Palladium and Graphene Oxide Doped ZnO for Aqueous Acetamiprid Degradation under Visible Light
Abstract
:1. Introduction
2. Results and Discussion
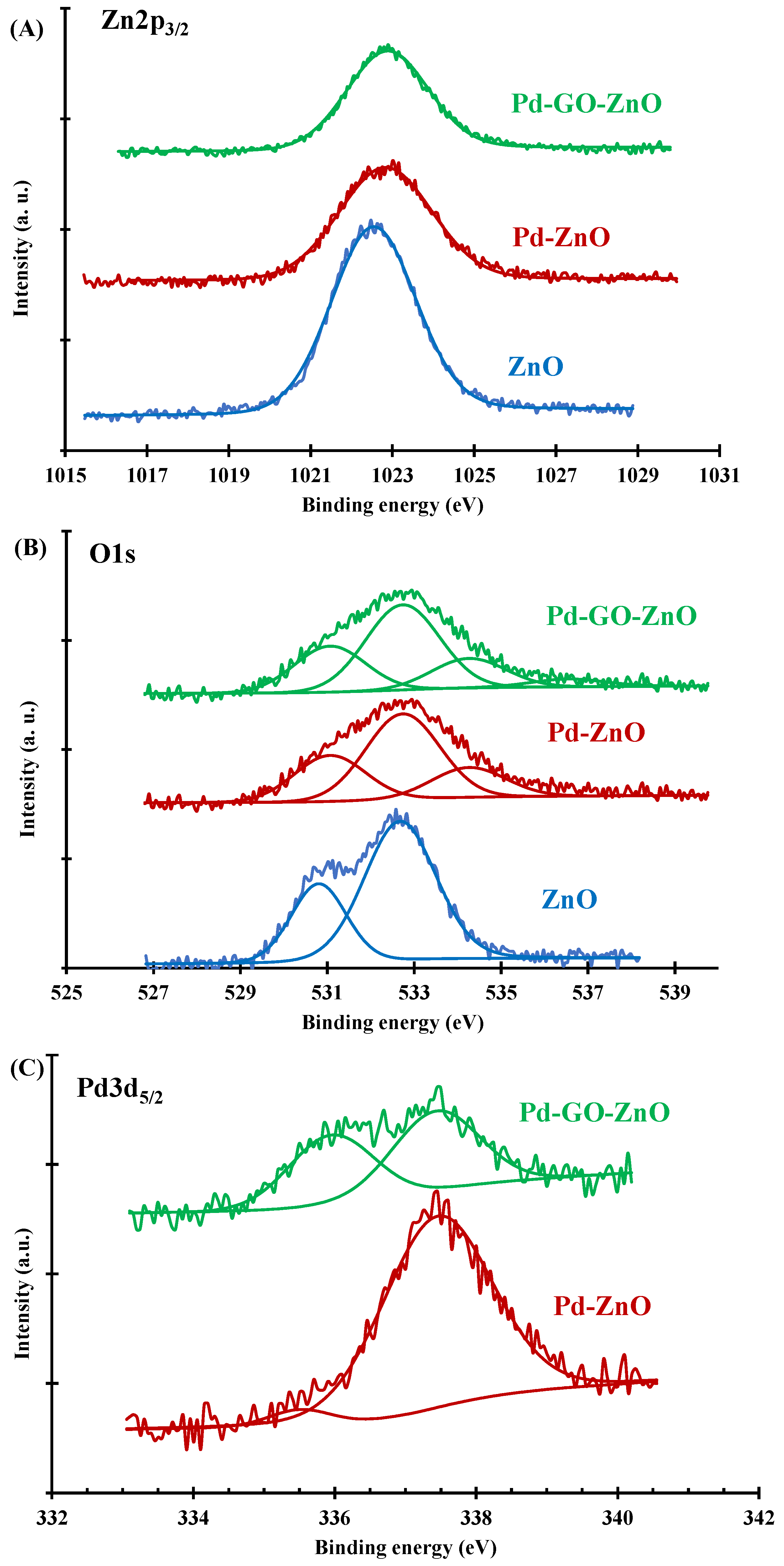
2.1. Photocatalysts Characterization
2.2. Photocatalytic Photodegradation of Aqueous Acetamiprid
2.3. Photocatalyst Stability
3. Experimental Methods
3.1. Materials
3.2. Synthesis of ZnO and Pd-ZnO Materials
3.3. Synthesis of the Pd-GO-ZnO Material
3.4. Photocatalysts Characterization
3.5. Photocatalytic Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garrido, I.; Flores, P.; Hellín, P.; Vela, N.; Navarro, S.; Fenoll, J. Solar reclamation of agro-wastewater polluted with eight pesticides by heterogeneous photocatalysis using a modular facility. A case study. Chemosphere 2020, 249, 126156. [Google Scholar] [CrossRef]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid contamination of global surfacewaters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Kanjilal, T.; Bhattacharjee, C.; Datta, S. Biodegradation of acetamiprid from wetland wastewater using indigenous Micrococcus luteus strain SC 1204: Optimization, evaluation of kinetic parameter and toxicity. J. Water Proc. Eng. 2015, 6, 21–31. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gusain, R.; Gupta, K.; Joshi, P.; Khatri, O.P. Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: A comprehensive review. Adv. Colloid Interf. Sci. 2019, 272, 102009. [Google Scholar] [CrossRef]
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; El Aatik, A.; Garrido, I.; Pérez-Lucas, G.; Navarro, S. Removal of Pesticides with Endocrine Disruptor Activity in Wastewater Effluent by Solar Heterogeneous Photocatalysis Using ZnO/Na2S2O8. Water Air Soil Poll. 2019, 230, 134. [Google Scholar] [CrossRef]
- Miyashiro, C.S. Développement de Catalyseurs Hétérogènes Pour la Photodégradation du Néonicotinoïde Acétamipride Dans L’eau. Ph.D. Thesis, Université Laval, Québec, QC, Canada, 2021. Available online: https://corpus.ulaval.ca/jspui/handle/20.500.11794/71024 (accessed on 17 June 2022).
- Júnior, O.G.; Neto, W.B.; Machado, A.E.; Daniel, D.; Trovó, A.G. Optimization of fipronil degradation by heterogeneous photocatalysis: Identification of transformation products and toxicity assessment. Water Res. 2017, 110, 133–140. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sust. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Ahn, Y.Y.; Yun, E.T.; Seo, J.W.; Lee, C.; Kim, S.H.; Kim, J.H.; Lee, J. Activation of peroxymonosulfate by surface-loaded noble metal nanoparticles for oxidative degradation of organic compounds. Environ. Sci. Technol. 2016, 50, 10187–10197. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Younesi, H.; Zinatizadeh, A.A. Kinetics and thermodynamics of photocatalytic degradation of organic pollutants in petroleum refinery wastewater over nano-TiO2 supported on Fe-ZSM-5. J. Taiwan Inst. Chem. Eng. 2016, 65, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Hasija, V.; Raizada, P.; Sudhaik, A.; Sharma, K.; Kumar, A.; Singh, P.; Thakur, V.K. Recent advances in noble metal free doped graphitic carbon nitride based nanohybrids for photocatalysis of organic contaminants in water: A review. Appl. Mater. Today 2019, 15, 494–524. [Google Scholar] [CrossRef]
- Vaiano, V.; Jaramillo-Paez, C.A.; Matarangolo, M.; Navío, J.A.; Hidalgo, M.C. UV and visible-light driven photocatalytic removal of caffeine using ZnO modified with different noble metals (Pt, Ag and Au). Mater. Res. Bull. 2019, 112, 251–260. [Google Scholar] [CrossRef]
- Verma, N.; Jagota, V.; Alguno, A.C.; Alimuddin; Rakhra, M.; Kumar, P.; Dugbakie, B.N. Characterization of fabricated gold-doped ZnO nanospheres and their use as a photocatalyst in the degradation of DR-31 dye. J. Nanomater. 2022, 2022, 7532332. [Google Scholar] [CrossRef]
- Wageh, S.; Almazroai, L.; Alshahrie, A.; Al-Ghamdi, A.A. Enhanced visible light photo-catalytic activity of ZnO and Ag-doped ZnO (ZnO:Ag) nanoparticles. J. Nanosci. Nanotechnol. 2018, 18, 7682–7690. [Google Scholar] [CrossRef]
- Kumar, R.; Rana, D.; Umar, A.; Sharma, P.; Chauhan, S.; Chauhan, M.S. Ag-doped ZnO nanoellipsoids: Potential scaffold for photocatalytic and sensing applications. Talanta 2015, 137, 204–213. [Google Scholar] [CrossRef]
- Alahmadi, N.; Amin, M.S.; Mohamed, R. Superficial visible-light-responsive Pt@ZnO nanorods photocatalysts for effective remediation of ciprofloxacin in water. J. Nanopart. Res. 2020, 22, 230. [Google Scholar] [CrossRef]
- Güy, N.; Çakar, S.; Özacar, M. Comparison of palladium/zinc oxide photocatalysts prepared by different palladium doping methods for Congo red degradation. J. Colloid Interf. Sci. 2016, 466, 128–137. [Google Scholar] [CrossRef]
- Najafidoust, A.; Abdollahi, B.; Abbasi Asl, I.; Karimi, R. Synthesis and characterization of novel M@ZnO/UiO-66 (M = Ni, Pt, Pd and mixed Pt&Pd) as an efficient photocatalyst under solar light. J. Mol. Struct. 2022, 1256, 132580. [Google Scholar]
- Li, B.; Liu, T.; Wang, Y.; Wang, Z. ZnO/graphene-oxide nanocomposite with remarkably enhanced visible-light-driven photocatalytic performance. J. Colloid Interf. Sci. 2012, 377, 114–121. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Noor, N.H.B.M.; Umar, K.; Adnan, R.; Ibrahim, M.N.M.; Rashid, M. Graphene oxide–ZnO nanocomposite: An efficient visible light photocatalyst for degradation of rhodamine B. Appl. Nanosci. 2021, 11, 1291–1302. [Google Scholar] [CrossRef]
- Raliya, R.; Avery, C.; Chakrabarti, S.; Biswas, P. Photocatalytic degradation of methyl orange dye by pristine titanium dioxide, zinc oxide, and graphene oxide nanostructures and their composites under visible light irradiation. Appl. Nanosci. 2017, 7, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Hasnidawani, J.N.; Azlina, H.N.; Norita, H.; Bonnia, N.N.; Ratim, S.; Ali, E.S. Synthesis of ZnO nanostructures using sol-gel method. Procedia Chem. 2016, 19, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Sabri, N.S.; Yahya, A.K.; Talari, M.K. Emission properties of Mn doped ZnO nanoparticles prepared by mechanochemical processing. J. Lumin. 2012, 132, 1735–1739. [Google Scholar] [CrossRef]
- Bai, X.; Li, L.; Liu, H.; Tan, L.; Liu, T.; Meng, X. Solvothermal synthesis of ZnO nanoparticles and anti-infection application in vivo. ACS Appl. Mater. Int. 2015, 7, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Sharma, S.; Kaith, B.S.; Rajput, J.; Kaur, M. Synthesis of ZnO nanoparticles using surfactant free in-air and microwave method. Appl. Surf. Sci. 2011, 257, 9661–9672. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, L.; Cheng, H.; Zhu, Y. Significantly enhanced photocatalytic performance of ZnO via graphene hybridization and the mechanism study. Appl. Catal. B 2011, 101, 382–387. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Zhang, Y.; Liu, J.; Duan, Y. Facile synthesis of hierarchical nanocomposites of aligned polyaniline nanorods on reduced graphene oxide nanosheets for microwave absorbing materials. RSC Adv. 2017, 7, 54031–54038. [Google Scholar] [CrossRef] [Green Version]
- Khurshid, F.; Jeyavelan, M.; Hudson, M.S.L.; Nagarajan, S. Ag-doped ZnO nanorods embedded reduced graphene oxide nanocomposite for photo-electrochemical applications. R. Soc. Open Sci. 2019, 6, 181764. [Google Scholar] [CrossRef] [Green Version]
- Castellazzi, P.; Groppi, G.; Forzatti, P.; Baylet, A.; Marecot, P.; Duprez, D. Role of Pd loading and dispersion on redox behaviour and CH4 combustion activity of Al2O3 supported catalysts. Catal. Today 2010, 155, 18–26. [Google Scholar] [CrossRef]
- Romeiro, F.C.; Rodrigues, M.A.; Silva, L.A.; Catto, A.C.; da Silva, L.F.; Longo, E.; Lima, R.C. rGO-ZnO nanocomposites for high electrocatalytic effect on water oxidation obtained by microwave-hydrothermal method. Appl. Surf. Sci. 2017, 423, 743–751. [Google Scholar] [CrossRef] [Green Version]
- Hosseini-Sarvari, M.; Razmi, Z.; Doroodmand, M.M. Palladium supported on zinc oxide nanoparticles: Synthesis, characterization, and application as heterogeneous catalyst for Mizoroki–Heck and Sonogashira reactions under ligand-free and air atmosphere conditions. Appl. Catal. A Gen. 2014, 475, 477–486. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, H.J. Preparation and characterization of nanocrystalline ZnO particles from a hydrothermal process. J. Nanopart. Res. 2008, 10, 401–407. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z. Facile synthesis of quasi spherical ZnO nanoparticles with excellent photocatalytic activity. J. Clust. Sci. 2015, 26, 1187–1201. [Google Scholar] [CrossRef]
- Rajeswari, R.; Prabu, H.G. Palladium–Decorated reduced graphene oxide/zinc oxide nanocomposite for enhanced antimicrobial, antioxidant and cytotoxicity activities. Proc. Biochem. 2020, 93, 36–47. [Google Scholar] [CrossRef]
- Yu, J.; Yu, X. Hydrothermal synthesis and photocatalytic activity of zinc oxide hollow spheres. Environ. Sci. Technol. 2008, 42, 4902–4907. [Google Scholar] [CrossRef]
- Zhang, J.; Suo, X.; Liu, X.; Liu, B.; Xue, Y.; Mu, L.; Shi, H. PdO loaded WO3 composite with Na2W4O13 flake: A 2-D heterostructure composite material. Mater. Lett. 2016, 184, 25–28. [Google Scholar] [CrossRef]
- Meng, L.J.; de Sá, C.P.M.; Dos Santos, M.P. Study of the structural properties of ZnO thin films by x-ray photoelectron spectroscopy. Appl. Surf. Sci. 1994, 78, 57–61. [Google Scholar] [CrossRef]
- Boulares, N.; Guergouri, K.; Zouaghi, R.; Tabet, N.; Lusson, A.; Sibieude, F.; Monty, C. Photoluminescence and photodissociation properties of pure and InO3 doped ZnO nanophases. Phys. Status Solidi 2004, 201, 2319–2328. [Google Scholar] [CrossRef]
- Weng, Y.C.; Hsiao, K.T.; Chiu, K.C.; Su, Y.F. The identification and characterization of Ptx-Zn1-xO photocatalysts for photoelectrochemical water splitting applications. Int. J. Hydrogen Energy 2016, 41, 22997–23006. [Google Scholar] [CrossRef]
- Kemache, N.; Hamoudi, S.; Arul, J.; Belkacemi, K. Activity and selectivity of nanostructured sulfur-doped Pd/SBA-15 catalyst for vegetable oil hardening. Ind. Eng. Chem. Res. 2010, 49, 971–979. [Google Scholar] [CrossRef]
- Prakhar, S.; Jitendra, K. Fabrication of Graphene-Supported Palladium Nanoparticles-Decorated Zinc Oxide Nanorods for Potential Application. J. Superconduct. Nov. Magn. 2019, 32, 721–728. [Google Scholar]
- Al-Amshany, Z.M.; Hussein, M.A. Novel Pd/ZnWO4 nanocomposite materials for photocatalytic degradation of atrazine. Appl. Nanosci. 2018, 8, 527–536. [Google Scholar] [CrossRef]
- Ali, A.; Biswas, M.R.U.D.; Oh, W.C. Novel and simple process for the photocatalytic reduction of CO2 with ternary Bi2O3–graphene–ZnO nanocomposite. J. Mater. Sci. Mater. Electron. 2018, 29, 10222–10233. [Google Scholar] [CrossRef]
- Liu, X.; Pan, L.; Lv, T.; Lu, T.; Zhu, G.; Sun, Z.; Sun, C. Microwave-assisted synthesis of ZnO–graphene composite for photocatalytic reduction of Cr (VI). Catal. Sci. Technol. 2011, 1, 1189–1193. [Google Scholar] [CrossRef]
- Seddigi, Z.S.; Ahmed, S.A.; Bumajdad, A.; Danish, E.Y.; Shawky, A.M.; Gondal, M.A.; Soylak, M. The efficient photocatalytic degradation of methyl tert-butyl ether under Pd/ZnO and visible light irradiation. Photochem. Photobiol. 2015, 91, 265–271. [Google Scholar] [CrossRef]
- Umukoro, E.H.; Madyibi, S.S.; Peleyeju, M.G.; Tshwenya, L.; Viljoen, E.H.; Ngila, J.C.; Arotiba, O.A. Photocatalytic application of Pd-ZnO-exfoliated graphite nanocomposite for the enhanced removal of acid orange 7 dye in water. Solid State Sci. 2017, 74, 118–124. [Google Scholar] [CrossRef]
- Andrade, M.B.; Santos, T.R.; Silva, M.F.; Vieira, M.F.; Bergamasco, R.; Hamoudi, S. Graphene oxide impregnated with iron oxide nanoparticles for the removal of atrazine from the aqueous medium. Sep. Sci. Technol. 2019, 54, 2653–2670. [Google Scholar] [CrossRef]
- Yousaf, S.; Kousar, T.; Taj, M.B.; Agboola, P.O.; Shakir, I.; Warsi, M.F. Synthesis and characterization of double heterojunction-graphene nano-hybrids for photocatalytic applications. Ceram. Int. 2019, 45, 17806–17817. [Google Scholar] [CrossRef]
- Gengenbach, T.R.; Major, G.H.; Linford, M.R.; Easton, C.D. Practical guides for x-ray photoelectron spectroscopy (XPS): Interpreting the carbon 1s spectrum. J. Vac. Sci. Technol. A 2021, 39, 013204. [Google Scholar] [CrossRef]
- Berens, M.J.; Capel, P.D.; Arnold, W.A. Neonicotinoid Insecticides in Surface Water, Groundwater, and Wastewater Across Land-Use Gradients and Potential Effects. Environ. Toxicol. Chem. 2021, 40, 1017–1033. [Google Scholar] [CrossRef] [PubMed]
- Zoumenou, B.G.Y.M.; Aïna, M.P.; Imorou Toko, I.; Igout, A.; Douny, C.; Brose, F.; Schiffers, B.; Gouda, I.; Sika, K.C.; Kestemont, P.; et al. Occurrence of Acetamiprid Residues in Water Reservoirs in the Cotton Basin of Northern Benin. Bull. Environ. Contam. Toxicol. 2019, 102, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Suganthi, A.; Bhuvaneswari, K.; Ramya, M. Determination of neonicotinoid insecticide residues in sugarcane juice using LC-MS-MS. Food Chem. 2018, 241, 275–280. [Google Scholar] [CrossRef] [PubMed]

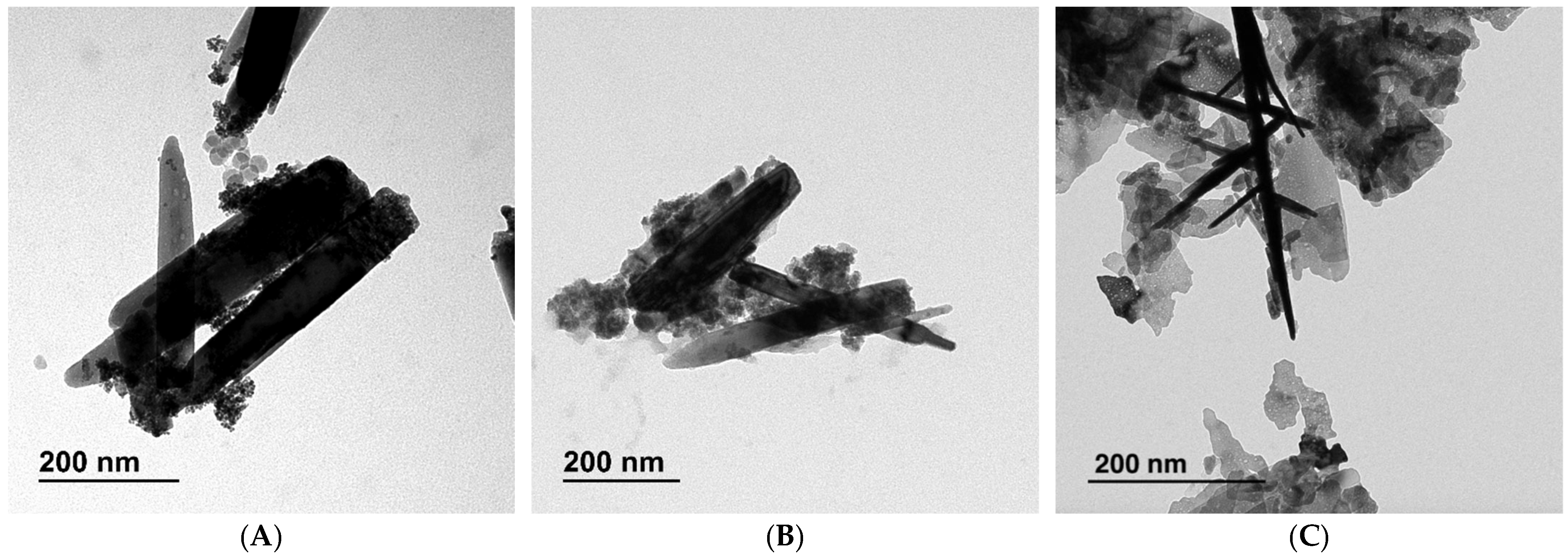
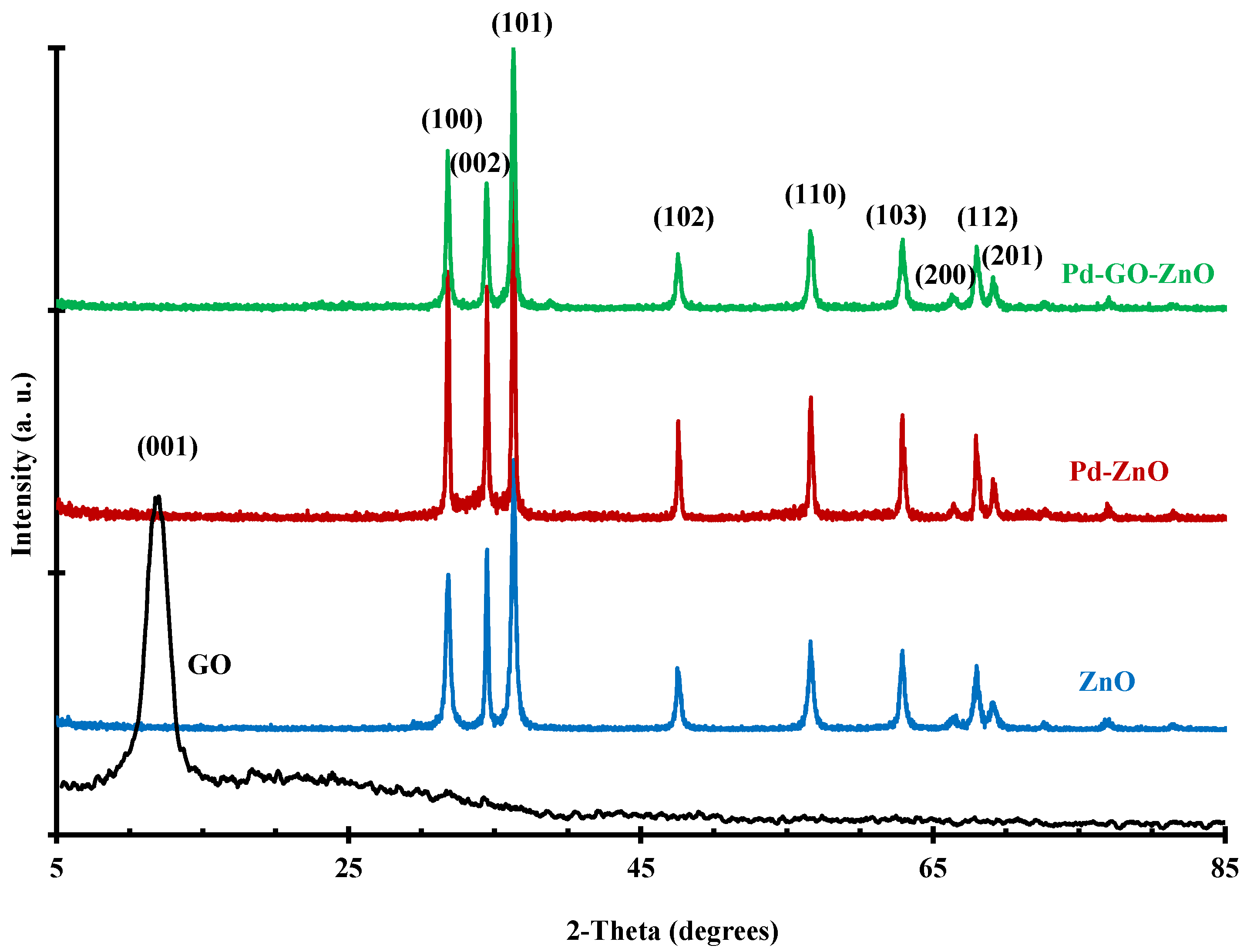
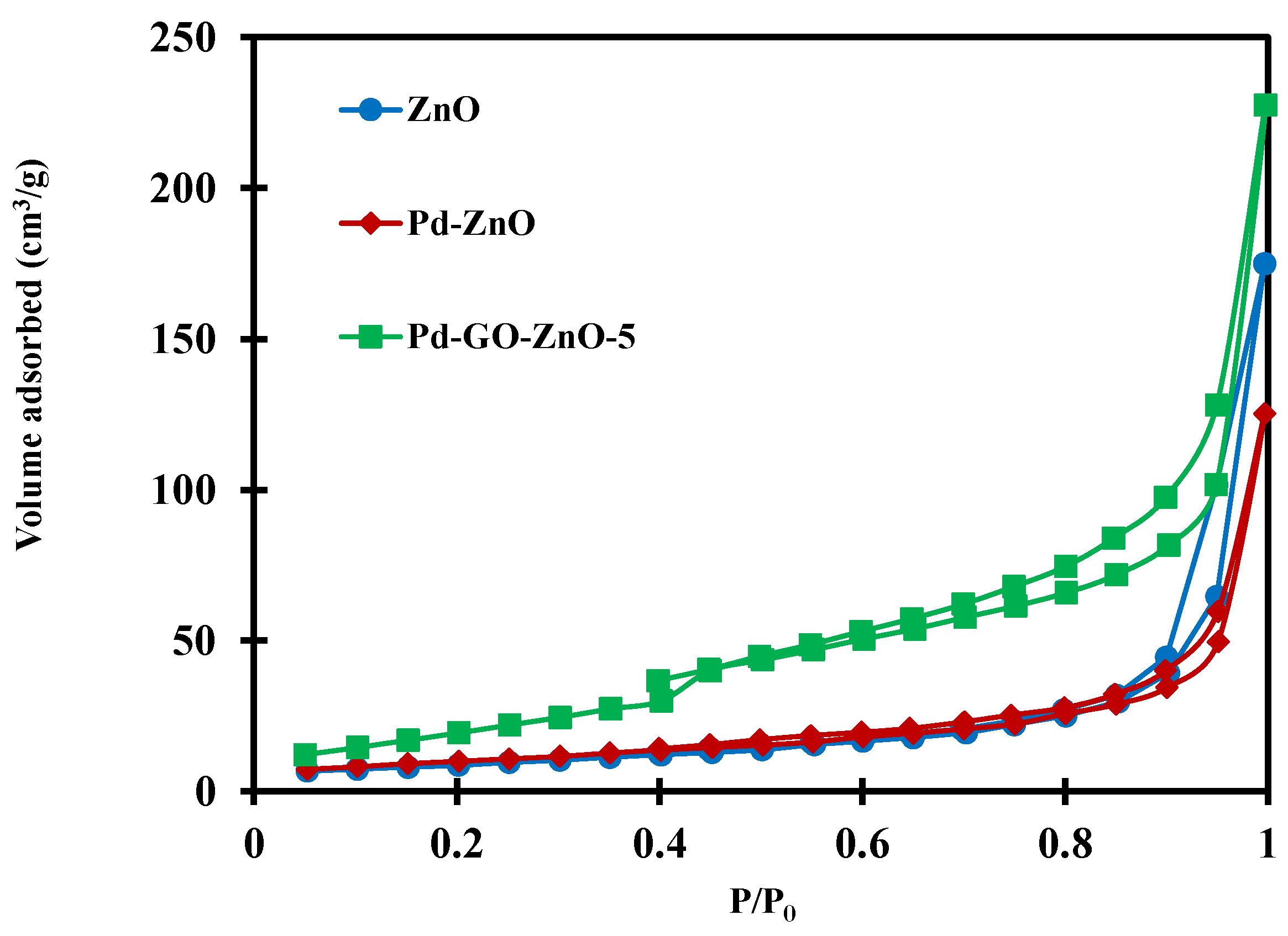

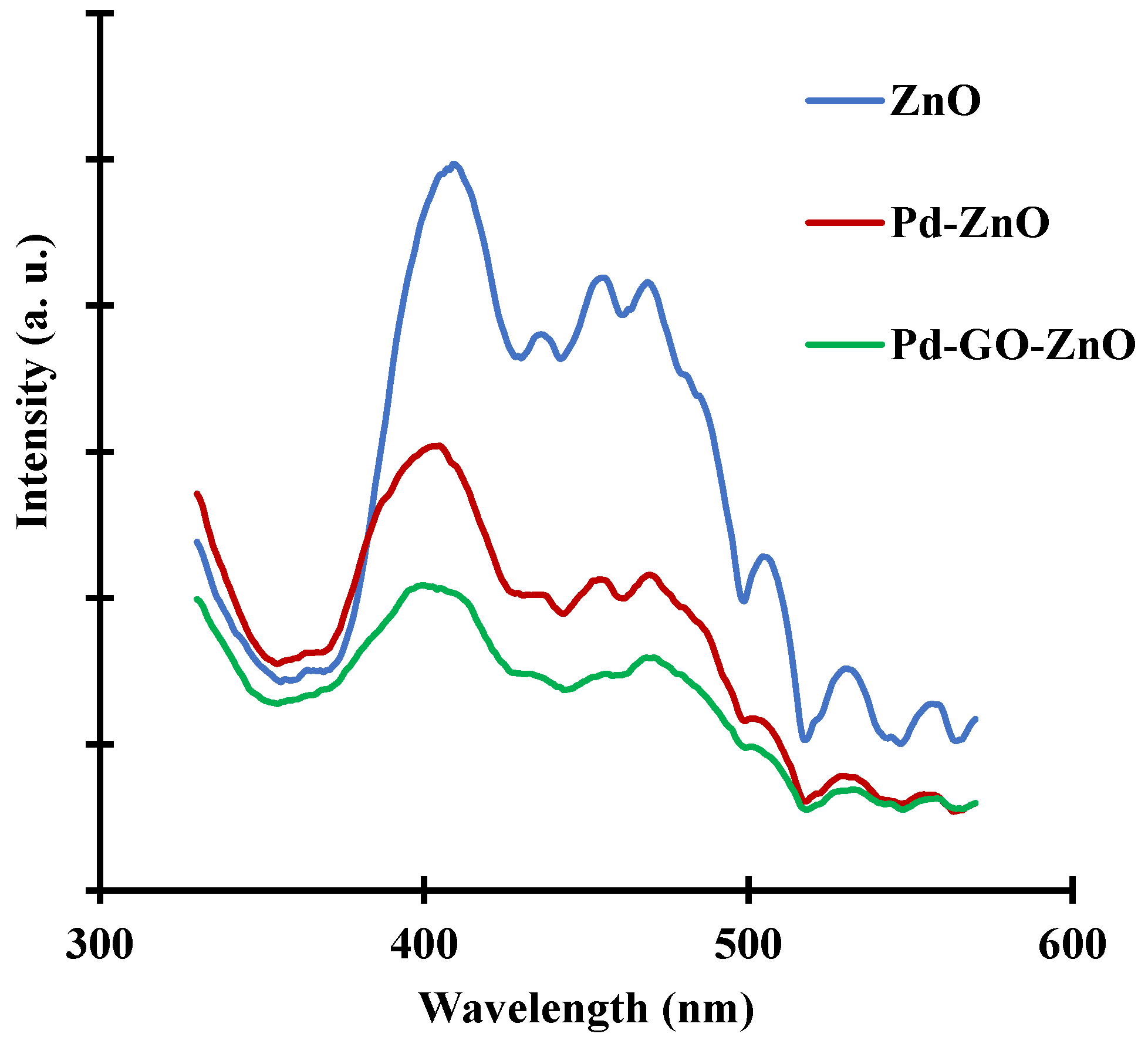



| Material | Surface Area (m2/g) | Pore Volume (mL/g) | Average Pore Size (nm) |
|---|---|---|---|
| ZnO | 30.90 | 0.27 | 6.38 |
| Pd-ZnO | 36.27 | 0.19 | 8.81 |
| Pd-GO-ZnO | 49.52 | 0.18 | 9.53 |
| Chemical Formula | C₁₀H₁₁ClN₄ |
|---|---|
| Chemical structure | 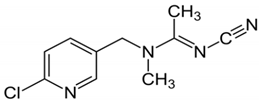 |
| Melting point (°C) | 98.9 |
| Density (g/mL) | 1.33 |
| Solubility in water at 25 °C (mg/L) | 4.25 × 103 |
| Octanol/water partition coefficient (KOW) | 6.27 |
| pKa at 25 °C | 0.7 |
| Vapor pressure at 20 °C (mPa) | 1.73 × 10−4 |
| Volatility | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayury Miyashiro, C.; Hamoudi, S. Palladium and Graphene Oxide Doped ZnO for Aqueous Acetamiprid Degradation under Visible Light. Catalysts 2022, 12, 709. https://doi.org/10.3390/catal12070709
Sayury Miyashiro C, Hamoudi S. Palladium and Graphene Oxide Doped ZnO for Aqueous Acetamiprid Degradation under Visible Light. Catalysts. 2022; 12(7):709. https://doi.org/10.3390/catal12070709
Chicago/Turabian StyleSayury Miyashiro, Carolina, and Safia Hamoudi. 2022. "Palladium and Graphene Oxide Doped ZnO for Aqueous Acetamiprid Degradation under Visible Light" Catalysts 12, no. 7: 709. https://doi.org/10.3390/catal12070709
APA StyleSayury Miyashiro, C., & Hamoudi, S. (2022). Palladium and Graphene Oxide Doped ZnO for Aqueous Acetamiprid Degradation under Visible Light. Catalysts, 12(7), 709. https://doi.org/10.3390/catal12070709






