Evaluating CO2 Desorption Activity of Tri-Solvent MEA + EAE + AMP with Various Commercial Solid Acid Catalysts
Abstract
:1. Introduction
2. Theory
2.1. The Coordinative Effects within MEA vs. EAE
2.2. The Mechanism of Catalytic CO2 Desorption
2.3. The Average Desorption Rate, Heat Duty and Desorption Factor of CO2 Desorption
3. Results and Discussion
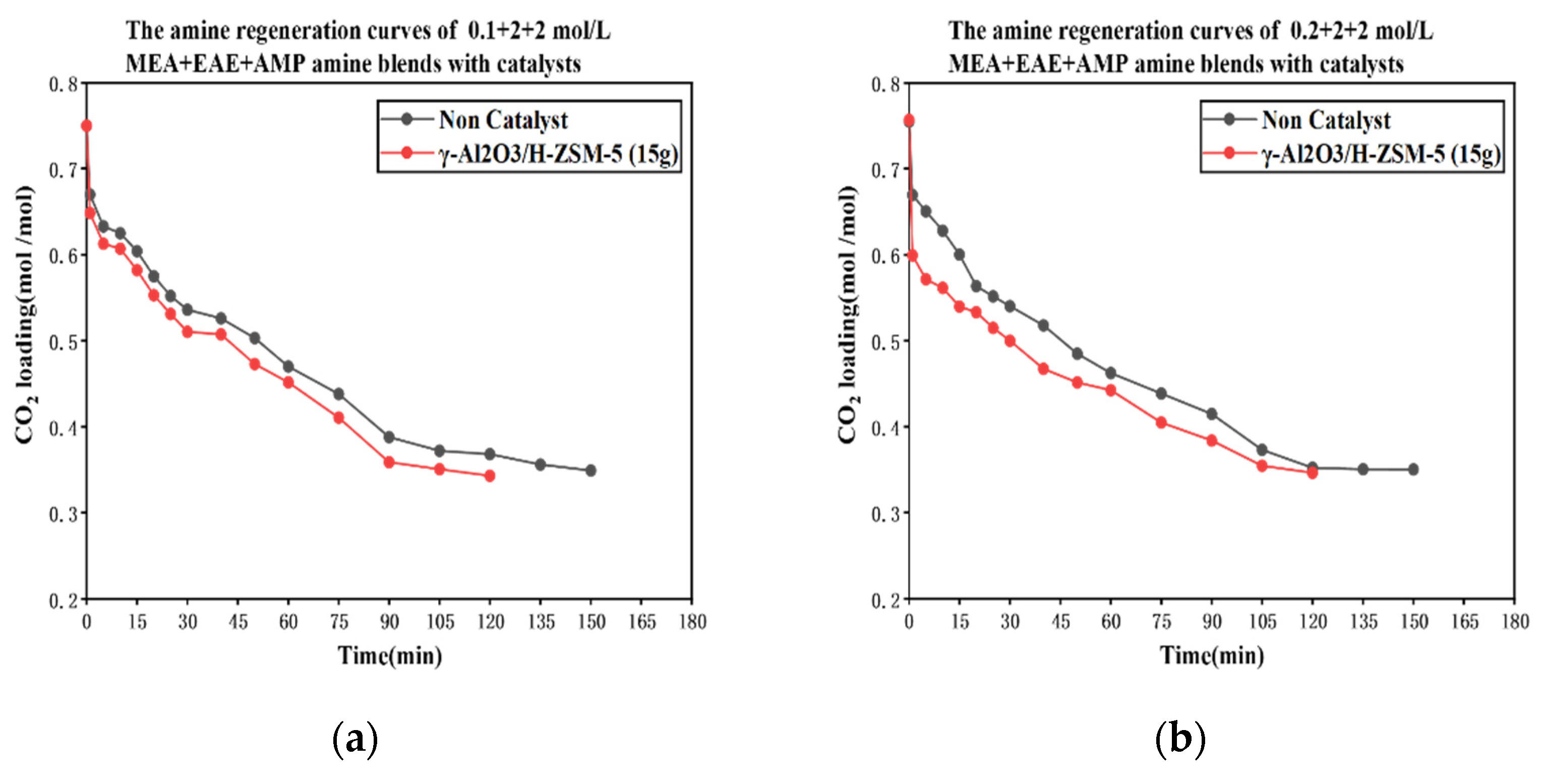
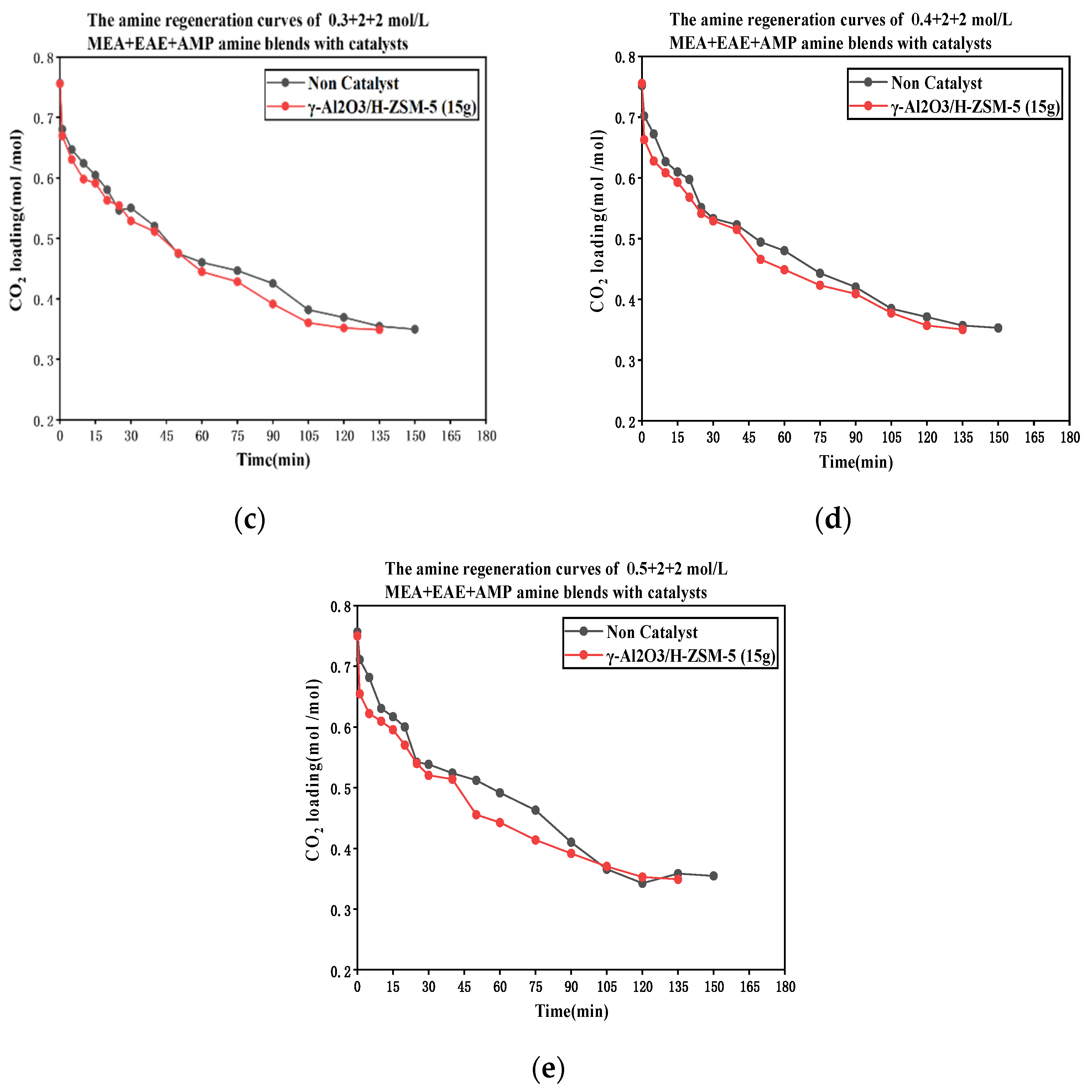
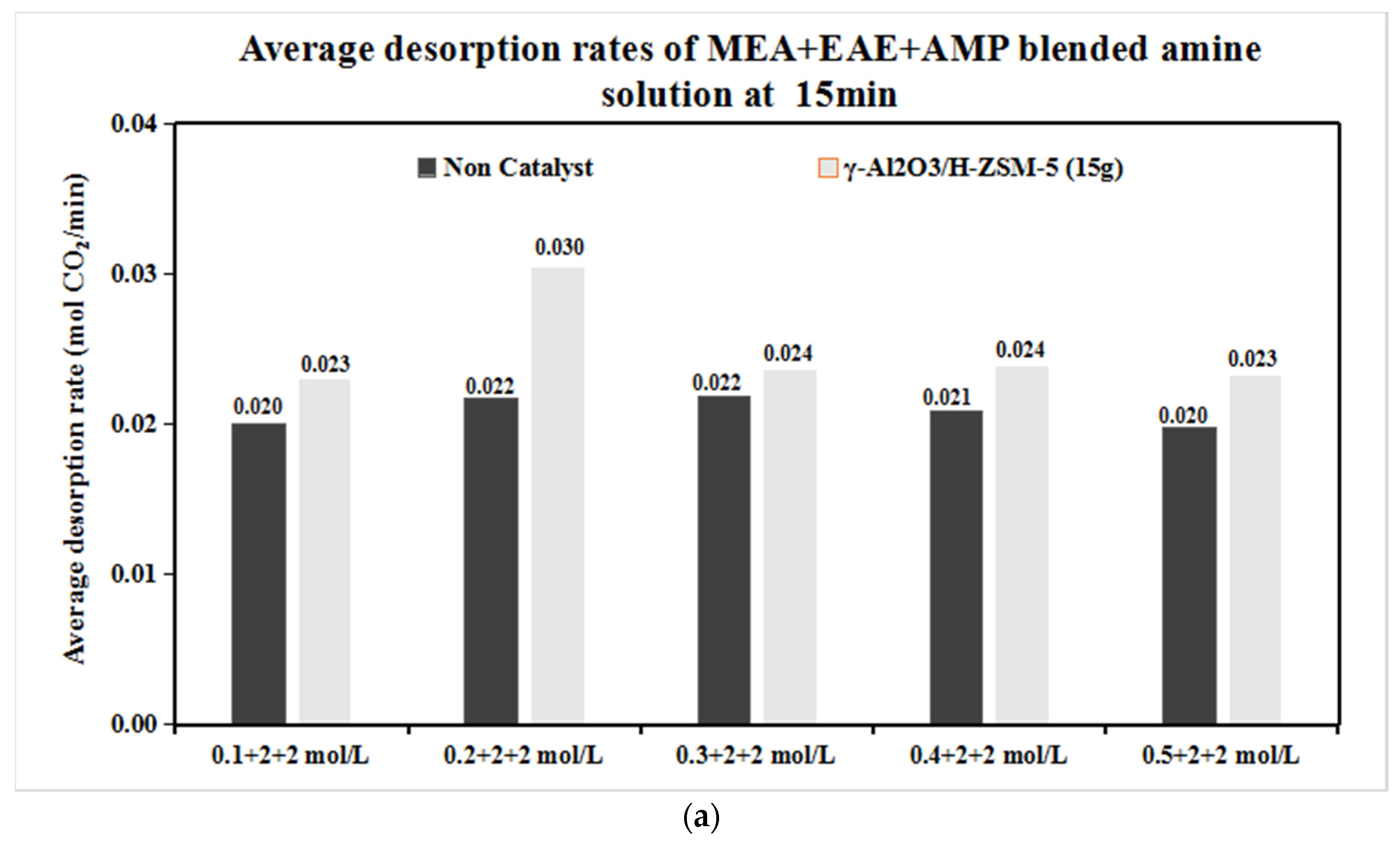
3.1. CO2 Desorption of Tri-Solvents MEA + EAE + AMP with Blended γ-Al2O3/HZSM-5 Catalyst
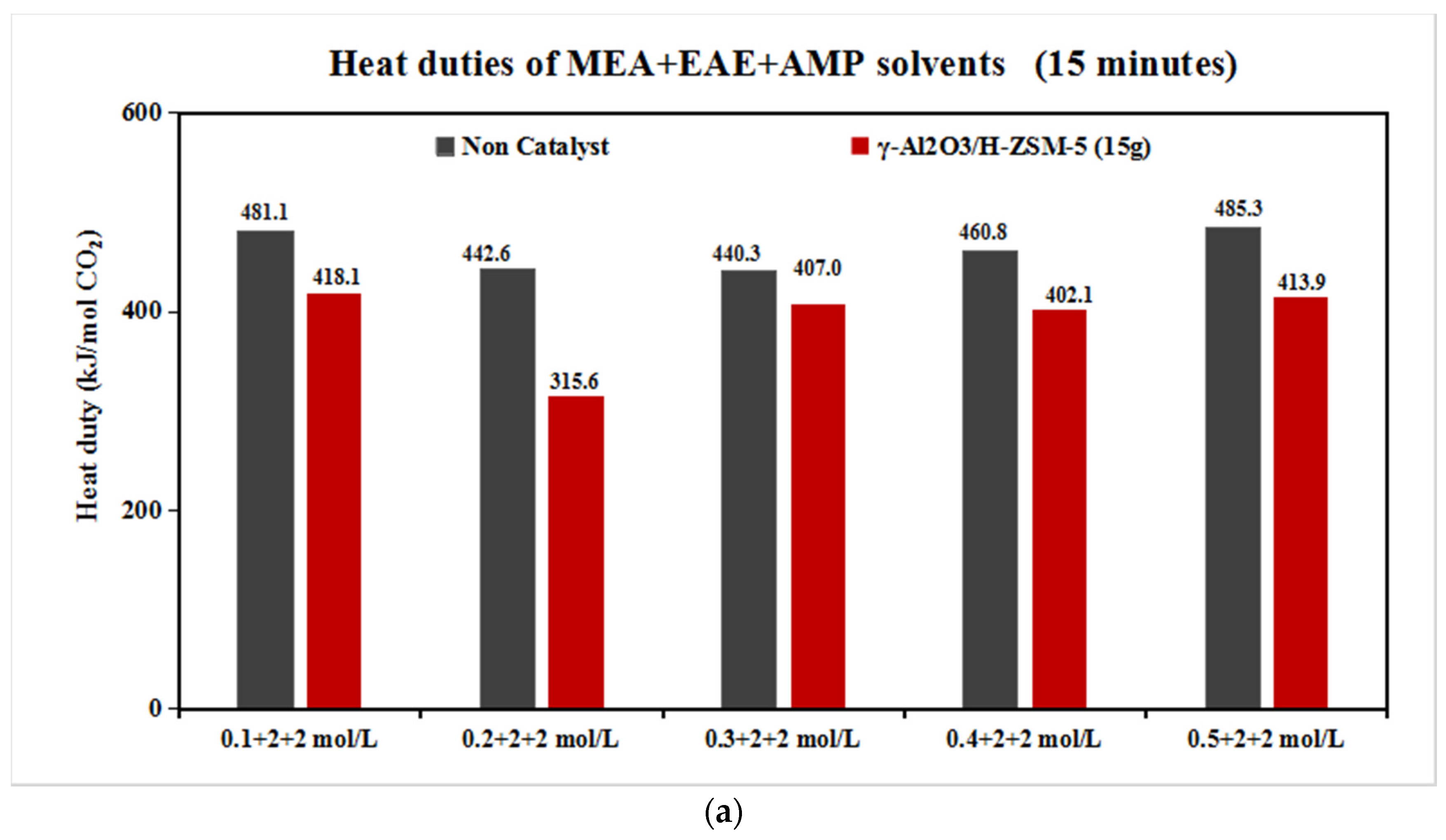
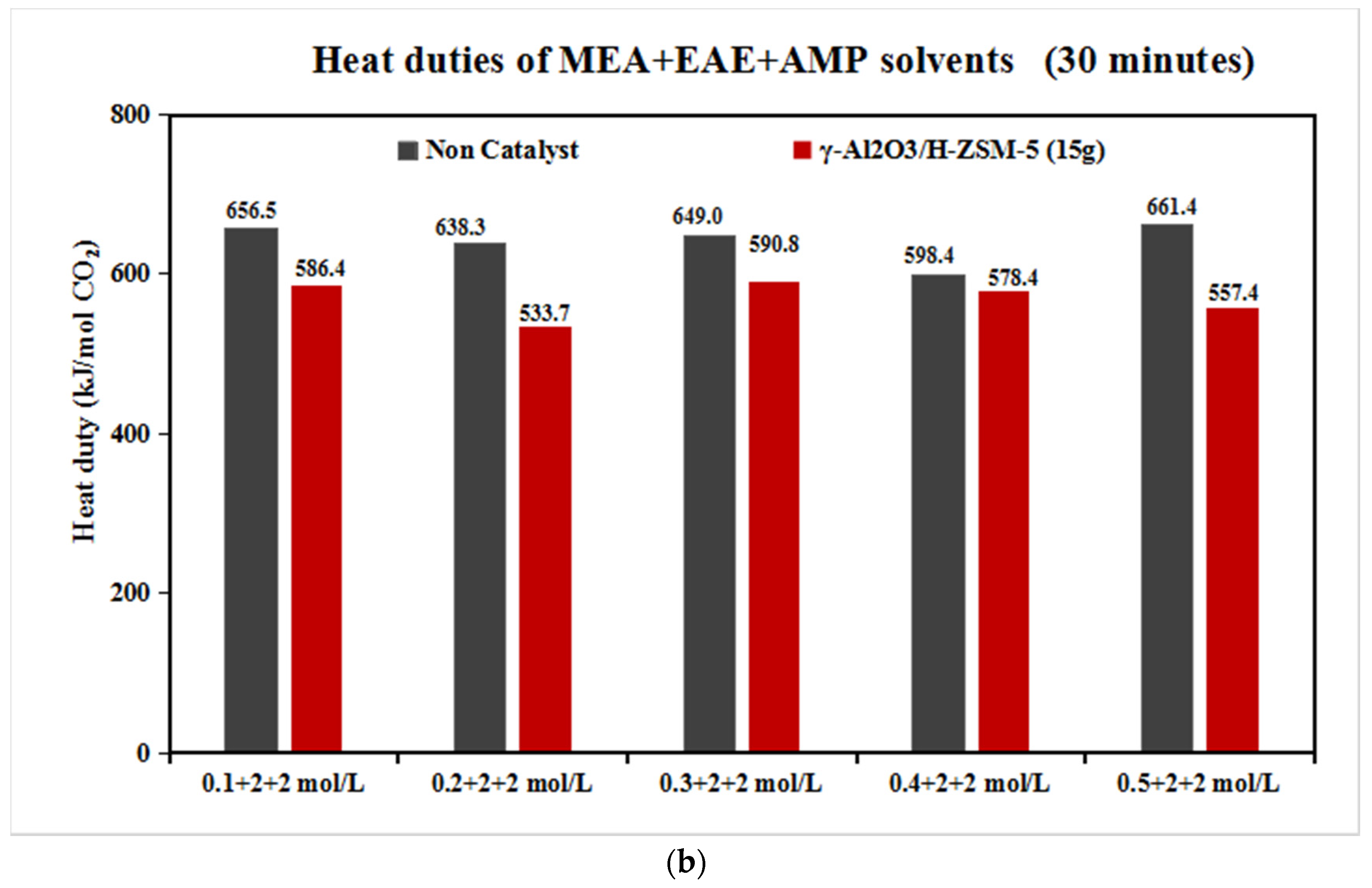
The Catalytic CO2 Desorption of MEA + EAE + AMP with Direct Heating
3.2. Catalytic CO2 Desorption of Tri-Solvents with Temperature Programming
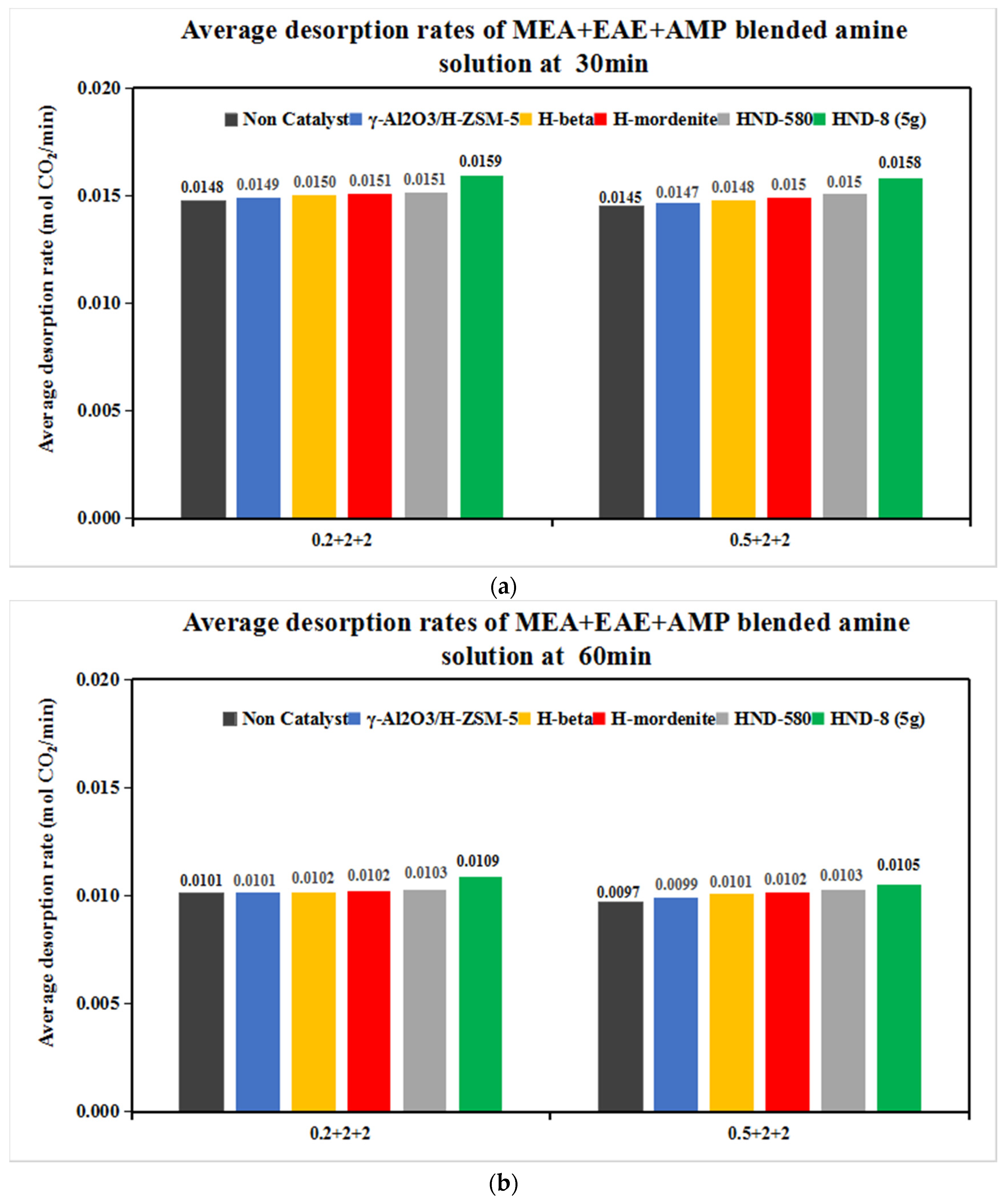
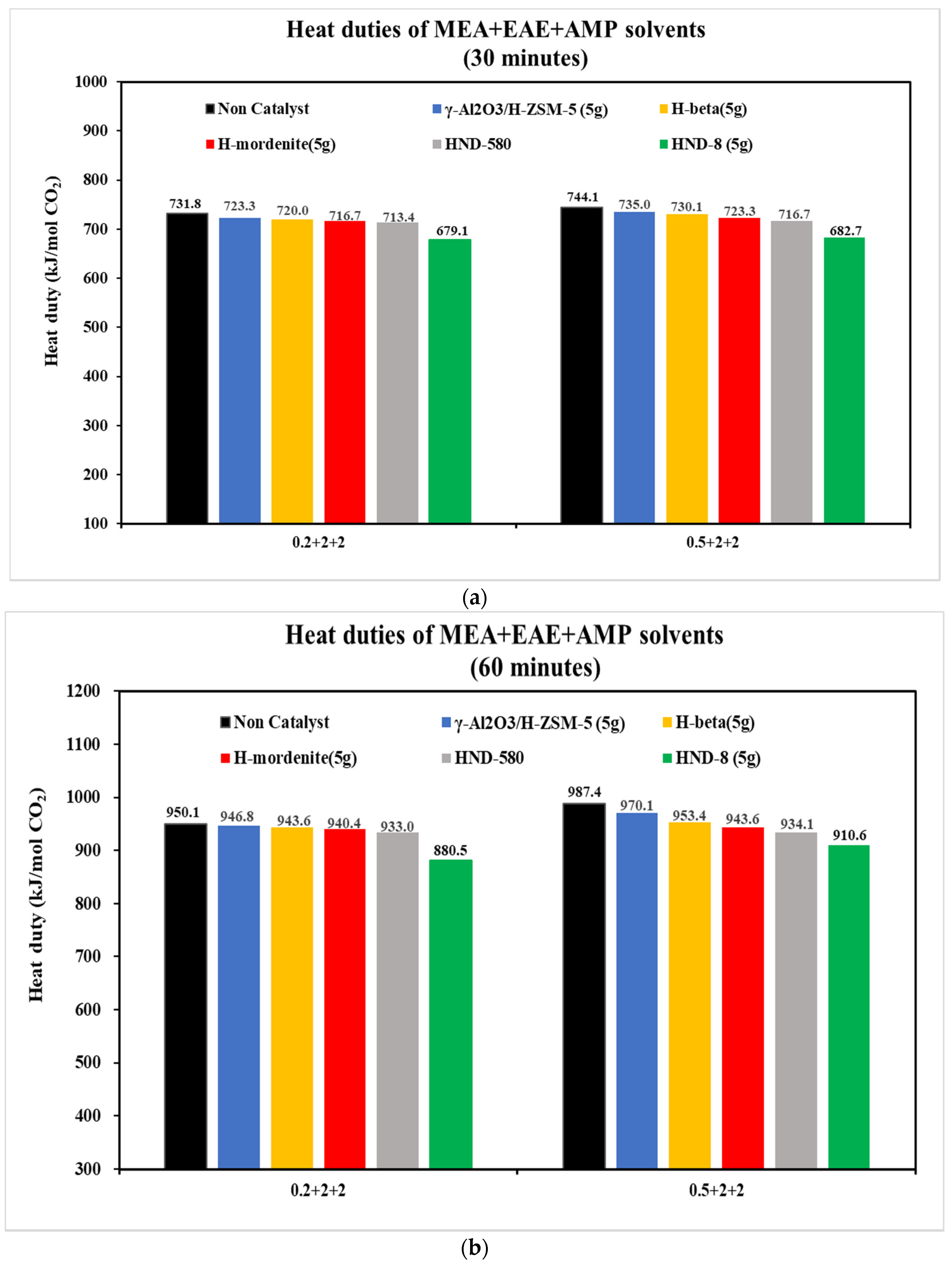
3.2.1. The CO2 Desorption of 0.2 + 2 + 2 and 0.5 + 2 + 2 mol/L Solvents with Five Catalysts
3.2.2. The CO2 Desorption of 0.3 + 1.5 + 2.5 and 0.2 + 1 + 3 mol/L Solvents with Catalysts
3.3. The Optimized Combination of Tri-Solvent MEA + EAE + AMP with Catalysts at Four Different Concentrations
3.4. The Structure–Activity Correlations of Various Catalysts
4. Materials and Methods
4.1. Chemicals, Solid Acid Catalysts and CO2 Loading Tests
4.2. Experimental Procedures for CO2 Desorption for Temperature Programming
5. Conclusions
- (1)
- The coordinative effect of MEA and EAE indicated that 0.2 + 2 + 2 mol/L was the best among 0.1–0.5 + 2 + 2 mol/L scenarios with blended catalyst γ-Al2O3/H-ZSM-5.
- (2)
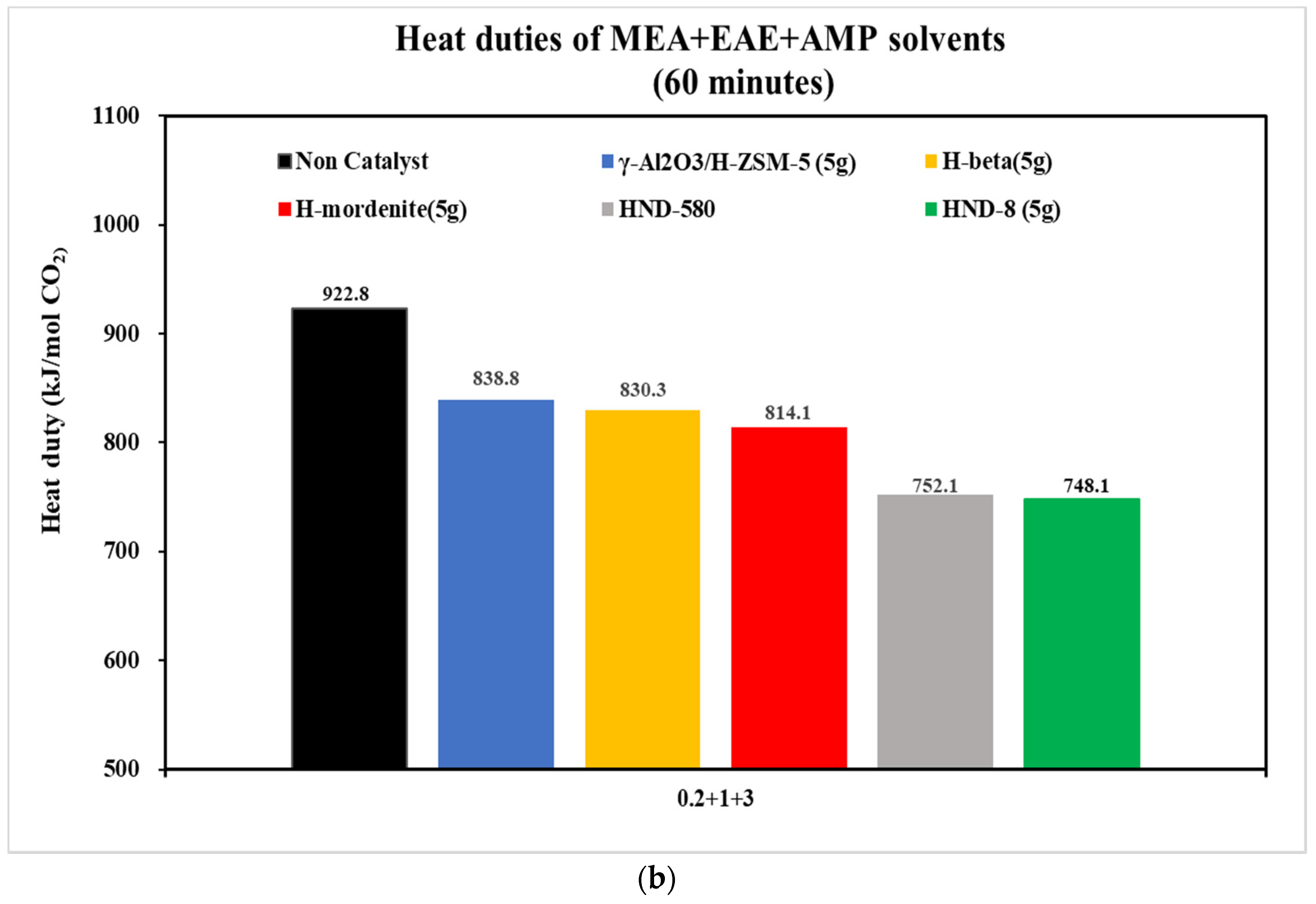
- With the aid of five commercial solid acid catalysts, the tri-solvents at various concentrations (0.2 + 1 + 3, 0.2 + 2 + 2, 0.5 + 2 + 2 and 0.3 + 1.5 + 2.5 mol/L) were consistent in the order of heat duty performance: blank > γ-Al2O3/H-ZSM-5 > H-mordenite > Hβ > HND-580 > HND-8. The lower, the better. HND-8 was the best solid acid catalyst due to its super acidity by strength. The structure–activity correlations of these catalysts await further studies.
- (3)
- With HND-8 as an energy efficient catalyst, the order of DF with various amine concentrations was: 0.2 + 1 + 3 > 0.2 + 2 + 2 > 0.5 + 2 + 2 > 0.3 + 1.5 + 2.5. The bigger, the better. The optimized desorption condition was 0.2+1+3 MEA+EAE+AMP with HND-8 at 95 °C. This combination of 0.2 + 1 + 3 + HND-8 + 95 °C is quite applicable for industrial CO2 capture processes.
- (4)
- The order of HD with different ratios of EAE/AMP was: 1/3 > 1/1 > 1.5/2.5. Generally, higher AMP concentration resulted in better desorption performance. The abnormal case happened when EAE/AMP was 1.5/2.5, which resulted from its lower operation temperature with inadequate heat input, which led to much less CO2 desorption.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| CA:L | concentration of solute A in the bulk liquid (k mol/m3) (mol/L) |
| DF | Desorption Factor |
| HD | Heat duty |
| P | Total system pressure (kPa) |
| Qinput | Heat input |
Greek Symbols
| A | CO2 loading (mol CO2/mol amine) |
| αeq | CO2 loading of solution in equilibrium with PCO2 |
Abbreviation
| AMP | 2-amino-2-methyl-1-propanol |
| BEA | Butylethanol amine |
| DEA | Diethanol amine |
| DEEA | (N, N-diethylethanolamine |
| EAE | 2-(ethylamino)ethanol |
| MEA | monoethanol amine |
| PZ | Piperizine |
References
- Rochelle, G. Amine Scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-combustion CO2 capture with chemical absorption: A state-of-the-art review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef] [Green Version]
- Gelowitz, D.; Supap, T.; Abdulaziz, N.; Sema, T.; Idem, R.; Tontiwachwuthikul, P. Part 8: Post-combustion CO2 capture: Pilot plant operation issues. Carbon Manag. 2013, 4, 215–231. [Google Scholar] [CrossRef]
- Idem, R.; Supap, T.; Shi, H.; Gelowitz, D.; Ball, M.; Campbell, C.; Tontiwachwuthikul, P. Practical experience in post-combustion CO2 capture using reactive solvents in large pilot and demonstration plants. Int. J. Greenh. Gas Control 2015, 40, 6–25. [Google Scholar] [CrossRef]
- Liang, Z.; Rongwong, W.; Liu, H.; Fu, K.; Gao, H.; Cao, F.; Zhang, R.; Sema, T.; Henni, A.; Sumon, K.Z.; et al. Recent progress and new developments in post-combustion carbon-capture technology with amine based solvents. Int. J. Greenh. Gas Control 2015, 40, 26–54. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Keener, T.C. A review: Desorption of CO2 from rich solutions in chemical absorption processes. Int. J. Greenh. Gas Control 2016, 51, 290–304. [Google Scholar] [CrossRef]
- Liang, Z.; Fu, K.; Idem, R.; Tontiwachwuthikul, P. Review on current advances, future challenges and consideration issues for post-combustion CO2 capture using amine-based absorbents. Chin. J. Chem. Eng. 2016, 24, 278–288. [Google Scholar] [CrossRef]
- Bernhardsen, I.M.; Knuutila, H.K. A review of potential amine solvents for CO2 absorption process: Absorption capacity, cyclic capacity and pKa. Int. J. Greenh. Gas Control 2017, 61, 27–48. [Google Scholar] [CrossRef]
- Zebardasti, A.; Dekamin, M.G.; Doustkhah, E.; Assadi, M.H.N. Carba-mate-Isocyanurate-Bridged Periodic Mesoporous Organosilica for van der Waals CO2 Capture. Inorg. Chem. 2020, 59, 11223–11227. [Google Scholar] [CrossRef]
- Sakwattanapong, R.; Amornvadee, A.; Veawab, A. Behavior of Reboiler heat duty for CO2 capture plants using re-generable single and blended alkanolamines. Ind. Eng. Chem. Res. 2005, 44, 4465–4473. [Google Scholar] [CrossRef]
- Idem, R.; Wilson, M.; Tontiwachwuthikul, P.; Chakma, A.; Veawab, A.; Aroonwilas, A.; Gelowitz, D. Pilot Plant Studies of the CO2 Capture Performance of Aqueous MEA and Mixed MEA/MDEA Solvents at the University of Regina CO2 Capture Technology Development Plant and the Boundary Dam CO2 Capture Demonstration Plant. Ind. Eng. Chem. Res. 2005, 45, 2414–2420. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- Sim, K.; Lee, N.; Kim, J.; Cho, E.-B.; Gunathilake, C.; Jaroniec, M. CO2 Adsorption on Amine-Functionalized Periodic Mesoporous Benzenesilicas. ACS Appl. Mater. Interfaces 2015, 7, 6792–6802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hong, J.; Liu, H.; Luo, X.; Olson, W.; Tontiwachwuthikul, P.; Liang, Z. SO42−/ZrO2 supported on γ-Al2O3 as a catalyst for CO2 desorption from CO2-loaded monoethanolamine solutions. AIChE J. 2018, 64, 3988–4001. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Liang, Z.; Idem, R.; Tontiwachwuthikul, P.; Al-Marri, M.J.; Benamor, A. Reducing energy consumption of CO2 desorption in CO2-loaded aqueous amine solution using Al2O3/HZSM-5 bifunctional catalysts. Appl. Energy 2018, 229, 562–576. [Google Scholar] [CrossRef]
- Alivand, M.S.; Mazaheri, O.; Wu, Y.; Stevens, G.W.; Scholes, C.A.; Mumford, K.A. Catalytic Solvent Regeneration for Ener-gy-Efficient CO2 Capture. ACS Sustain. Chem. Eng. 2020, 8, 18755–18788. [Google Scholar] [CrossRef]
- Nwaoha, C.; Saiwan, C.; Supap, T.; Idem, R.; Tontiwachwuthikul, P.; Rongwong, W.; Al-Marri, M.J.; Benamor, A. Carbon dioxide (CO2) capture performance of aqueous tri-solvent blends containing 2-amino-2-methyl-1-propanol (AMP) and methyldi-ethanolamine (MDEA) promoted by diethylenetriamine (DETA). Int. J. Greenh. Gas Control 2016, 53, 292–304. [Google Scholar] [CrossRef]
- Nwaoha, C.; Saiwan, C.; Tontiwachwuthikul, P.; Supap, T.; Rongwong, W.; Idem, R.; Al-Marri, M.J.; Benamor, A. Carbon dioxide (CO2) capture: Absorption-desorption capabilities of 2-amino-2-methyl-1-propanol (AMP), piperazine (PZ) and monoeth-anolamine (MEA) tri-solvent blends. J. Nat. Gas Sci. Eng. 2016, 33, 742–750. [Google Scholar] [CrossRef]
- Nwaoha, C.S.C.; Supap, T.; Idem, R.; Tontiwachwuthikul, P.; Al-Marri, M.J.; Benamor, A. Regeneration energy analysis of aqueous tri-solvent blends containing AMP, MDEA and DETA for CO2 capture. Energy Procedia 2017, 114, 2039–2046. [Google Scholar] [CrossRef]
- Nwaoha, C.; Idem, R.; Supap, T.; Saiwan, C.; Tontiwachwuthikul, P.; Rongwong, W.; Al-Marri, M.J.; Benamor, A. Heat duty, heat of absorption, sensible heat and heat of vaporization of 2–Amino–2–Methyl–1–Propanol (AMP), Piperazine (PZ) and Monoethanolamine (MEA) tri–solvent blend for carbon dioxide (CO2) capture. Chem. Eng. Sci. 2017, 170, 26–35. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, X.; Yang, Q.; Yu, H.; Liang, Z.; Luo, X. Analysis of the reduction of energy cost by using MEA-MDEA-PZ solvent for post-combustion CO2 capture (PCC). Appl. Energy 2017, 205, 1002–1011. [Google Scholar] [CrossRef]
- Nwaoha, C.; Beaulieu, M.; Tontiwachwuthikul, P.; Gibson, M.D. Techno-economic analysis of CO2 capture from a 1.2 million MTPA cement plant using AMP-PZ-MEA blend. Int. J. Greenh. Gas Control 2018, 78, 400–412. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Liu, H.; Gao, H.; Liang, Z. Evaluating CO2 desorption performance in CO2-loaded aqueous tri-solvent blend amines with and without solid acid catalysts. Appl. Energy 2018, 218, 417–429. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Gao, H.; Luo, X.; Liang, Z.; Tontiwachwuthikul, P. Zeolite catalyst-aided tri-solvent blend amine regeneration: An alternative pathway to reduce the energy consumption in amine-based CO2 capture process. Appl. Energy 2019, 240, 827–841. [Google Scholar] [CrossRef]
- Shi, H.; Cui, M.; Fu, J.; Dai, W.; Huang, M.; Han, J.; Quan, L.; Tontiwachwuthikul, P.; Liang, Z. Application of “coordinative ef-fect” into tri-solvent MEA+BEA+AMP blends at concentrations of 0.1 + 2 + 2∼0.5 + 2 + 2 mol/L with absorption, desorption and mass transfer analyses. Int. J. Greenh. Gas Control 2021, 107, 103267. [Google Scholar] [CrossRef]
- Shi, H.; Yang, X.; Feng, H.; Fu, J.; Zou, T.; Yao, J.; Wang, Z.; Jiang, L.; Tontiwachwuthikul, P. Evaluating Energy-Efficient Solu-tions of CO2 Capture within Tri-solvent MEA+BEA+AMP within 0.1 + 2 + 2–0.5 + 2 + 2 mol/L Combining Heterogeneous Acid–Base Catalysts. Ind. Eng. Chem. Res. 2021, 60, 7352–7366. [Google Scholar] [CrossRef]
- Shi, H.; Cheng, X.; Peng, J.; Feng, H.; Tontiwachwuthikul, P.; Hu, J. The CO2 absorption and desorption analysis of tri-solvent MEA + EAE + AMP compared with MEA + BEA + AMP along with “coordination effects” evaluation. Environ. Sci. Pollut. Res. 2022, 29, 40686–40700. [Google Scholar] [CrossRef]
- Muchan, P.; Saiwan, C.; Narku-Tetteh, J.; Idem, R.; Supap, T.; Tontiwachwuthikul, P. Screening tests of aqueous alkanolamine solutions based on primary, secondary, and tertiary structure for blended aqueous amine solution selection in post com-bustion CO2 capture. Chem. Eng. Sci. 2017, 170, 574–582. [Google Scholar] [CrossRef]
- Shi, H.; Naami, A.; Idem, R.; Tontiwachwuthikul, P. Catalytic and non catalytic solvent regeneration during absorption-based CO2 capture with single and blended reactive amine solvents. Int. J. Greenh. Gas Control 2014, 26, 39–50. [Google Scholar] [CrossRef]
- Liang, Z.; Idem, R.; Tontiwachwuthikul, P.; Yu, F.; Liu, H.; Rongwong, W. Experimental study on the solvent regeneration of a CO2-loaded MEA solution using single and hybrid solid acid catalysts. AIChE J. 2015, 62, 753–765. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Gao, H.; Liang, Z.; Idem, R.; Tontiwachwuthikul, P. Investigation of CO2 Regeneration in Single and Blended Amine Solvents with and without Catalyst. Ind. Eng. Chem. Res. 2017, 56, 7656–7664. [Google Scholar] [CrossRef]
- Bairq, Z.A.S.; Gao, H.; Huang, Y.; Zhang, H.; Liang, Z. Enhancing CO2 desorption performance in rich MEA solution by addi-tion of SO42-/ZrO2/SiO2 bifunctional catalyst. Appl. Energy 2019, 252, 113440. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Z.; Sun, X.; Yang, J.; Gao, H.; Huang, Y.; Luo, X.; Liang, Z.; Tontiwachwuthikul, P. Reducing Energy Penalty of CO2 Capture Using Fe Promoted SO42-/ZrO2/MCM-41 Catalyst. Environ. Sci. Technol. 2019, 53, 6094–6102. [Google Scholar] [CrossRef] [PubMed]
- Bairq, Z.A.S.; Gao, H.X.; Murshed, F.A.M.; Tontiwachwuthikul, P.; Liang, Z.W. Modified Heterogeneous Catalyst-Aided Re-generation of CO2 Capture Amines: A Promising Perspective for a Drastic Reduction in Energy Consumption. ACS Sustain. Chem. Eng. 2020, 8, 9526–9536. [Google Scholar] [CrossRef]
- Gao, H.; Huang, Y.; Zhang, X.; Bairq, Z.A.S.; Huang, Y.; Tontiwachwuthikul, P.; Liang, Z. Catalytic performance and mechanism of SO42−/ZrO2/SBA-15 catalyst for CO2 desorption in CO2-loaded monoethanolamine solution. Appl. Energy 2020, 259, 114179. [Google Scholar] [CrossRef]
- Xing, L.; Wei, K.; Li, Q.; Wang, R.; Zhang, S.; Wang, L. One-Step Synthesized SO42-/ZrO2-HZSM-5 Solid Acid Catalyst for Car-bamate Decomposition in CO2 Capture. Environ. Sci. Technol. 2020, 54, 13944–13952. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Yang, J.; Gao, H.; Huang, Y.; Luo, X.; Liang, Z.; Tontiwachwuthikul, P. Amine-based CO2 capture aided by acid-basic bifunctional catalyst: Advancement of amine regeneration using metal modified MCM-41. Chem. Eng. J. 2019, 383, 123077. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Luo, X.; Gao, H.; Bairq, Z.A.S.; Tontiwachwuthikul, P.; Liang, Z. Catalytic Performance and Mechanism of Meso–Microporous Material β-SBA-15-Supported FeZr Catalysts for CO2 Desorption in CO2-Loaded Aqueous Amine Solu-tion. Ind. Eng. Chem. Res. 2021, 60, 2698–2709. [Google Scholar] [CrossRef]
- Sun, Q.; Li, T.; Mao, Y.; Gao, H.; Sema, T.; Wang, S.; Liu, L.; Liang, Z. Reducing Heat Duty of MEA Regeneration Using a Sulfonic Acid-Functionalized Mesoporous MCM-41 Catalyst. Ind. Eng. Chem. Res. 2021, 60, 18304–18315. [Google Scholar] [CrossRef]
- Biernacki, P.; Steinigeweg, S.; Paul, W.; Brehm, A. Eco-Efficiency Analysis of Biomethane Production. Ind. Eng. Chem. Res. 2014, 53, 19594–19599. [Google Scholar] [CrossRef]
- El Hadri, N.; Quang, D.V.; Goetheer, E.L.; Abu Zahra, M.R. Aqueous amine solution characterization for post-combustion CO2 capture process. Appl. Energy 2017, 185, 1433–1449. [Google Scholar] [CrossRef]
- Gao, H.; Gao, G.; Liu, H.; Luo, X.; Liang, Z.; Idem, R.O. Density, Viscosity, and Refractive Index of Aqueous CO2-Loaded and -Unloaded Ethylaminoethanol (EAE) Solutions from 293.15 to 323.15 K for Post Combustion CO2 Capture. J. Chem. Eng. Data 2017, 62, 4205–4214. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, J.; Kim, H.; Lee, K.S. Solubility of Carbon Dioxide in Aqueous Solutions of Three Secondary Amines: 2-(Butylamino)ethanol, 2-(Isopropylamino)ethanol, and 2-(Ethylamino)ethanol Secondary Alkanolamine Solutions. J. Chem. Eng. Data 2017, 62, 2428–2435. [Google Scholar] [CrossRef]
- Liu, S.; Gao, H.; He, C.; Liang, Z. Experimental evaluation of highly efficient primary and secondary amines with lower en-ergy by a novel method for post-combustion CO2 capture. Appl. Energy 2019, 233–234, 443–452. [Google Scholar] [CrossRef]
- Gao, H.; Wang, N.; Du, J.; Luo, X.; Liang, Z. Comparative kinetics of carbon dioxide (CO2) absorption into EAE, 1DMA2P and their blends in aqueous solution using the stopped-flow technique. Int. J. Greenh. Gas Control 2020, 94, 102948. [Google Scholar] [CrossRef]
- Pandey, D.; Mondal, M.K. Thermodynamic modeling and new experimental CO2 solubility into aqueous EAE and AEEA blend, heat of absorption, cyclic absorption capacity and desorption study for post-combustion CO2 capture. Chem. Eng. J. 2021, 410, 128334. [Google Scholar] [CrossRef]
- Pandey, D.; Mondal, M.K. Experimental data and modeling for density and viscosity of carbon dioxide (CO2)-loaded and -unloaded aqueous blend of 2-(ethylamino)ethanol (EAE) and aminoethylethanolamine (AEEA) for post-combustion CO2 capture. J. Mol. Liq. 2021, 330, 115678. [Google Scholar] [CrossRef]
- Ciftja, A.F.; Hartono, A.; Svendsen, H.F. Experimental study on carbamate formation in the AMP–CO2–H2O system at differ-ent temperatures. Chem. Eng. Sci. 2014, 107, 317–327. [Google Scholar] [CrossRef]
- Hairul, N.A.H.; Shariff, A.M.; Bustam, M.A. Process behaviour in a packed absorption column for high pressure CO2 absorp-tion from natural gas using PZ + AMP blended solution. Fuel Processing Technol. 2017, 157, 20–28. [Google Scholar] [CrossRef]
- Liu, F.; Jing, G.; Zhou, X.; Lv, B.; Zhou, Z. Performance and Mechanisms of Triethylene Tetramine (TETA) and 2-Amino-2-methyl-1-propanol (AMP) in Aqueous and Nonaqueous Solutions for CO2 Capture. ACS Sustain. Chem. Eng. 2017, 6, 1352–1361. [Google Scholar] [CrossRef]
- Hassankiadeh, M.N.; Jahangiri, A. Application of aqueous blends of AMP and piperazine to the low CO2 partial pressure capturing: New experimental and theoretical analysis. Energy 2018, 165, 164–178. [Google Scholar] [CrossRef]
- Jahangiri, A.; Hassankiadeh, M.N. Effects of piperazine concentration and operating conditions on the solubility of CO2 in AMP solution at low CO2 partial pressure. Sep. Sci. Technol. 2018, 54, 1067–1078. [Google Scholar] [CrossRef]
- Nwaoha, C.; Tontiwachwuthikul, P.; Benamor, A. A comparative study of novel activated AMP using 1,5-diamino-2-methylpentane vs MEA solution for CO2 capture from gas-fired power plant. Fuel 2018, 234, 1089–1098. [Google Scholar] [CrossRef]
- Nwaoha, C.; Tontiwachwuthikul, P.; Benamor, A. CO2 capture from lime kiln using AMP-DA2MP amine solvent blend: A pilot plant study. J. Environ. Chem. Eng. 2018, 6, 7102–7110. [Google Scholar] [CrossRef]
- Pakzad, P.; Mofarahi, M.; Izadpanah, A.A.; Afkhamipour, M.; Lee, C.-H. An experimental and modeling study of CO2 solubili-ty in a 2-amino-2-methyl-1-propanol (AMP) + N-methyl-2-pyrrolidone (NMP) solution. Chem. Eng. Sci. 2018, 175, 365–376. [Google Scholar] [CrossRef]
- Wai, S.K.; Nwaoha, C.; Saiwan, C.; Idem, R.; Supap, T. Absorption heat, solubility, absorption and desorption rates, cyclic ca-pacity, heat duty, and absorption kinetic modeling of AMP–DETA blend for post–combustion CO2 capture. Sep. Purif. Technol. 2018, 194, 89–95. [Google Scholar] [CrossRef]
- Shi, H.; Zheng, L.; Huang, M.; Zuo, Y.; Li, M.; Jiang, L.; Idem, R. Tontiwachwuthikul, CO2 desorption tests of blended mo-noethanolamine-diethanolamine solutions to discover novel energy efficient solvents. Asia-Pac. J. Chem. Eng. 2018, 13, e2186. [Google Scholar] [CrossRef]
- Shi, H.; Feng, H.; Yang, X.; Zou, T.; Tontiwachwuthikul, P.; Jiang, L. Study of “coordinative effect” within bi-blended amine MEA + AMP and MEA + BEA at 0.1 + 2–0.5 + 2 mol/L with absorption–desorption parameter analyses. Asia-Pac. J. Chem. Eng. 2021, 16, e2645. [Google Scholar] [CrossRef]
- Shi, H.; Cheng, X.; Peng, J.; Feng, H.; Yang, X.; Quan, L.; Jiang, L.; Tontiwachwuthikul, P. Structure–Activity Correlation Analyses of MEA + 3A1P/MAE Isomers with a Coordinative Effect Study. Ind. Eng. Chem. Res. 2022, 61, 3091–3103. [Google Scholar] [CrossRef]
- Shi, H.; Zheng, L.; Huang, M.; Zuo, Y.; Kang, S.; Huang, Y.; Idem, R.; Tontiwachwuthikul, P. Catalytic-CO2-Desorption Studies of DEA and DEA–MEA Blended Solutions with the Aid of Lewis and Brønsted Acids. Ind. Eng. Chem. Res. 2018, 57, 11505–11516. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, H.; Gao, H.; Xiao, M.; Luo, X.; Idem, R.; Tontiwachwuthikul, P.; Liang, Z. Kinetics and mechanism study of homo-geneous reaction of CO2 and blends of diethanolamine and monoethanolamine using the stopped-flow technique. Chem.-Cal. Eng. J. 2017, 316, 592–600. [Google Scholar] [CrossRef]
- Liu, H.; Li, M.; Luo, X.; Liang, Z.; Idem, R.; Tontiwachwuthikul, P. Investigation mechanism of DEA as an activator on aqueous MEA solution for postcombustion CO2 capture. AICHE J. 2018, 64, 2515–2525. [Google Scholar] [CrossRef]
- Shi, H.; Fu, J.; Wu, Q.; Huang, M.; Jiang, L.; Cui, M.; Idem, R.; Tontiwachwuthikul, P. Studies of the coordination effect of DEA-MEA blended amines (within 1 + 4 to 2 + 3 M) under heterogeneous catalysis by means of absorption and desorption parameters. Sep. Purif. Technol. 2019, 236, 116179. [Google Scholar] [CrossRef]
- Shi, H.; Peng, J.; Cheng, X.; Yang, X.; Jin, J.; Hu, J. The CO2 desorption analysis of tri-solvent MEA + BEA + DEEA with several commercial solid acid catalysts. Int. J. Greenh. Gas Control 2022, 116, 103647. [Google Scholar] [CrossRef]
- Idem, R.; Shi, H.; Gelowitz, D.; Tontiwachwuthikul, P. Catalytic Method and Apparatus for Separating a Gaseous Component from an Incoming Gas Stream; W.I.P. Organization: Regina, SK, Canada, 2011. [Google Scholar]
- Zhao, B.; Liu, F.; Cui, Z.; Liu, C.; Yue, H.; Tang, S.; Liu, Y.; Lu, H.; Liang, B. Enhancing the energetic efficiency of MDEA/PZ-based CO2 capture technology for a 650 MW power plant: Process improvement. Appl. Energy 2017, 185, 362–375. [Google Scholar] [CrossRef]
- Akachuku, A.; Osei, P.A.; Decardi-Nelson, B.; Srisang, W.; Pouryousefi, F.; Ibrahim, H.; Idem, R. Experimental and kinetic study of the catalytic desorption of CO2 from CO2-loaded monoethanolamine (MEA) and blended monoethanolamine—Me-thyl-diethanolamine (MEA-MDEA) solutions. Energy 2019, 179, 475–489. [Google Scholar] [CrossRef]
- Afari, D.B.; Coker, J.; Narku-Tetteh, J.; Idem, R. Comparative Kinetic Studies of Solid Absorber Catalyst (K/MgO) and Solid Desorber Catalyst (HZSM-5)-Aided CO2 Absorption and Desorption from Aqueous Solutions of MEA and Blended Solu-tions of BEA-AMP and MEA-MDEA. Ind. Eng. Chem. Res. 2018, 57, 15824–15839. [Google Scholar] [CrossRef]
- Narku-Tetteh, J.; Afari, D.B.; Coker, J.; Idem, R. Evaluation of the Roles of Absorber and Desorber Catalysts in the Heat Duty and Heat of CO2 Desorption from BEA-AMP and MEA-MDEA Solvent Blends in a Bench-Scale CO2 Capture Pilot Plant. Energy Fuels 2018, 32, 9711–9726. [Google Scholar] [CrossRef]
- Natewong, P.; Prasongthum, N.; Reubroycharoen, P.; Idem, R.O. Evaluating the CO2 Capture Performance Using a BEA-AMP Biblend Amine Solvent with Novel High-Performing Absorber and Desorber Catalysts in a Bench-Scale CO2 Capture Pilot Plant. Energy Fuels 2019, 33, 3390–3402. [Google Scholar] [CrossRef]
- Prasongthum, N.; Natewong, P.; Reubroycharoen, P.; Idem, R. Solvent Regeneration of a CO2-Loaded BEA–AMP Bi-Blend Amine Solvent with the Aid of a Solid Brønsted Ce(SO4)2/ZrO2 Superacid Catalyst. Energy Fuels 2019, 33, 1334–1343. [Google Scholar] [CrossRef]
- Horwitz, W. Association of Official Analytical Chemists (AOAC) Methods; George Banta Co.: Menasha, WI, USA, 1975. [Google Scholar]
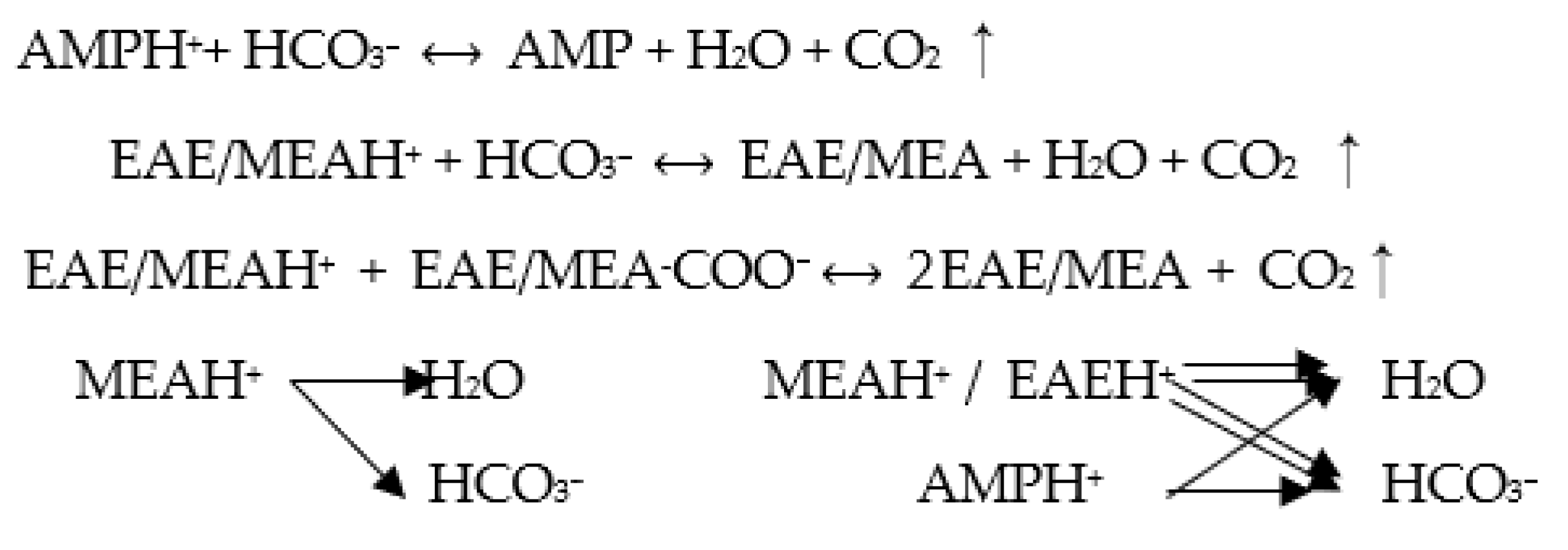



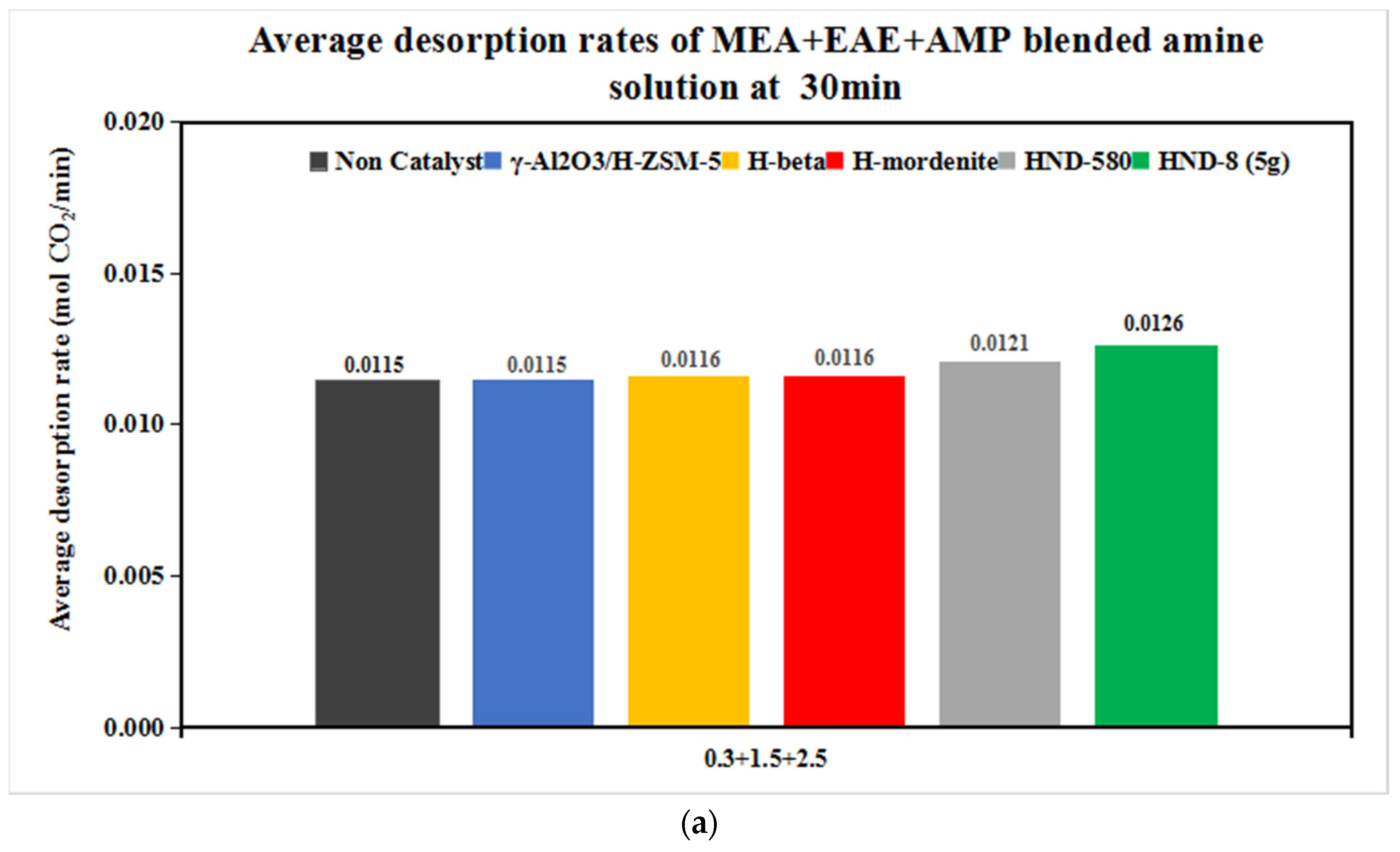
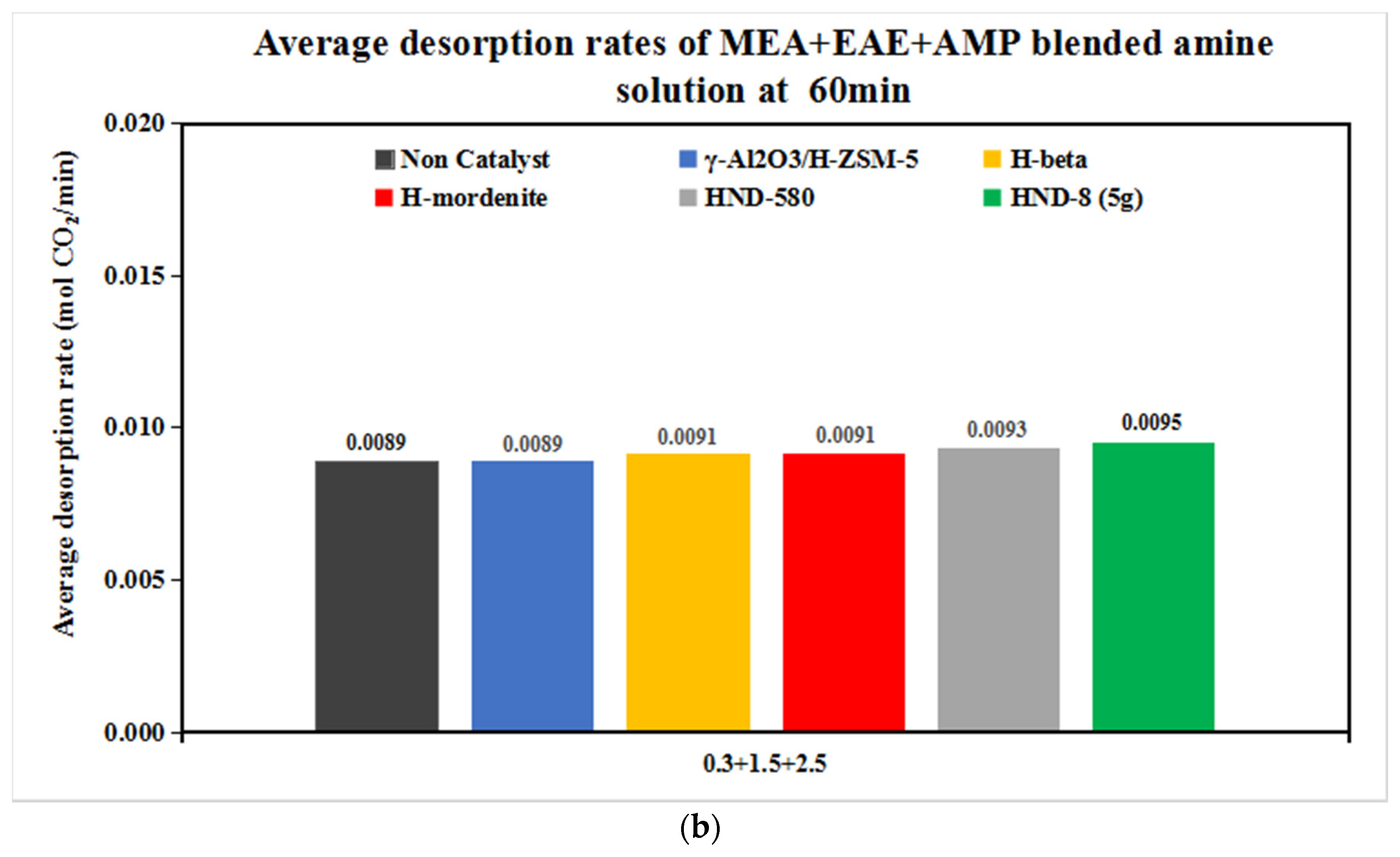
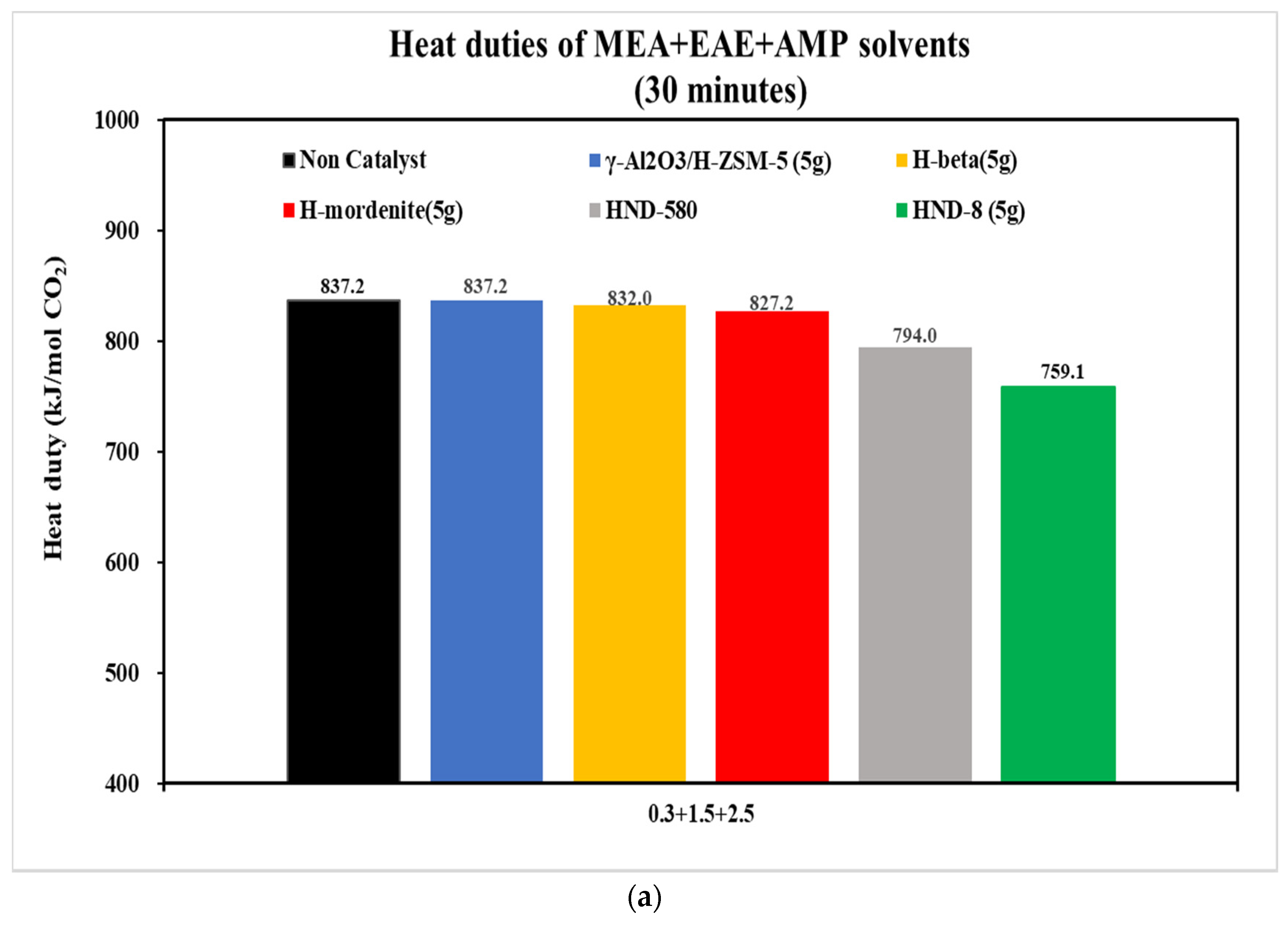
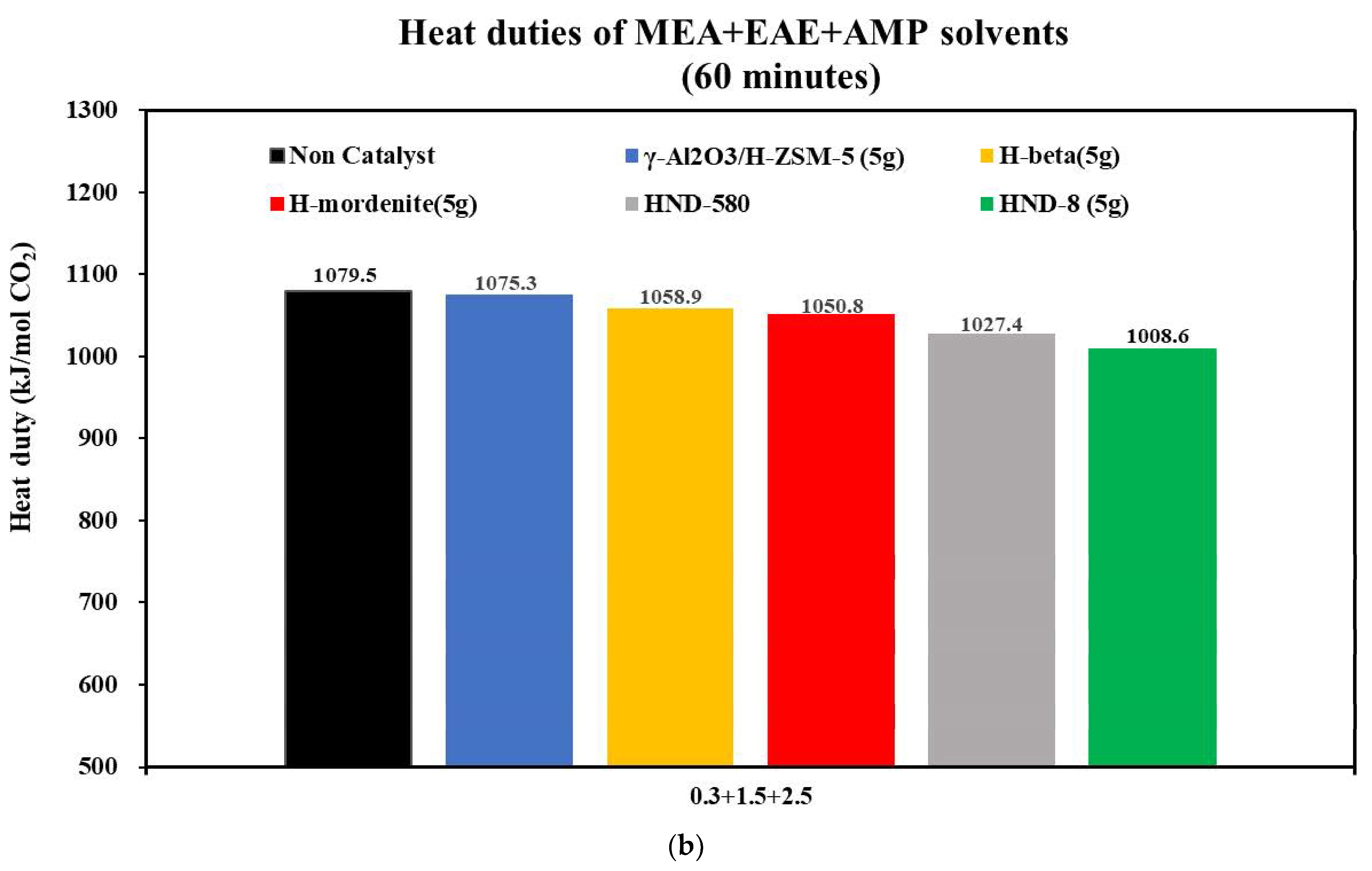
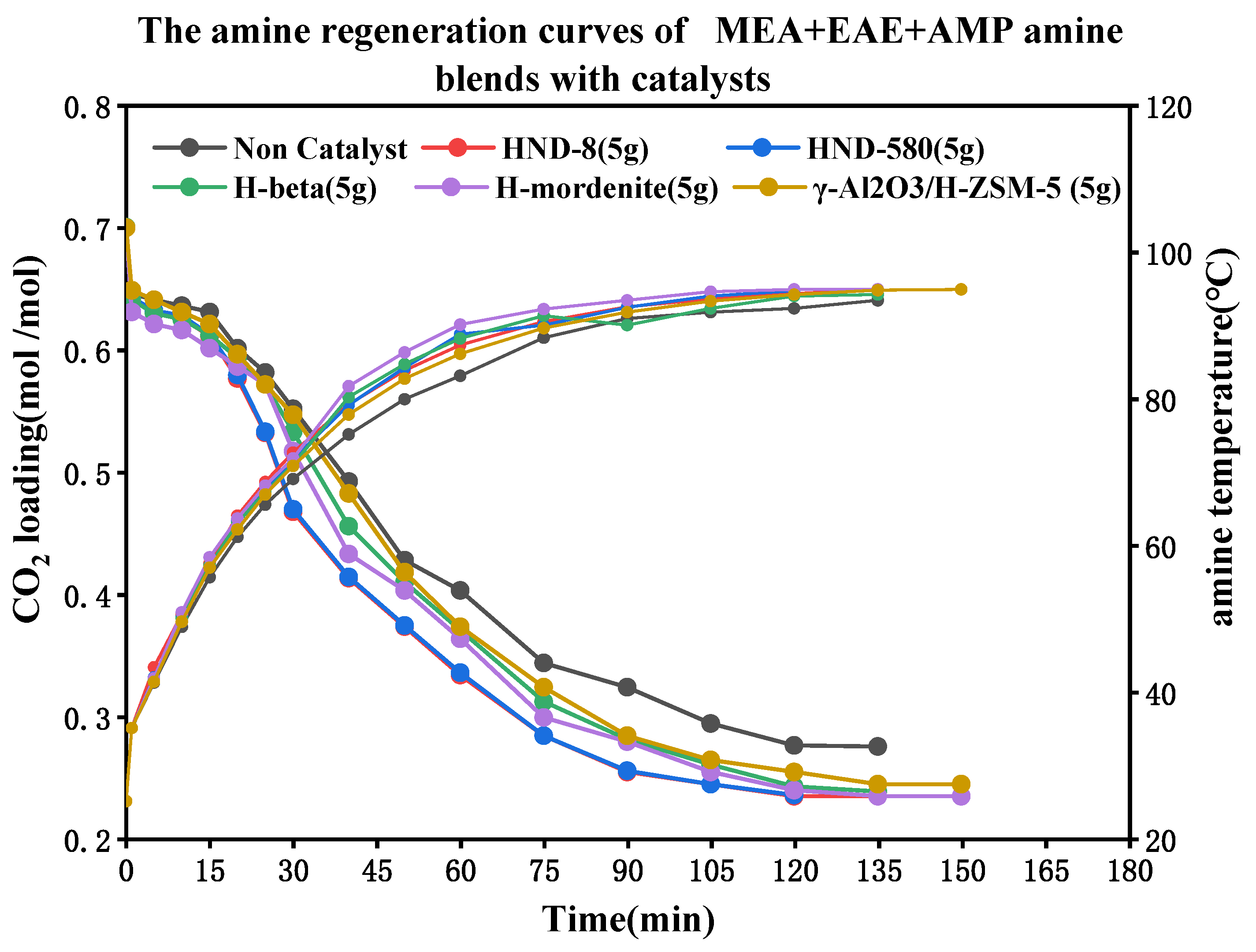
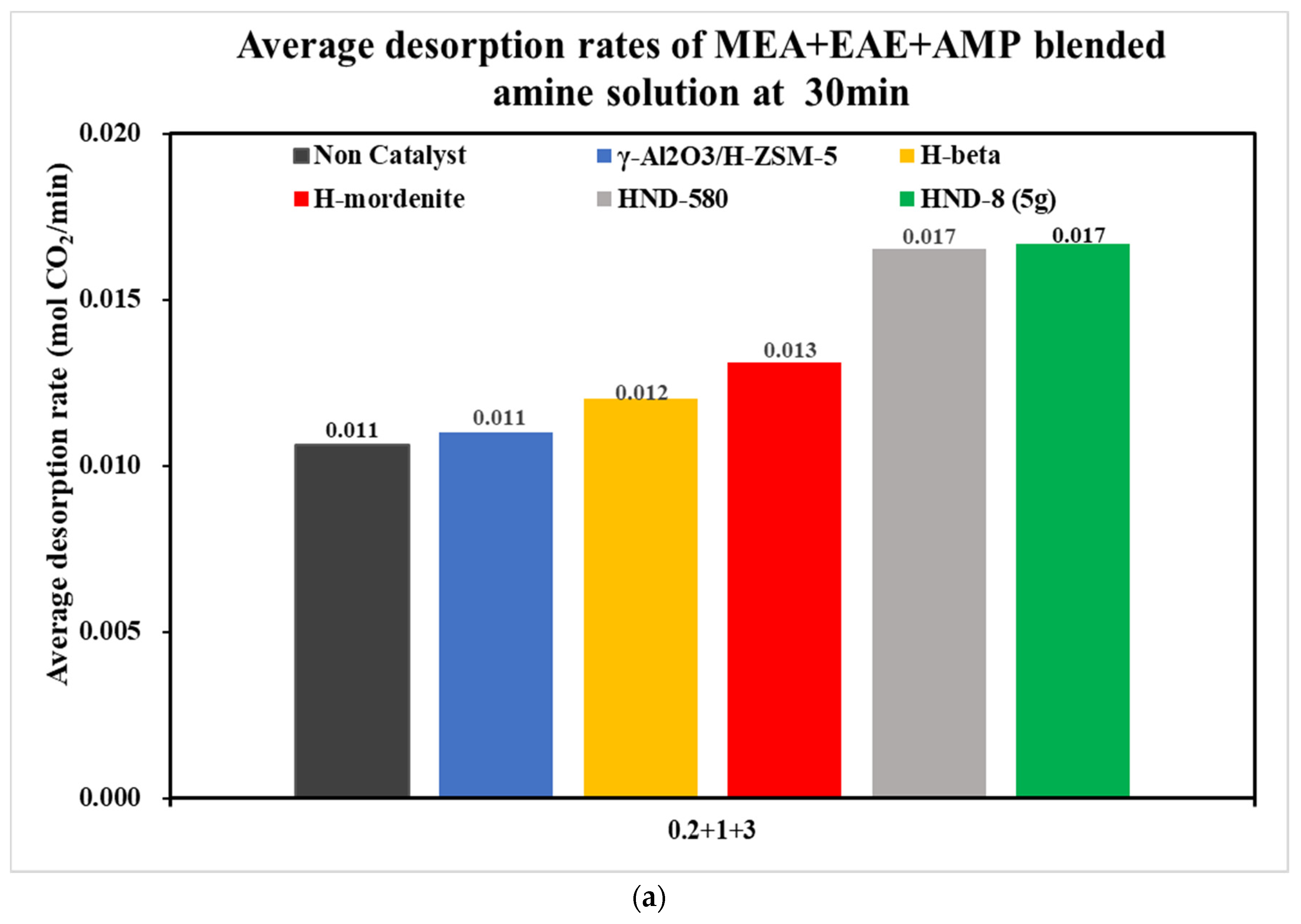
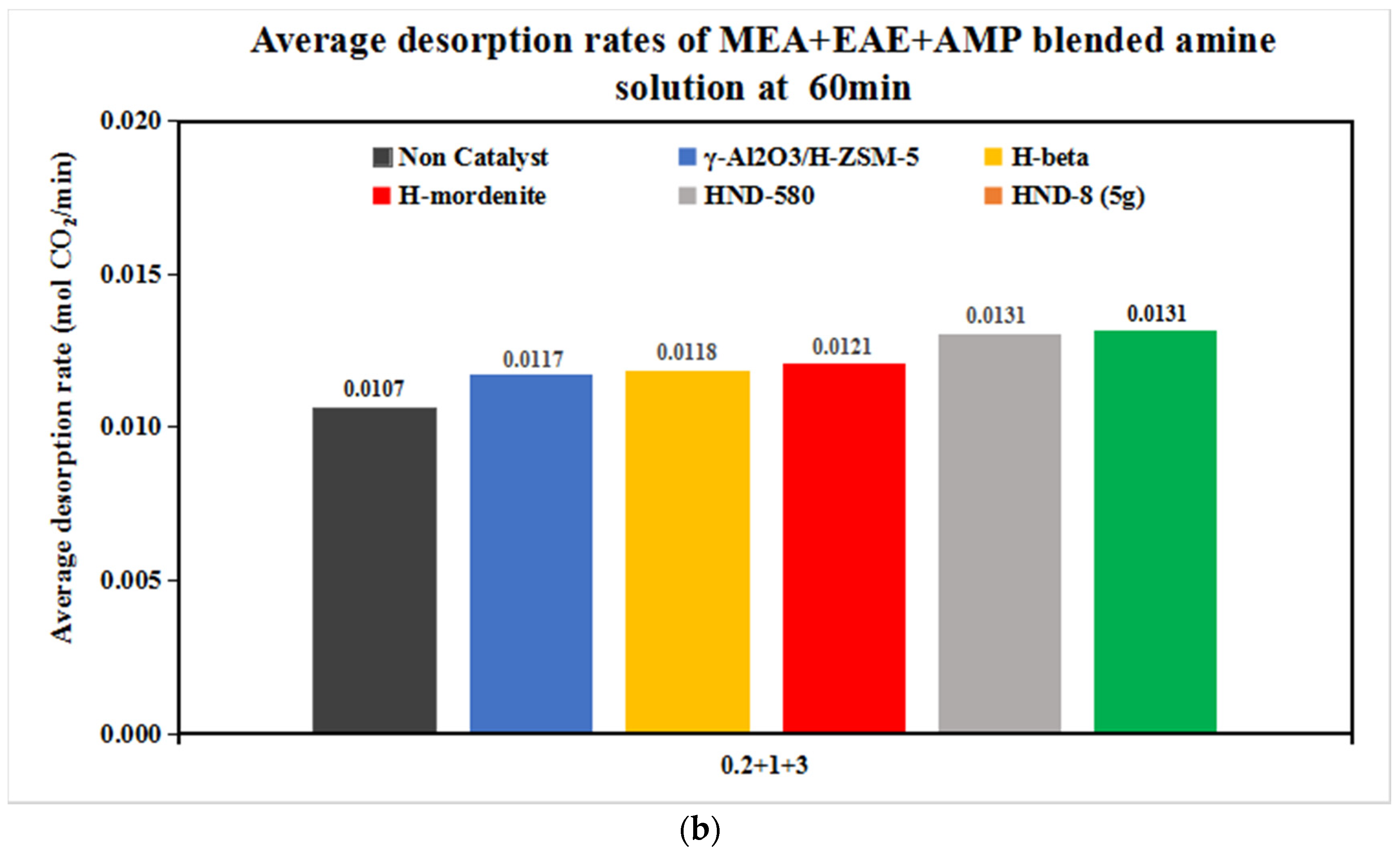
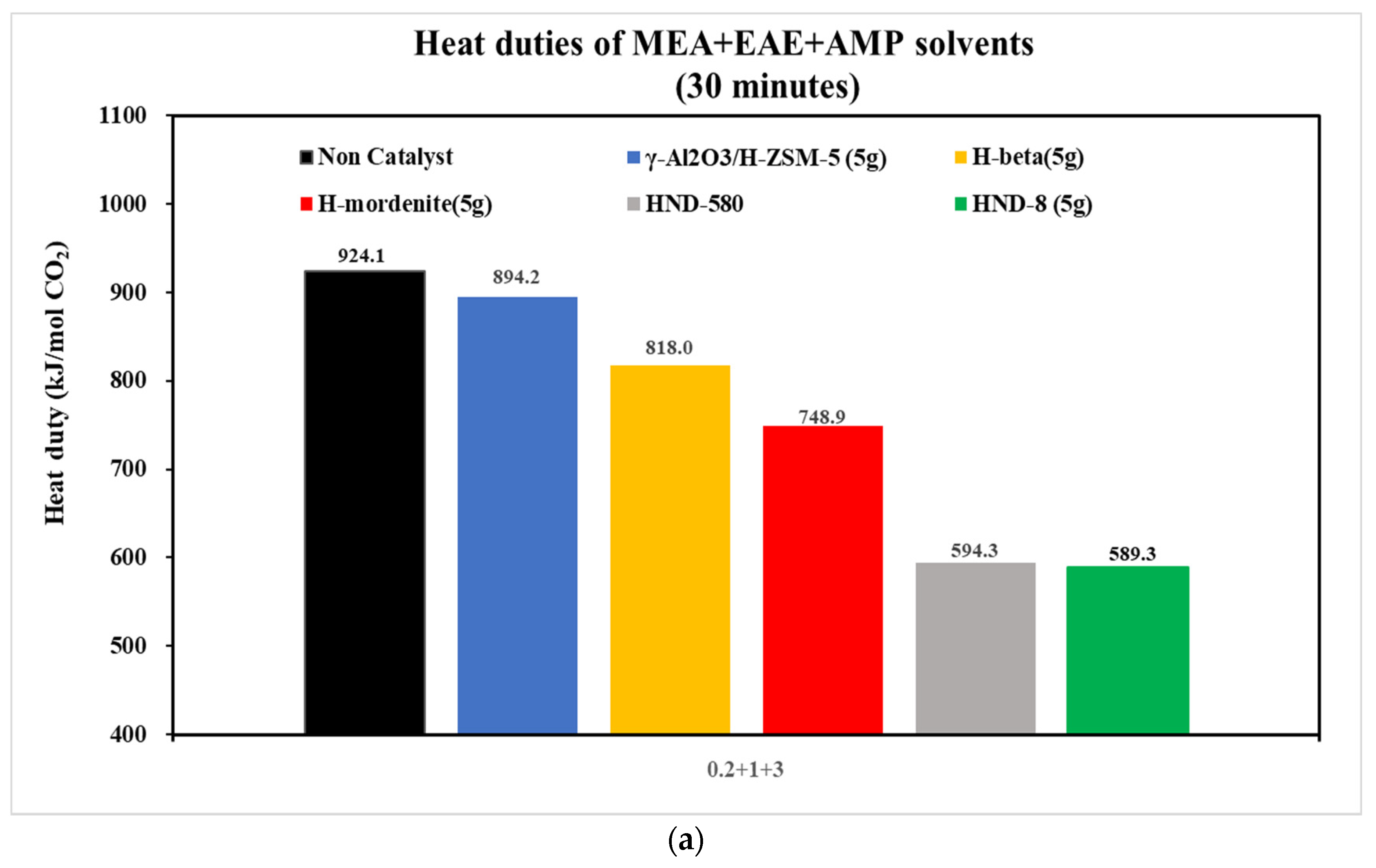
| Tri-Solvent | Concentration Range | Reference |
|---|---|---|
| MEA + MDEA + PZ | 3 + 1.5–2.5 + 0.5–1.5 mol/L | [19] |
| MEA + MDEA + PZ | 3 + 2.5 + 0.5 mol/L | [22] |
| MEA + AMP + PZ | 3 + 1.5–2.5 + 0.5–1.5 mol/L | [21] |
| AMP + PZ + MEA | 1.5–2.5 + 0.5–1.5 + 3 mol/L | [15] |
| AMP + PZ + MEA | 1.5–2.5 + 0.5–1.5 + 3 mol/L | [16] |
| AMP + PZ + MEA | 2 + 1 + 2 mol/L | [18,20] |
| MEA + BEA + AMP | 0.1–0.5 + 2 + 2 mol/L | [23,24] |
| MEA + BEA + DEEA | 0.1–0.5 + 2 + 2 mol/L | Accepted in IJGGC |
| MEA + EAE + AMP | 0.1–0.5 + 2 + 2 mol/L | [25] |
| MEA + EAE + AMP | 0.1–0.5 + 1–2 + 2–3 mol/L | This study |
| MEA + EAE + AMP | Desorption Factor (10−3 mol CO2)3/L2 kJ min | |||
|---|---|---|---|---|
| 15 min | 30 min | |||
| (mol/L) | Blank | γ-Al2O3/H-ZSM-5 (15 g) | Blank | γ-Al2O3/H-ZSM-5 (15 g) |
| 0.1 + 2 + 2 | 0.025 | 0.038 | 0.020 | 0.027 |
| 0.2 + 2 + 2 | 0.032 | 0.088 | 0.021 | 0.036 |
| 0.3 + 2 + 2 | 0.032 | 0.041 | 0.020 | 0.027 |
| 0.4 + 2 + 2 | 0.029 | 0.043 | 0.026 | 0.029 |
| 0.5 + 2 + 2 | 0.027 | 0.039 | 0.019 | 0.032 |
| MEA + EAE + AMP | Desorption Factor (10−3 mol CO2)3/L2 kJ min | |||||
|---|---|---|---|---|---|---|
| (mol/L) | Non Catalyst | γ-Al2O3/H-ZSM-5 | H-Beta | H-Mordenite | HND-8 | HND-580 |
| 0.2 + 2 + 2 | 0.0179 | 0.0185 | 0.0188 | 0.0190 | 0.0223 | 0.0193 |
| 0.5 + 2 + 2 | 0.0170 | 0.0176 | 0.0180 | 0.0185 | 0.0220 | 0.0190 |
| MEA + EAE + AMP | Desorption Factor (10−3 mol CO2)3/L2 kJ min | |||||
|---|---|---|---|---|---|---|
| (mol/L) | Non Catalyst | γ-Al2O3/H-ZSM-5 | H-Beta | H-Mordenite | HND-8 | HND-580 |
| 0.5 + 1.5 + 2.5 | 0.0094 | 0.0094 | 0.0097 | 0.0098 | 0.0126 | 0.0110 |
| 0.2 + 1 + 3 | 0.0072 | 0.0079 | 0.0103 | 0.0135 | 0.0277 | 0.0270 |
| Parameters | Catalyst | |
|---|---|---|
| HND-8 | HND-580 | |
| Acidity by strength (mmol/g) | 24.75 | ≥4.95 |
| Wet apparent density (g/mL) | 0.75–0.85 | 0.55–0.65 |
| Wet true density (g/mL) | 1.18–1.28 | 1.18–1.28 |
| Average pore diameter (nm) | ≥15 | ≥15 |
| Surface area (m2/g) | >20 | ≥20 |
| Pore volume (cm3/g) | 0.2–0.4 | 0.2–0.45 |
| Particle size between 0.315–1.25 mm (%) | >90 | ≥90 |
| Water content (%) | ≤3 (dry), 50–57 (wet) | ≤3 |
| Maximum service temperature (°C) | 150 | 170 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Peng, J.; Li, Y.; Shi, H.; Jin, J.; Hu, J.; Lu, S. Evaluating CO2 Desorption Activity of Tri-Solvent MEA + EAE + AMP with Various Commercial Solid Acid Catalysts. Catalysts 2022, 12, 723. https://doi.org/10.3390/catal12070723
Zhang B, Peng J, Li Y, Shi H, Jin J, Hu J, Lu S. Evaluating CO2 Desorption Activity of Tri-Solvent MEA + EAE + AMP with Various Commercial Solid Acid Catalysts. Catalysts. 2022; 12(7):723. https://doi.org/10.3390/catal12070723
Chicago/Turabian StyleZhang, Binbin, Jiacheng Peng, Ye Li, Huancong Shi, Jing Jin, Jiawei Hu, and Shijian Lu. 2022. "Evaluating CO2 Desorption Activity of Tri-Solvent MEA + EAE + AMP with Various Commercial Solid Acid Catalysts" Catalysts 12, no. 7: 723. https://doi.org/10.3390/catal12070723
APA StyleZhang, B., Peng, J., Li, Y., Shi, H., Jin, J., Hu, J., & Lu, S. (2022). Evaluating CO2 Desorption Activity of Tri-Solvent MEA + EAE + AMP with Various Commercial Solid Acid Catalysts. Catalysts, 12(7), 723. https://doi.org/10.3390/catal12070723









