Electrocatalysis of 2,6-Dinitrophenol Based on Wet-Chemically Synthesized PbO-ZnO Microstructures
Abstract
:1. Introduction
2. Results and Discussion
2.1. Binding Energy and Ionization States of PbO-Doped ZnO MSs
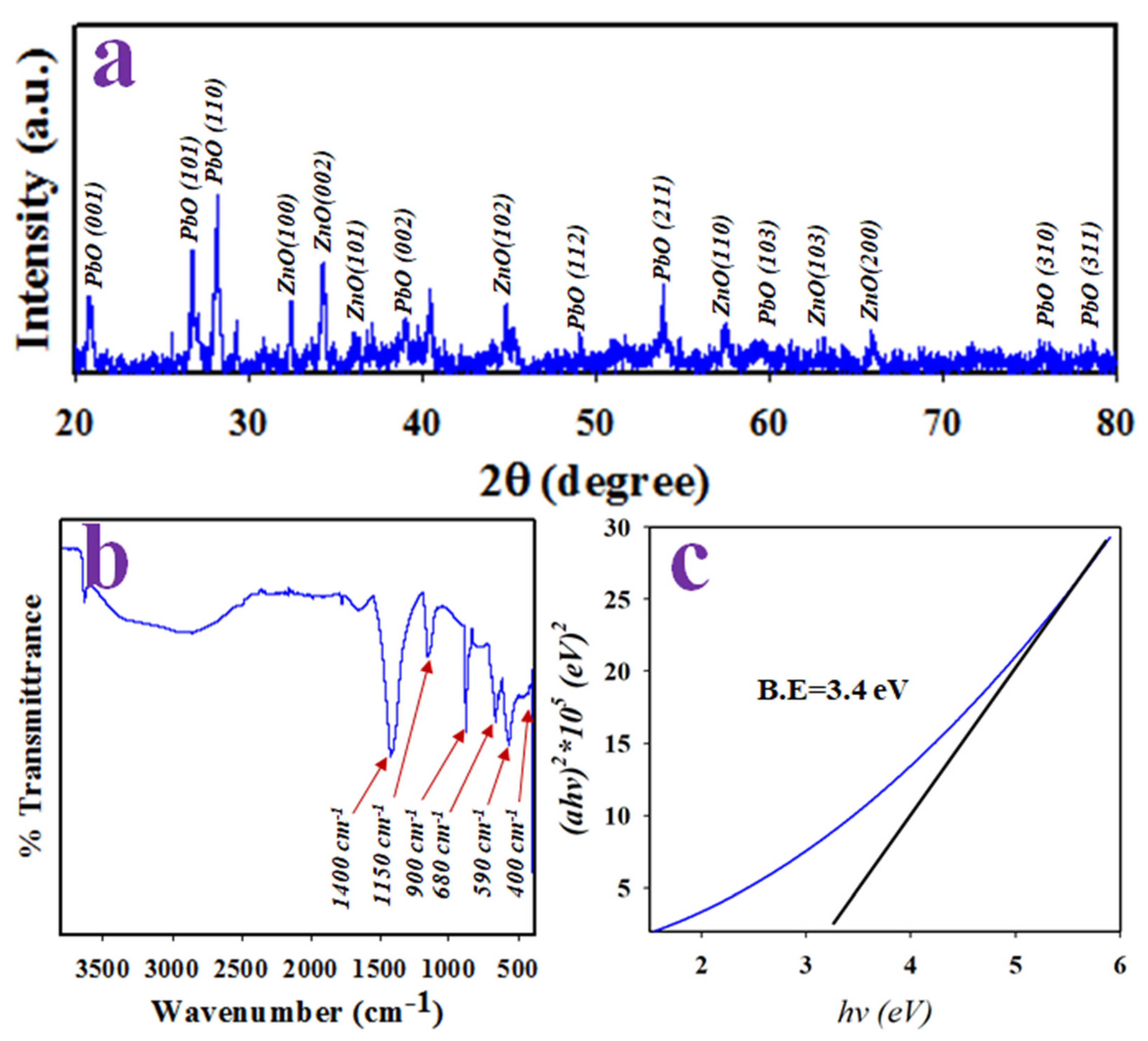
2.2. Crystallinity, Functional Groups and Optical Bandgap of PbO-Doped ZnO MSs
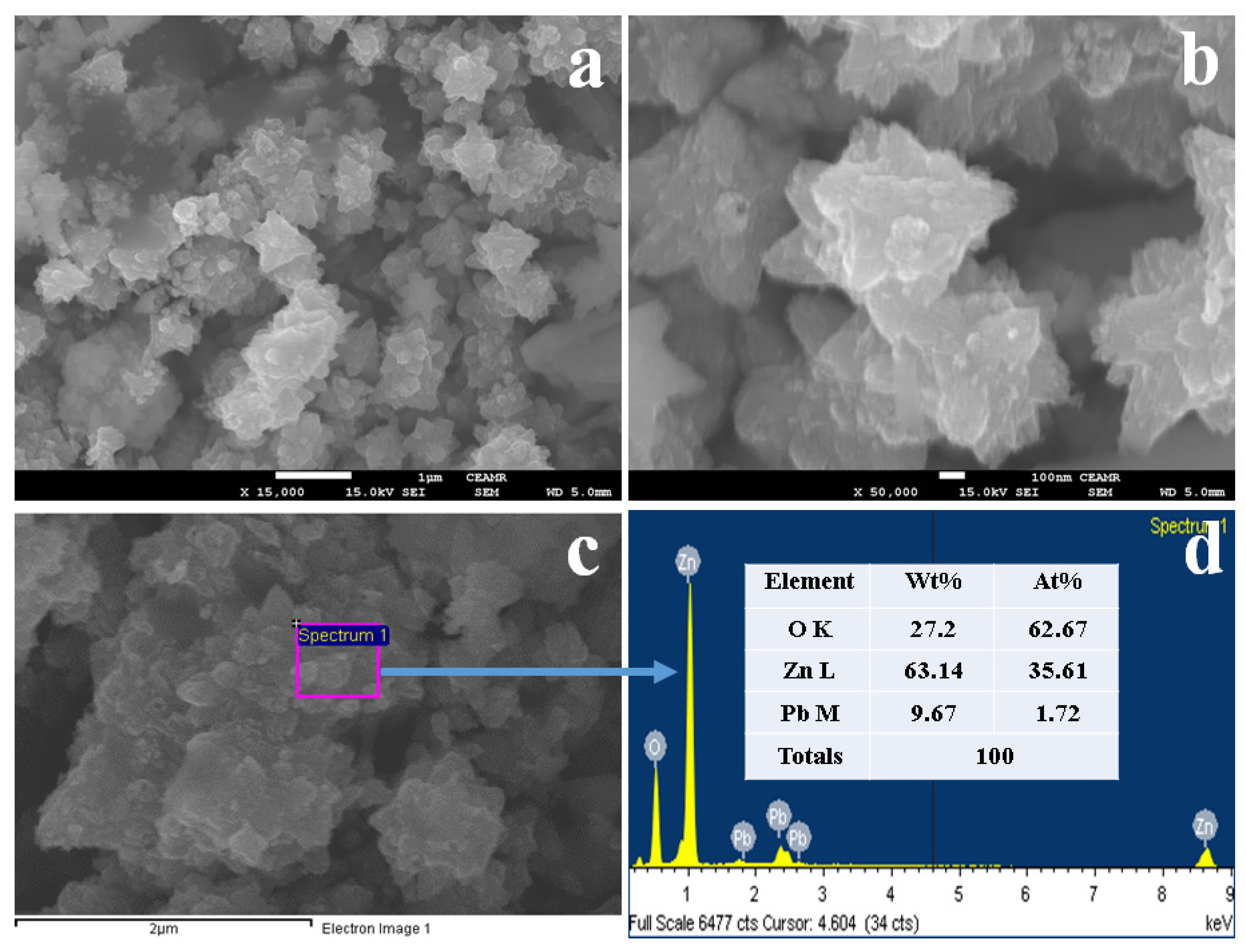
2.3. FESEM and EDS Analysis of PbO-Doped ZnO MSs
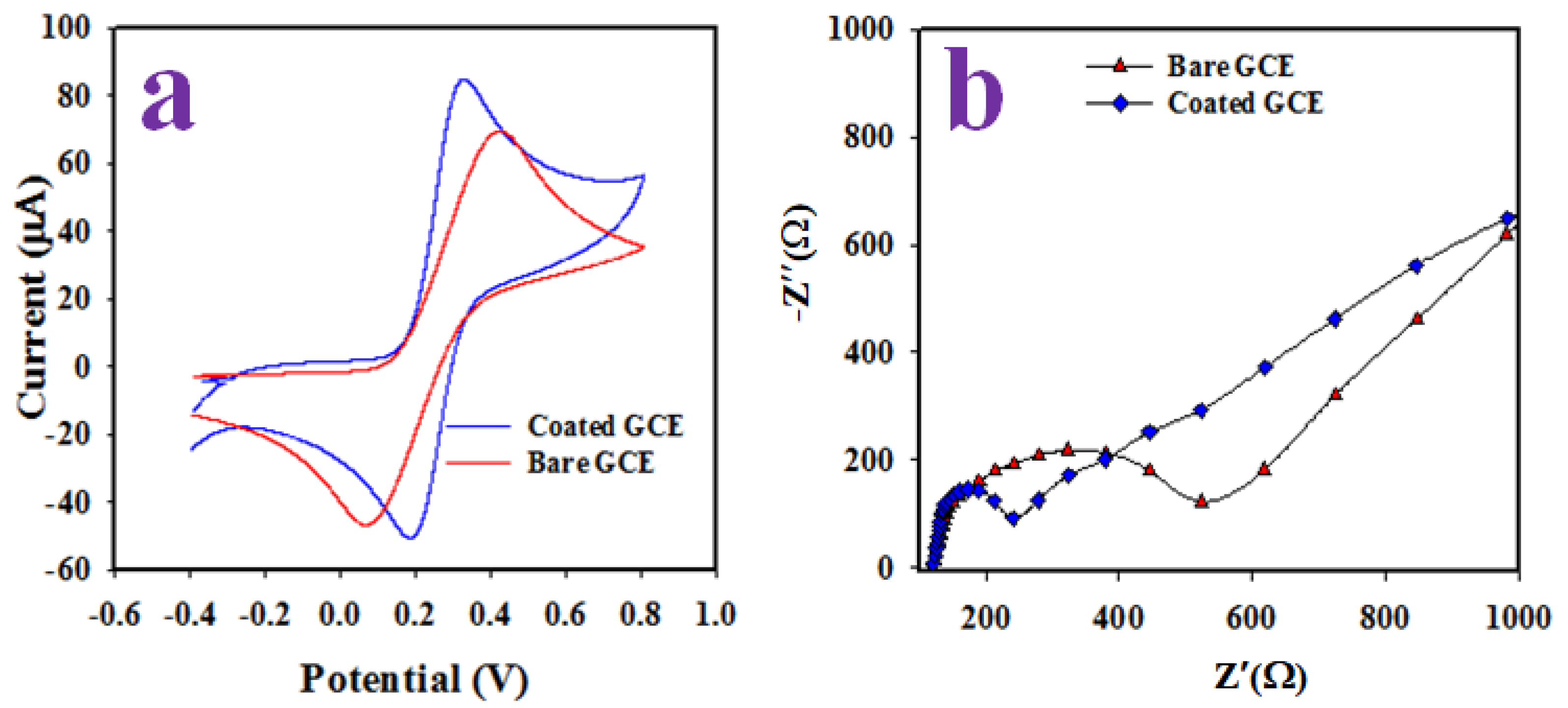
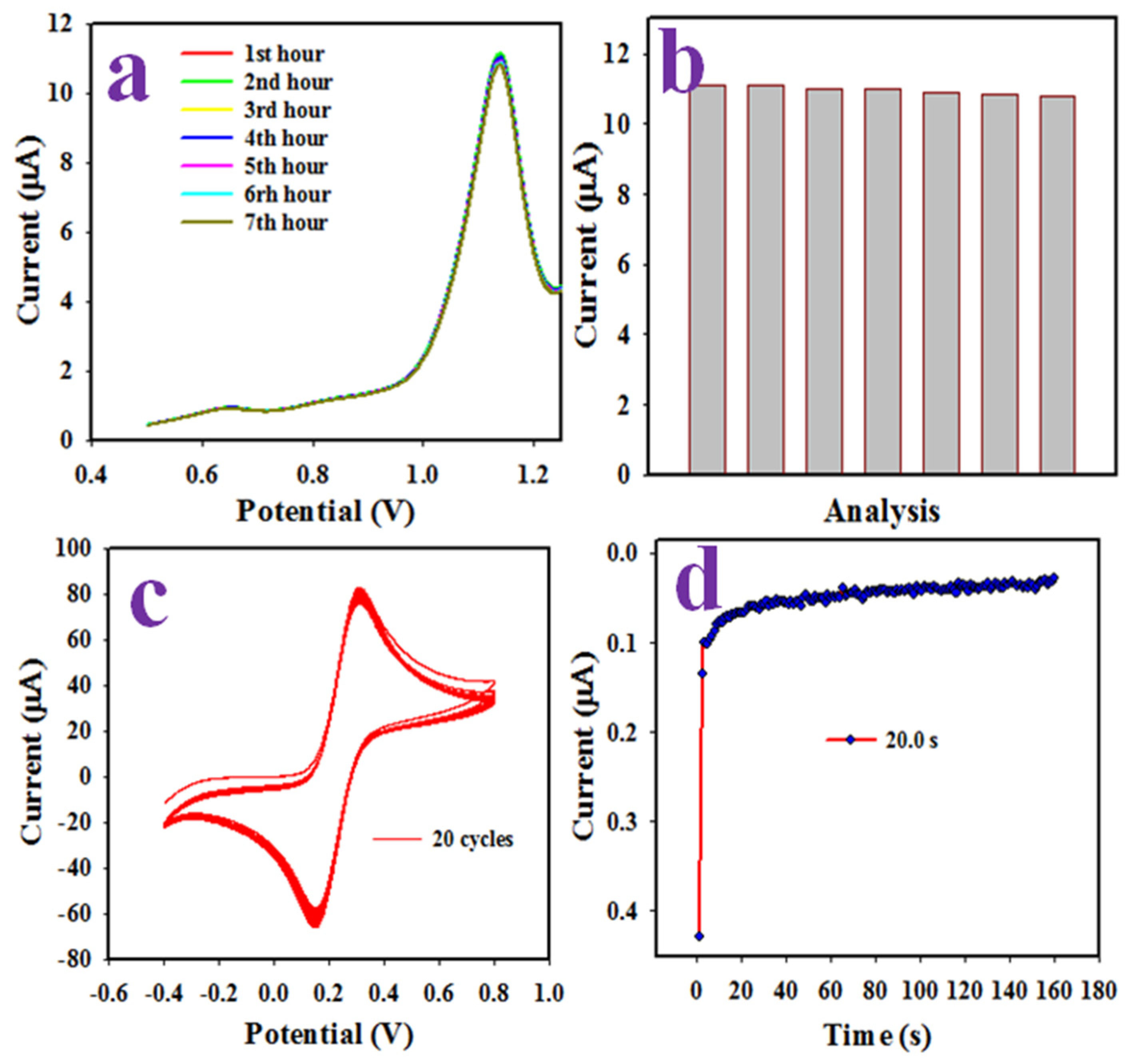
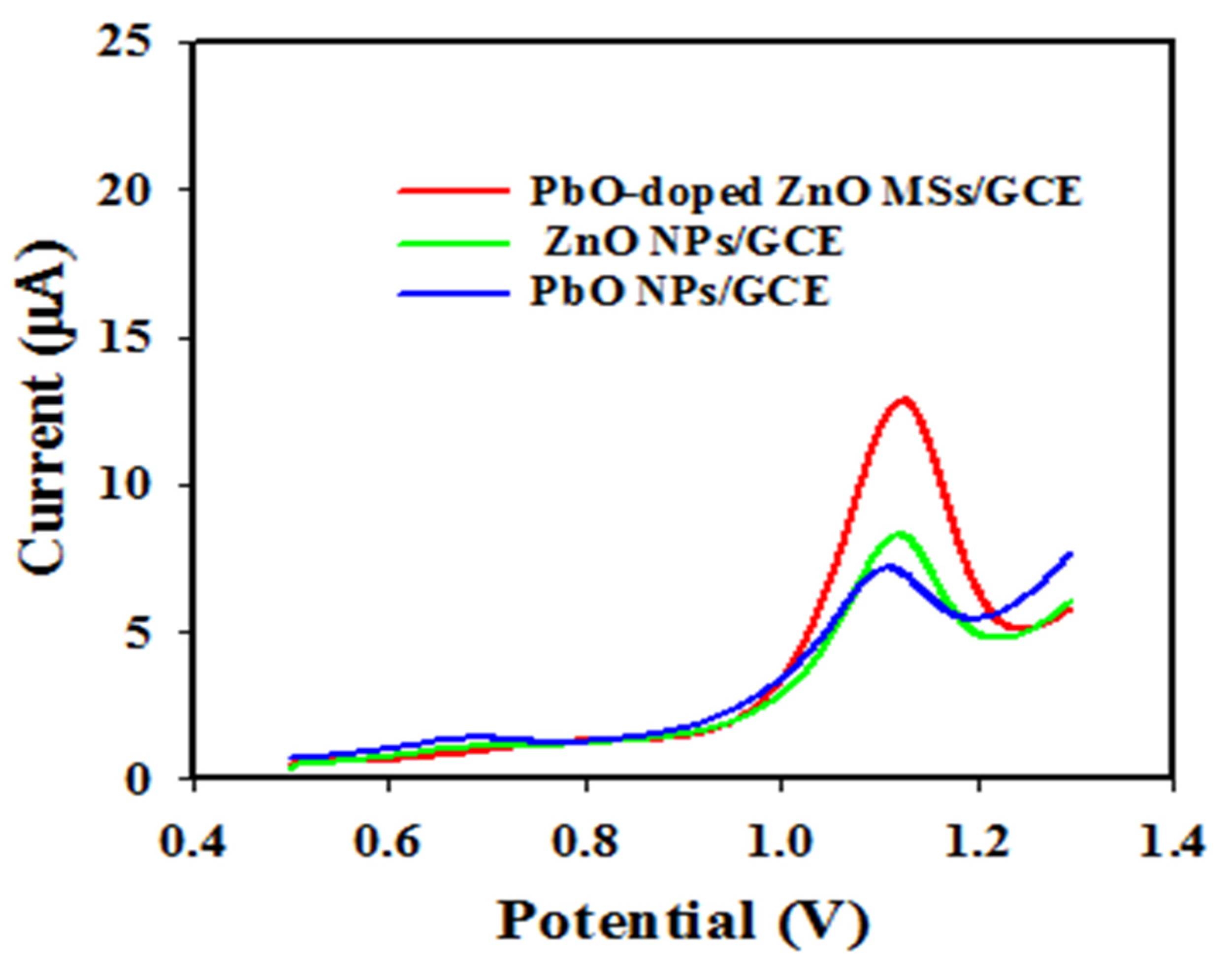
2.4. Elecrochemical Characterizationof PbO-Doped ZnO MSs as Sensing Substrates on GCE
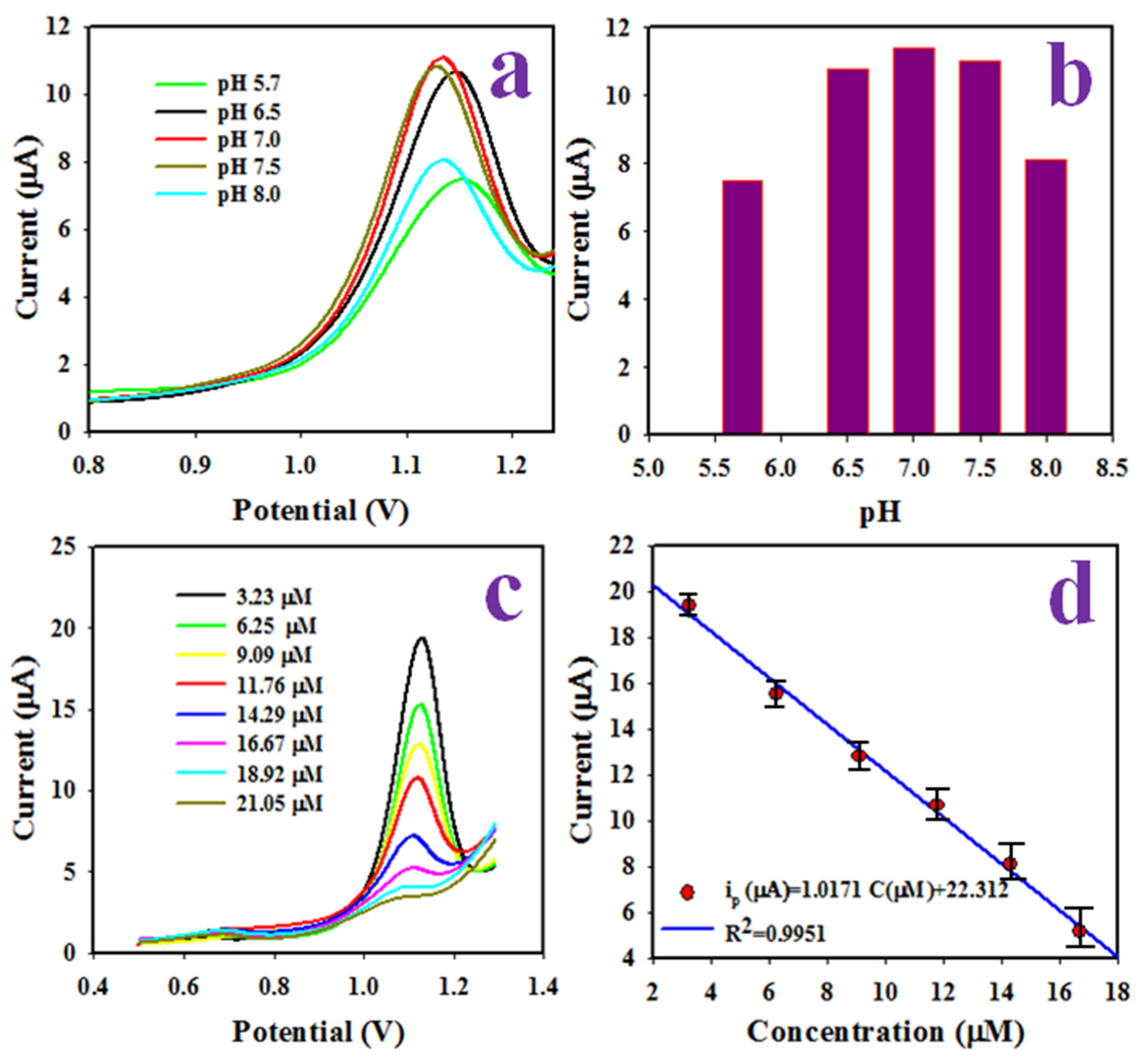
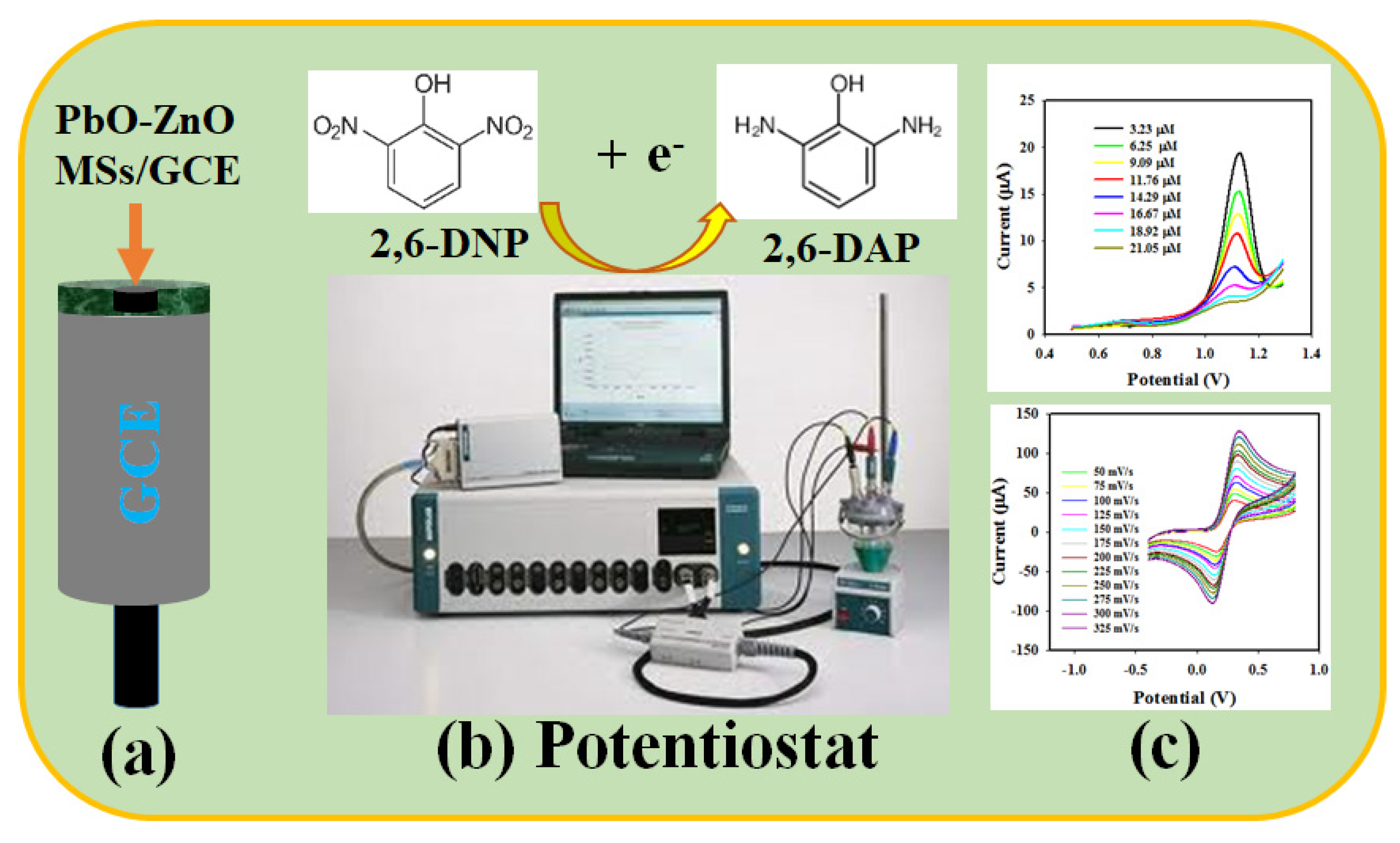
2.5. Detection of 2,6-DNP Applying DPV Method
2.6. The Recovery Method to Real Samples Analysis
3. Experimental
3.1. Materials and Methods
3.2. Synthesis of PbO-Doped ZnO MSs
3.3. GCE Modification by PbO-Doped ZnO MSs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asiri, A.M.; Adeosun, W.A.; Marwani, H.M. Electrocatalytic reduction of 2, 6-dinitrophenol on polycongo red decorated glassy carbon electrode for sensing application. J. Environ. Chem. Eng. 2020, 8, 104378. [Google Scholar] [CrossRef]
- Zaharia, M.; Tudorachi, L.; Pintilie, O.; Drochioi, C.; Gradinaru, R.; Murariu, M. Banned dinitrophenols still trigger both legal and forensic issues. Environ. Forensics 2016, 17, 120–130. [Google Scholar] [CrossRef]
- Gemini, V.L.; Gallego, A.; Tripodi, V.; Corach, D.; Planes, E.I.; Korol, S.E. Microbial degradation and detoxification of 2,4-dinitrophenol in aerobic and anoxic processes. Int. Biodeterior. Biodegrad. 2007, 60, 226–230. [Google Scholar] [CrossRef]
- She, Z.; Gao, M.; Jin, C.; Chen, Y.; Yu, J. Toxicity and biodegradation of 2,4-dinitrophenol and 3-nitrophenol in anaerobic systems. Process Biochem. 2005, 40, 3017–3024. [Google Scholar] [CrossRef]
- Wahid, A.; Asiri, A.M.; Rahman, M.M. One-step facile synthesis of Nd2O3/ZnO nanostructures for an efficient selective 2,4-dinitrophenol sensor probe. Appl. Surf. Sci. 2019, 487, 1253–1261. [Google Scholar] [CrossRef]
- Kamour, A.; George, N.; Gwynnette, D.; Cooper, G.; Lupton, D.; Eddleston, M.; Thompson, J.P.; Vale, J.A.; Thanacoody, H.K.R.; Hill, S. Increasing frequency of severe clinical toxicity after use of 2,4-dinitrophenol in the UK: A report from the National Poisons Information Service. Emerg. Med. J. 2014, 32, 383–386. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, H.; Zhao, L.; Lin, J.M. A selective optical chemical sensor for 2,6-dinitrophenol based on fluorescence quenching of a novel functional polymer. Talanta 2006, 70, 160–168. [Google Scholar] [CrossRef]
- National Recommended Water Quality Criteria; Environmental Protection Agency (EPA): Washington, WA, USA, 2004.
- Belloli, R.; Barletta, B.; Bolzacchini, E.; Meinardi, S.; Orlandi, M.; Rindone, B. Determination of toxic nitrophenols in the atmosphere by high-performance liquid chromatography. J. Chromatogr. A 1999, 846, 277–281. [Google Scholar] [CrossRef]
- Kaniansky, D.; Krcmova, E.; Madajova, V.; Mas’ar, M.; Mar’ak, J.; Onuska, F.I. Determination of nitrophenols by capillary zone electrophoresis in a hydrodynamically closed separation compartment. J. Chromatogr. A 1997, 772, 327–337. [Google Scholar] [CrossRef]
- Roseboom, H.; Berkhoff, C.J.; Wammes, J.I.J.; Wegman, R.C.C. Reversed-phase ion-pair high-performance liquid chromatography of nitrophenols. J. Chromatogr. 1981, 208, 331–337. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alfaifi, S.Y. Fabrication of novel and potential selective 4-Cyanophenol chemical sensor probe based on Cu-doped Gd2O3 nanofiber materials modified PEDOT:PSS polymer mixtures with Au/μ-Chip for effective monitoring of environmental contaminants from various water samples. Polymers 2021, 13, 3379. [Google Scholar] [PubMed]
- Ahmed, M.; Hameed, S.; Ihsan, A.; Naseer, M.M. Fluorescent thiazol-substituted pyrazoline nanoparticles for sensitive and highly selective sensing of explosive 2,4,6-trinitrophenol in aqueous medium. Sens. Actuators B Chem. 2017, 248, 57–62. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Desai, A.V.; Ghosh, S.K. Engineering metal–organic frameworks for aqueous phase 2,4,6-trinitrophenol (TNP) sensing. CrystEngComm 2016, 18, 2994–3007. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Joarder, B.; Chaudhari, A.K.; Mukherjee, S.; Ghosh, S.K. Highly Selective Detection of Nitro Explosives by a Luminescent Metal–Organic Framework. Angew. Chem. 2013, 125, 2953–2957. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Ni, Y.; Kokot, S. A sensor based on blue luminescent graphene quantum dots for analysis of a common explosive substance and an industrial intermediate, 2,4,6-trinitrophenol. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 1213–1221. [Google Scholar] [CrossRef]
- Pınar, P.T.; Allahverdiyev, S.; Yardım, Y.; Şentürk, Z. Voltammetric sensing of dinitrophenolic herbicide dinoterb on cathodically pretreated boron-doped diamond electrode in the presence of cationic surfactant. Microchem. J. 2020, 155, 104772. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam, M.M.; Alfaifi, S.Y.; Asiri, A.M.; Ali, M.M. Sensitive detection of thiourea hazardous unsafe toxin with sandwich type Nafion/CuO/ZnO nanospikes/Glassy carbon composite electrodes. Polymers 2021, 13, 3998. [Google Scholar] [CrossRef]
- Majidian, M.; Raoof, J.B.; Fischer, J.; Barek, J. Differential Pulse Voltammetric Determination of 2-Methyl-4,6-Dinitrophenol using Bismuth Bulk Electrode. Electroanalysis 2020, 32, 317–322. [Google Scholar] [CrossRef]
- Jiranek, I.; Peckova, K.; Kralova, Z.; Moreir, J.C.; Barek, J. The use of silver solid amalgam electrode for voltammetric and amperometric determination of nitroquinolines. Electrochim. Acta 2009, 54, 1939–1947. [Google Scholar] [CrossRef]
- Wan, Q.; Li, Q.H.; Chen, Y.J.; Wang, T.H. Fabrication and ethanol sensing characteristics of ZnO nanowire gas sensors. Appl. Phys. Lett. 2004, 84, 18. [Google Scholar] [CrossRef] [Green Version]
- Al-Hardan, N.; Abdullah, M.J.; Aziz, A.A. Impedance spectroscopy of undoped and Cr-doped ZnO gas sensors under different oxygen concentrations. Appl. Surf. Sci. 2011, 257, 8993–8997. [Google Scholar] [CrossRef]
- Hung, C.M.; Phuong, H.V.; Duy, N.V.; Hoa, N.D.; Hieu, N.D. Comparative effects of synthesis parameters on the NO2 gas-sensing performance of on-chip grown ZnO and Zn2SnO4 nanowire sensors. J. Alloys Compd. 2018, 765, 1237–1242. [Google Scholar] [CrossRef]
- Carotta, M.C.; Cervi, A.; di Natale, V.; Gherardi, S.; Giberti, A.; Guidi, V.; Puzzovio, D.; Vendemiati, B.; Martinelli, G.; Sacerdoti, M.; et al. ZnO gas sensors: A comparison between nanoparticles and nanotetrapods-based thick films. Sens. Actuators B Chem. 2009, 137, 164–169. [Google Scholar] [CrossRef]
- Hjiri, M.; El Mir, L.; Leonardi, S.G.; Pistone, A.; Mavili, L.; Neri, G. Al-doped ZnO for highly sensitive CO gas sensors. Sens. Actuators B Chem. 2014, 196, 413–420. [Google Scholar] [CrossRef]
- Law, J.B.K.; Thong, J.T.L. Improving the NH3 gas sensitivity of ZnO nanowire sensors by reducing the carrier concentration. Nanotechnology 2008, 19, 205502. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.M.; Asiri, A.M.; Hasnat, M.A.; Rahman, M.M. Detection of L-Aspartic Acid with Ag-Doped ZnO Nanosheets Using Differential Pulse Voltammetry. Biosensors 2022, 12, 379. [Google Scholar] [CrossRef]
- Alam, M.M.; Asiri, M.A.; Rahman, M.M. Fabrication of phenylhydrazine sensor with V2O5 doped ZnO nanocomposites. Mater. Chem. Phys. 2020, 243, 122658. [Google Scholar] [CrossRef]
- Alam, M.M.; Asiri, A.M.; Uddin, J.; Rahman, M.M. Selective 1,4-dioxane chemical sensor development with doped ZnO/GO nanocomposites by electrochemical approach. J. Mater Sci. Mater. Electron. 2022, 33, 4360–4374. [Google Scholar] [CrossRef]
- Li, C.; Lin, H.; Jing, M.; Zhang, L.Y.; Yuan, W.; Li, C.M. ZnO nanowire arrays with in situ sequentially self-assembled vertically oriented CdS nanosheets as superior photoanodes for photoelectrochemical water splitting. Sustain. Energy Fuels 2022, 6, 3240–3248. [Google Scholar] [CrossRef]
- Zhong, X.; Yuan, W.; Kang, Y.; Xie, J.; Hu, F.; Li, C.M. Biomass-Derived Hierarchical Nanoporous Carbon with Rich Functional Groups for Direct-Electron-Transfer-Based Glucose Sensing. ChemElectroChem 2016, 3, 144–151. [Google Scholar] [CrossRef]
- Yuan, W.; Lu, Z.; Li, C.M. Self-assembling microsized materials to fabricate multifunctional hierarchical nanostructures on macroscale substrate. J. Mater. Chem. A 2013, 1, 6416–6424. [Google Scholar] [CrossRef]
- Pasha, S.K.; Chidambaram, K.; Kennedy, L.J.; Vijaya, J.J. Lead Oxide—PbO Humidity Sensor. Sens. Transducers. J. 2010, 122, 113–119. [Google Scholar]
- Hyun, S.K.; Sun, G.J.; Lee, J.K.; Lee, C.; Lee, W.I.; Kim, H.W. Ethanol gas sensing using a networked PbO-decorated SnO2 nanowires. Thin Solid Films 2017, 637, 21–26. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Pandey, P.; Mishra, V.N.; Dwivedi, R. Structural and micro structural studies of PbO-doped SnO2 sensor for detection of methanol, propanol and acetone. J. Nat. Gas Chem. 2011, 20, 179–183. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Su, L.; Bellagamba, M.; Zhang, H.; Lei, Y. Electrospun Co3O4 nanofibers for sensitive and selective glucose detection. Biosens. Bioelectron. 2010, 26, 542–548. [Google Scholar] [CrossRef]
- Ibrahim, Y.; Meslam, M.; Eid, K.; Salah, B.; Abdullah, A.M.; Ozoemena, K.I.; Elzatahry, A.; Sharaf, M.A.; Sillanpä, M. A review of MXenes as emergent materials for dye removal from wastewater. Sep. Purif. Technol. 2022, 282, 120083. [Google Scholar] [CrossRef]
- Eid, K.; Sliem, M.H.; Abdullah, A.M. Tailoring the defects of sub-100 nm multipodal titanium nitride/oxynitride nanotubes for efficient water splitting performance. Nanoscale Adv. 2021, 3, 5016–5026. [Google Scholar] [CrossRef]
- Sheng, X.; Xu, X.; Wu, Y.; Zhang, X.; Lin, P.; Eid, K.; Abdullah, A.M.; Li, Z.; Yang, T.; Nanjundan, A.K.; et al. Nitrogenization of Biomass-Derived Porous Carbon Microtubes Promotes Capacitive Deionization Performance. Bull. Chem. Soc. Jpn. 2022, 95, 774–795. [Google Scholar] [CrossRef]
- Li, S.S.; Su, Y.K. Improvement of the performance in Cr-doped ZnO memory devices via control of oxygen defects. RSC Adv. 2019, 9, 2941–2947. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Fan, D.; Shen, W. Catalyst-free direct vapor-phase growth of Zn1−xCuxO micro-cross structures and their optical properties. Nanoscale Res. Lett. 2013, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Claros, M.; Setka, M.; Jimenez, Y.P.; Vallejos, S. AACVD Synthesis and Characterization of Iron and Copper Oxides Modified ZnO Structured Films. Nanomaterials 2020, 10, 471. [Google Scholar] [CrossRef] [Green Version]
- Panchariya, D.K.; Rai, R.K.; Kumar, E.A.; Singh, S.K. Core-Shell Zeolitic Imidazolate Frameworks for Enhanced Hydrogen Storage. ACS Omega 2018, 3, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mu, S.; Weng, X.; Zhao, Y.; Song, S. Rutile flotation with Pb2+ ions as activator: Adsorption of Pb2+ atrutile/water interface. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 431–437. [Google Scholar] [CrossRef]
- Szafraniak, I.; Połomska, M.; Hilczer, B.; Talik, E.; Kepiński, L. Characterization of PbTiO3 Nanopowders Obtained by Room Temperature Synthesis. Ferroelectrics 2006, 336, 279–287. [Google Scholar] [CrossRef]
- Muhammad, W.; Ullah, N.; Haroon, M.; Abbasi, B.H. Optical, morphological and biological analysis of zinc oxide nanoparticles (ZnO NPs) using Papaver somniferum L. RSC Adv. 2019, 9, 29541–29548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtar, M.J.; Ahamed, M.; Kumar, S.; Khan, M.M.; Ahmad, J.; Alrokayan, S.A. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int. J. Nanomed. 2012, 7, 845–857. [Google Scholar]
- Arulmozhi, K.T.; Mythili, N. Studies on the chemical synthesis and characterization of lead oxide nanoparticles with different organic capping agents. AIP Adv. 2013, 3, 122122. [Google Scholar] [CrossRef]
- Ranjbar, M.; Yousefi, M. Sonochemical Synthesis and Characterization of a Nano-Sized Lead (II) Coordination Polymer; A New Precursor for the Preparation of PbO Nanoparticles. Int. J. Nanosci. Nanotechnol. 2016, 12, 109–118. [Google Scholar]
- Anzlovar, A.; Orel, Z.C.; Kogej, K.; Zigon, M. Polyol-Mediated Synthesis of Zinc Oxide Nanorods and Nanocomposites with Poly(methyl methacrylate). J. Nanomater. 2012, 2022, 760872. [Google Scholar] [CrossRef] [Green Version]
- Rezvani, M.A.; Khandan, S.; Sabahi, N. Oxidative Desulfurization of Gas Oil Catalyzed by (TBA) 4PW11Fe@PbO as an Efficient and Recoverable Heterogeneous Phase-Transfer Nanocatalyst. Energy Fuels 2017, 31, 5472–5481. [Google Scholar] [CrossRef]
- Yu, L.; He, R.; Zhang, Y.; Gao, J. Effect of Surface Treatment on Flexural and Tribological Properties of Poly (p-phenylene Benzobisoxazole)/Polyimide Composites under Normal and Elevated Temperature. Materials 2018, 11, 2131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaraju, G.; Udayabhanu; Shivaraj; Prashanth, S.A.; Shastri, M.; Yathish, K.V.; Anupama, C.; Rangappa, D. Electrochemical heavy metal detection, photocatalytic, photoluminescence, biodiesel production and antibacterial activities of Ag–ZnO nanomaterial. Mater. Res. Bull. 2017, 94, 54–63. [Google Scholar] [CrossRef]
- Naeem, R.; Yahya, R.; Pandikumar, A.; Ming, H.N.; Mazhar, M. Optical and optoelectronic properties of morphology and structure controlled ZnO, CdO and PbO thin films deposited by electric field directed aerosol assisted CVD. J. Mater. Sci. Mater. Electron. 2017, 28, 868–877. [Google Scholar] [CrossRef]
- Asiri, A.M.; Adeosun, W.A.; Marwani, H.M.; Rahman, M.M. Homopolymerization of 3-aminobenzoic acid for enzyme-free electrocatalytic assay of nitrite ions. New J. Chem. 2020, 44, 2022–2032. [Google Scholar] [CrossRef]
- Alizadeh, T.; Azizi, S. Graphene/graphite paste electrode incorporated with molecularly imprinted polymer nanoparticles as a novel sensor for differential pulse voltammetry determination of fluoxetine. Biosens. Bioelectron. 2016, 81, 198–206. [Google Scholar] [CrossRef]
- Ahmed, J.; Rakib, R.H.; Rahman, M.M.; Asiri, A.M.; Siddiquey, I.A.; Islam, S.S.; Hasnat, M.A. Electrocatalytic Oxidation of 4-Aminophenol Molecules at the Surface of an FeS2/Carbon Nanotube Modified Glassy Carbon Electrode in Aqueous Medium. ChemPlusChem 2019, 84, 175–182. [Google Scholar] [CrossRef]
- Lenke, H.; Knackmuss, H.J. Initial Hydrogenation and Extensive Reduction of Substituted 2,4-Dinitrophenols. Appl. Environ. Microbiol. 1996, 62, 784–790. [Google Scholar] [CrossRef] [Green Version]
- Reinado, E.P.; Roldán, M.D.; Castillo, F.; Vivián, C.M. The NprA nitroreductase required for 2,4-dinitrophenol reduction in Rhodobacter capsulatus is a dihydropteridine reductase. Environ. Microbiol. 2008, 10, 3174–3183. [Google Scholar] [CrossRef]


| Modified GCE | *LOD | #LDR | Sensitivity | Ref. |
|---|---|---|---|---|
| PCR/GCE | 0.1 µM | 0.5~65 μM | --- | [1] |
| Functional PVC | 23.0 nM | 2.5 µM~mM | --- | [7] |
| GQDs | 0.091 µM | 0.1~15 µM | --- | [16] |
| PbO/ZnO MSs/GCE | 2.0 µM | 3.23~16.67 μM | 32.1867 µAµM−1cm−2 | This work |
| Real Samples | Added 2,6-DNP Conc. (µM) | Measured 2,6-DNP Conc.a by PbO-ZnO MSs /GCE (µM) | Average Recovery b (%) | RSD c (%) (n = 3) | ||
|---|---|---|---|---|---|---|
| R1 | R2 | R3 | ||||
| Mineral water | 2.09 | 2.97 | 2.99 | 2.95 | 98.68 | |
| Sea water | 2.09 | 2.94 | 2.96 | 2.98 | 98.53 | |
| Tap water | 2.09 | 2.97 | 2.00 | 2.93 | 98.64 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Alam, M.M.; Asiri, A.M.; Chowdhury, M.A.; Uddin, J. Electrocatalysis of 2,6-Dinitrophenol Based on Wet-Chemically Synthesized PbO-ZnO Microstructures. Catalysts 2022, 12, 727. https://doi.org/10.3390/catal12070727
Rahman MM, Alam MM, Asiri AM, Chowdhury MA, Uddin J. Electrocatalysis of 2,6-Dinitrophenol Based on Wet-Chemically Synthesized PbO-ZnO Microstructures. Catalysts. 2022; 12(7):727. https://doi.org/10.3390/catal12070727
Chicago/Turabian StyleRahman, Mohammed M., Md M. Alam, Abdullah M. Asiri, Mohammad Asaduzzaman Chowdhury, and Jamal Uddin. 2022. "Electrocatalysis of 2,6-Dinitrophenol Based on Wet-Chemically Synthesized PbO-ZnO Microstructures" Catalysts 12, no. 7: 727. https://doi.org/10.3390/catal12070727
APA StyleRahman, M. M., Alam, M. M., Asiri, A. M., Chowdhury, M. A., & Uddin, J. (2022). Electrocatalysis of 2,6-Dinitrophenol Based on Wet-Chemically Synthesized PbO-ZnO Microstructures. Catalysts, 12(7), 727. https://doi.org/10.3390/catal12070727









