; Liu, X.; Liang, Q.; Chen, Z. Rational design of MoSe2-NiSe@carbon heteronanostructures for efficient electrocatalytic hydrogen evolution in both acidic and alkaline media. Carbon 2018, 139, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Zhang, Y.; Jiang, W.; Zhang, Q.; Yang, Y.; Gu, L.; Hu, J.; Wan, L. Phase-Controlled Synthesis of 1T-MoSe2/NiSe Heterostructure Nanowire Arrays via Electronic Injection for Synergistically Enhanced Hydrogen Evolution. Small Methods 2019, 3, 1800317. [Google Scholar] [CrossRef]
- Wang, G.; Chen, W.; Chen, G.; Huang, J.; Song, C.; Chen, D.; Du, Y.; Li, C.; Ostrikov, K.K. Trimetallic Mo–Ni–Co selenides nanorod electrocatalysts for highly-efficient and ultra-stable hydrogen evolution. Nano Energy 2020, 71, 104637. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Dong, Q.; Geng, H.; Zheng, Y.; Liu, Y.; Wang, W.; Li, C.C.; Dong, X. Highly Dispersive MoP Nanoparticles Anchored on Reduced Graphene Oxide Nanosheets for an Efficient Hydrogen Evolution Reaction Electrocatalyst. ACS Appl. Mater. Interfaces 2018, 10, 26258–26263. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zheng, B.; Qi, F.; He, J.; Li, P.; Zhang, W. Few-layered WSe2 nanoflowers anchored on graphene nanosheets: A highly efficient and stable electrocatalyst for hydrogen evolution. Electrochim. Acta 2016, 222, 1293–1299. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, L.; Huang, Y.; Sun, Y.; Hu, T.; Xu, K.; Ma, F. Hydrothermal synthesis of N-doped RGO/MoSe2 composites and enhanced electro-catalytic hydrogen evolution. J. Mater. Sci. 2017, 52, 13561–13571. [Google Scholar] [CrossRef]
- Kuang, P.; Tong, T.; Fan, K.; Yu, J. In Situ Fabrication of Ni-Mo Bimetal Sulfide Hybrid as an Efficient Electrocatalyst for Hy-drogen Evolution over a Wide pH Range. ACS Catal. 2017, 7, 6179–6187. [Google Scholar] [CrossRef]
- Wu, M.; Huang, Y.; Cheng, X.; Geng, X.; Tang, Q.; You, Y.; Yu, Y.; Zhou, R.; Xu, J. Arrays of ZnSe/MoSe2 Nanotubes with Electronic Modulation as Efficient Electrocatalysts for Hydrogen Evolution Reaction. Adv. Mater. Interfaces 2017, 4, 1700948. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, K.; Wei, Z.; Li, H.; Zhang, T.; Li, X.; Cai, W.; Ma, J.; Fan, H.J.; Li, Y. Strong Electronic Interaction in Du-al-Cation-Incorporated NiSe2 Nanosheets with Lattice Distortion for Highly Efficient Overall Water Splitting. Adv. Mater. 2018, 30, 1802121. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Gong, Y.; Chen, Y.; Zhang, H.; Wang, Z.; Mao, S.; Xie, L.; Jiang, Z.; Wang, Y. Carbon vacancy defect-activated Pt cluster for hydrogen generation. J. Mater. Chem. A 2019, 7, 15364–15370. [Google Scholar] [CrossRef]
- Zhu, M.; Yan, Q.; Xue, Y.; Yan, Y.; Zhu, K.; Ye, K.; Yan, J.; Cao, D.; Xie, H.; Wang, G. Free-Standing P-Doped NiSe2/MoSe2 Catalyst for Efficient Hydrogen Evolution in Acidic and Alkaline Media. ACS Sustain. Chem. Eng. 2022, 10, 279–287. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Wang, X.; Zheng, B.; Zhang, W.; Chen, Y. Scalable synthesis of porous hollow CoSe2-MoSe2/carbon mi-crospheres for highly efficient hydrogen evolution reaction in acidic and alkaline media. J. Mater. Chem. A 2018, 6, 12701–12707. [Google Scholar] [CrossRef]
- Kadam, S.R.; Enyashin, A.N.; Houben, L.; Bar-Ziv, R.; Bar-Sadan, M. Ni-WSe2 nanostructures as efficient catalysts for electrochemical hydrogen evolution reaction (HER) in acidic and alkaline media. J. Mater. Chem. A 2020, 8, 1403–1416. [Google Scholar] [CrossRef]
- Long, A.; Li, W.; Zhou, M.; Gao, W.; Liu, B.; Wei, J.; Zhang, X.; Liu, H.; Liu, Y.; Zeng, X. MoS2 nanosheets grown on nickel chalcogenides: Controllable synthesis and electrocatalytic origins for the hydrogen evolution reaction in alkaline solution. J. Mater. Chem. A 2019, 7, 21514–21522. [Google Scholar] [CrossRef]
- Men, Y.; Li, P.; Zhou, J.; Cheng, G.; Chen, S.; Luo, W. Tailoring the Electronic Structure of Co2P by N Doping for Boosting Hydrogen Evolution Reaction at All pH Values. ACS Catal. 2019, 9, 3744–3752. [Google Scholar] [CrossRef]
- Anjum, M.A.R.; Okyay, M.S.; Kim, M.; Lee, M.H.; Park, N.; Lee, J.S. Bifunctional sulfur-doped cobalt phosphide electrocat-alyst outperforms all-noble-metal electrocatalysts in alkaline electrolyzer for overall water splitting. Nano Energy 2018, 53, 286–295. [Google Scholar] [CrossRef]
- Zhang, S.; Zhai, D.; Sun, T.; Han, A.; Zhai, Y.; Cheong, W.; Liu, Y.; Su, C.; Wang, D.; Li, Y. In situ embedding Co9S8 into nitrogen and sulfur codoped hollow porous carbon as a bifunctional electrocatalyst for oxygen reduction and hydrogen evolution reactions. Appl. Catal. B Environ. 2019, 254, 186–193. [Google Scholar] [CrossRef]
- Maity, S.; Das, B.; Samanta, M.; Das, B.K.; Ghosh, S.; Chattopadhyay, K.K. MoSe2-Amorphous CNT Hierarchical Hybrid Core–Shell Structure for Efficient Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2020, 3, 5067–5076. [Google Scholar] [CrossRef]
- Truong, Q.D.; Nakayasu, Y.; Nguyen, Q.T.; Nguyen, D.N.; Nguyen, C.T.; Devaraju, M.K.; Rangappa, D.; Nayuki, K.; Sasaki, Y.; Tran, P.D.; et al. Defect-rich exfoliated MoSe2 nanosheets by supercritical fluid process as an attractive catalyst for hydrogen evolution in water. Appl. Surf. Sci. 2020, 505, 144537. [Google Scholar] [CrossRef]
- Zimron, O.; Zilberman, T.; Kadam, S.R.; Ghosh, S.; Kolatker, S.; Neyman, A.; Bar-Ziv, R.; Bar-Sadan, M. Co-Doped MoSe2 Nanoflowers as Efficient Catalysts for Electrochemical Hydrogen Evolution Reaction (HER) in Acidic and Alkaline Media. Isr. J. Chem. 2020, 60, 624–629. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, Y.; Gao, T.; Yao, T.; Zhang, X.; Han, J.; Wang, X.; Zhang, Z.; Xu, P.; Zhang, P.; et al. Synergistic Phase and Disorder Engineering in 1T-MoSe2 Nanosheets for Enhanced Hydrogen-Evolution Reaction. Adv. Mater. 2017, 29, 1700311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, M.; Shi, Z.; Jiang, M.; Jian, W.; Xiao, Z.; Li, J.; Lee, C.; Xu, J. Composition and Interface Engineering of Alloyed MoS2xSe2(1-x) Nanotubes for Enhanced Hydrogen Evolution Reaction Activity. Small 2016, 12, 4379–4385. [Google Scholar] [CrossRef] [PubMed]
- OMeiron, E.; Kuraganti, V.; Hod, I.; Bar-Ziv, R.; Bar-Sadan, M. Improved catalytic activity of Mo1-xWxSe2 alloy nanoflowers promotes efficient hydrogen evolution reaction in both acidic and alkaline aqueous solutions. Nanoscale 2017, 9, 13998–14005. [Google Scholar] [CrossRef]
- Qu, B.; Li, C.; Zhu, C.; Wang, S.; Zhang, X.; Chen, Y. Growth of MoSe2nanosheets with small size and expanded spaces of (002) plane on the surfaces of porous N-doped carbon nanotubes for hydrogen production. Nanoscale 2016, 8, 16886–16893. [Google Scholar] [CrossRef]
- Wang, R.; Han, J.; Xu, P.; Gao, T.; Zhong, J.; Wang, X.; Zhang, X.; Li, Z.; Xu, L.; Song, B. Dual-Enhanced Doping in ReSe2 for Effi-ciently Photoenhanced Hydrogen Evolution Reaction. Adv. Sci. 2020, 7, 2000216. [Google Scholar] [CrossRef] [Green Version]
- Qu, B.; Yu, X.; Chen, Y.; Zhu, C.; Li, C.; Yin, Z.; Zhang, X. Ultrathin MoSe2 Nanosheets Decorated on Carbon Fiber Cloth as Binder-Free and High-Performance Electrocatalyst for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2015, 7, 14170–14175. [Google Scholar] [CrossRef]
- Chen, X.; Liu, G.; Zheng, W.; Feng, W.; Cao, W.; Hu, W.; Hu, P. Vertical 2D MoO2/MoSe2 Core-Shell Nanosheet Arrays as High-Performance Electrocatalysts for Hydrogen Evolution Reaction. Adv. Funct. Mater. 2016, 26, 8537–8544. [Google Scholar] [CrossRef]
- Lin, H.; Li, H.; Li, Y.; Liu, J.; Wang, X.; Wang, L. Hierarchical CoS/MoS2 and Co3S4/MoS2/Ni2P nanotubes for efficient electro-catalytic hydrogen evolution in alkaline media. J. Mater. Chem. A 2017, 5, 25410–25419. [Google Scholar] [CrossRef]
- Chouki, T.; Donkova, B.; Aktarla, B.; Stefanov, P.; Emin, S. Growth of MoSe2 electrocatalyst from metallic molybdenum nanopar-ticles for efficient hydrogen evolution. Mater. Today Commun. 2021, 26, 101976. [Google Scholar] [CrossRef]
- Poorahong, S.; Somnin, C.; Mahamadou, I.M.; Dubois, C.; Chergui, S.; Peng, Z.; Su, Y.; Tran, T.X.; Thammakhet-Buranachai, C.; Mazzah, A.; et al. Nanoporous Graphite-like Membranes Decorated with MoSe2 Nanosheets for Hydrogen Evolution. ACS Appl. Nano Mater. 2022, 5, 2769–2778. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, H.; Li, M.; Wang, Y.; Wang, D. Nanoflower-like 1T/2H mixed-phase MoSe2 as an efficient elec-trocatalyst for hydrogen evolution. J. Alloys Compd. 2021, 878, 160381. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; He, Y.; Li, H.; He, T.; Shi, H.; Ma, X.; Yang, Q.; Chen, L.; Chen, J. Self-supporting MoSe2/CoSe2@CFP electrocatalyst electrode for high-efficiency HER under alkaline solution. J. Solid State Chem. 2021, 298, 122108. [Google Scholar] [CrossRef]
- Yang, B.; Huang, Z.; Wu, H.; Hu, H.; Lin, H.; Nie, M.; Li, Q. Sea urchin-like CoSe2 nanoparticles modified graphene oxide as an efficient and stable hydrogen evolution catalyst. J. Electroanal. Chem. 2022, 907, 116037. [Google Scholar] [CrossRef]
- Li, G.; Feng, S.; Wang, C.; Deng, P.; Li, J. Co-NiSe2/NF nanosheet for efficient hydrogen evolution reaction. Catal. Commun. 2022, 165, 106443. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).