Indoor Air Photocatalytic Decontamination by UV–Vis Activated CuS/SnO2/WO3 Heterostructure
Abstract
:1. Introduction
2. Results and Discussion
2.1. Composition and Morphology
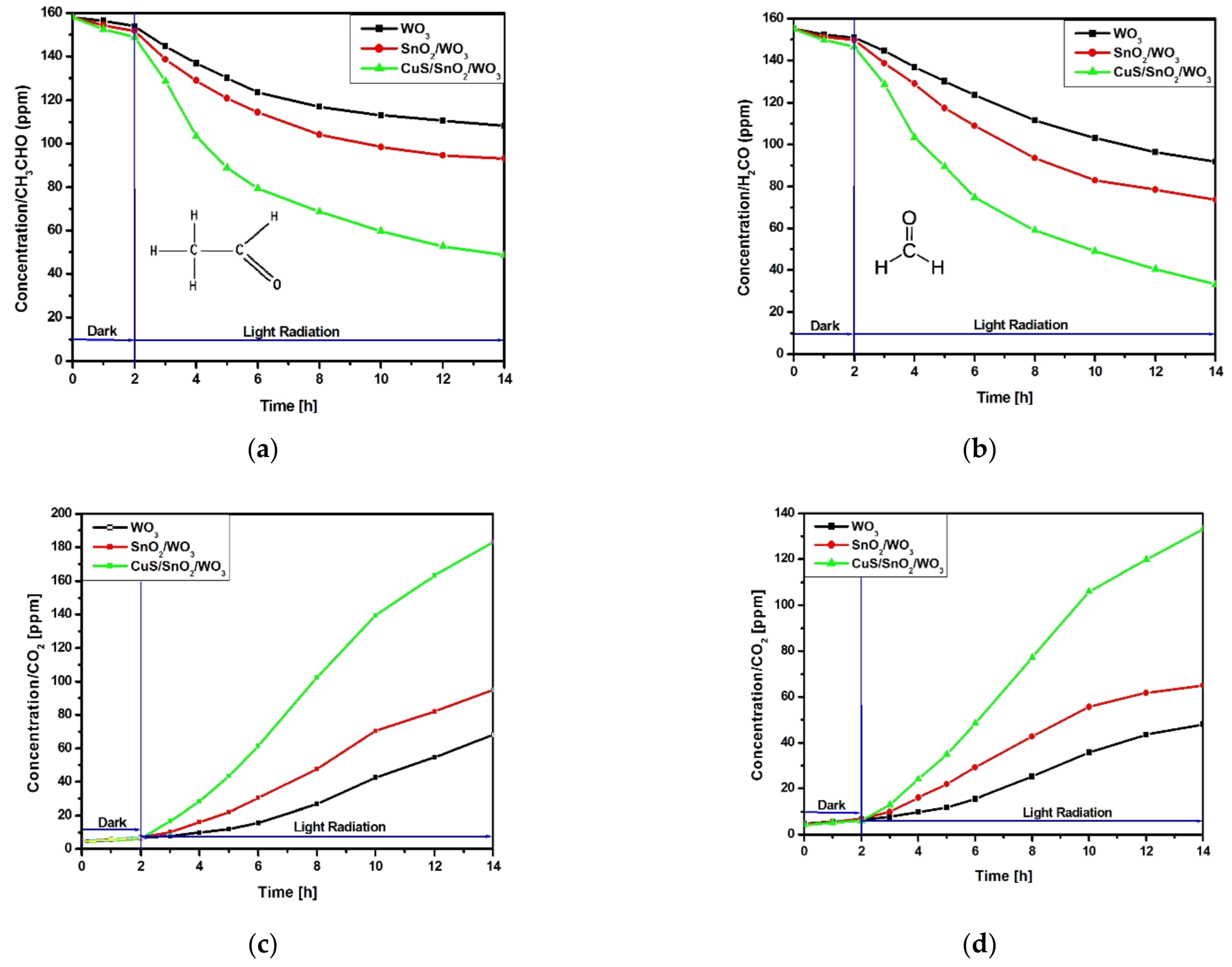
2.2. Indoor Air Photocatalytic Treatment
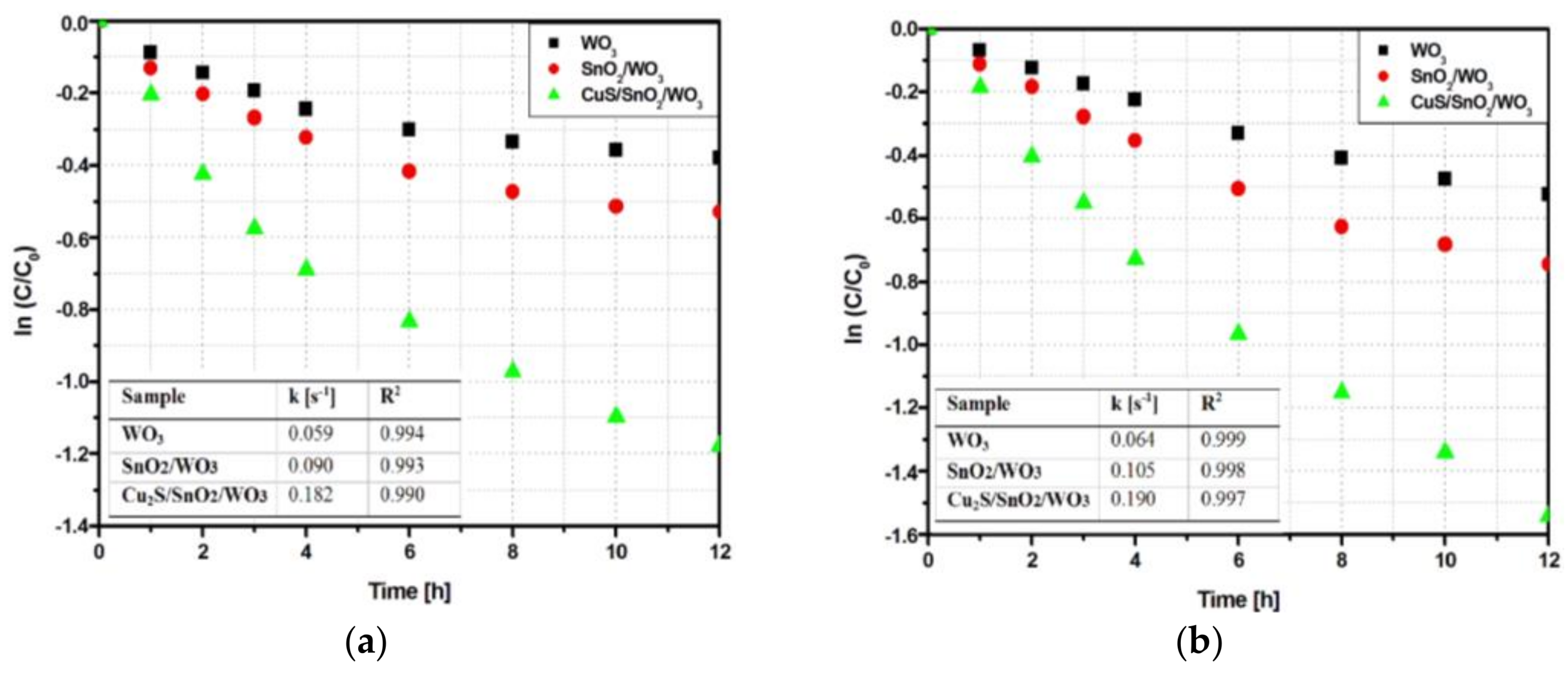
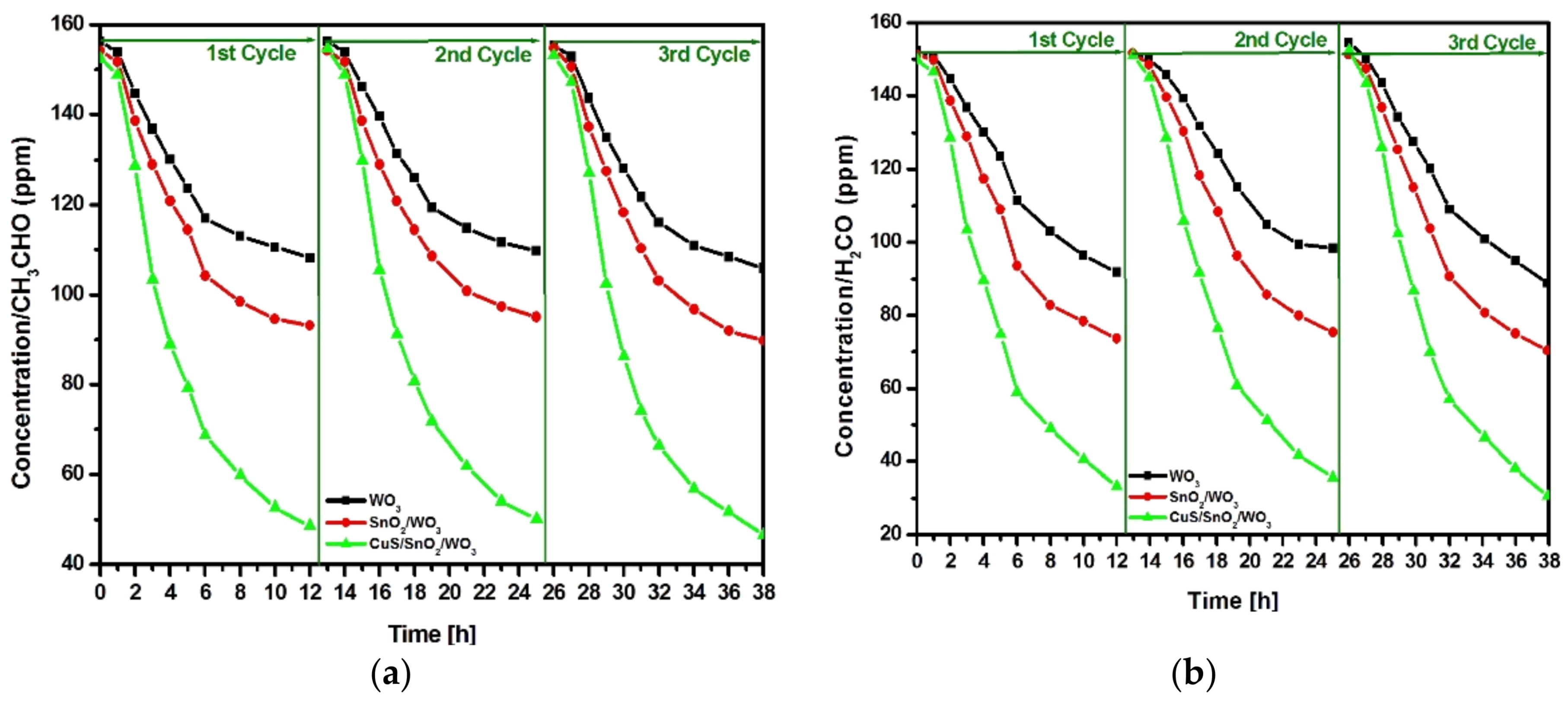
2.2.1. VOCs Degradation Efficiency and Kinetics
2.2.2. Heterostructure Photocatalytic Mechanism
3. Materials and Methods
3.1. Materials Synthesis and Heterostructure Deposition
3.1.1. Heterostructure Powder Synthesis
3.1.2. Film Deposition
3.2. Photocatatalytic Experiments
3.3. Materials Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, C.; Deng, M.; Deng, Q. Effect of outdoor air pollution and indoor environmental factors on small for gestational age. Build. Environ. 2021, 206, 108399. [Google Scholar] [CrossRef]
- Edginton, S.; O’Sullivan, D.E.; Lougheed, M.D. The effect of acute outdoor air pollution on peak expiratory flow in individuals with asthma: A systematic review and meta-analysis. Environ. Res. 2021, 192, 110296. [Google Scholar] [CrossRef] [PubMed]
- Havet, A.; Hulo, S.; Dauchet, L. Residential exposure to outdoor air pollution and adult lung function, with focus on small airway obstruction. Environ. Res. 2020, 183, 109161. [Google Scholar] [CrossRef]
- Ye, M.; Chen, L.; Liu, X.; Xu, W.; Zhu, T.; Chen, G. Catalytic Oxidation of Chlorobenzene over Ruthenium-Ceria Bimetallic Catalysts. Catalysts 2018, 8, 116. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Sun, L.; Sui, H.; He, L.; Yuan, W.; Han, Z. A Novel Polymeric Adsorbent Embedded with Phase Change Materials (PCMs) Microcapsules: Synthesis and Application. Nanomaterials 2019, 9, 736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.J.; Wi, S.; Jeong, S.-G.; Kim, S. Evaluation of the Adsorption Performance and Sustainability of Exfoliated Graphite Nanoplatelets (xGnP) for VOCs. Materials 2015, 8, 7615–7621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enesca, A.; Andronic, L.; Duta, A. Optimization of Opto-Electrical and Photocatalytic Properties of SnO2 Thin Films Using Zn2+ and W6+ Dopant Ions. Catal. Lett. 2012, 142, 224–230. [Google Scholar] [CrossRef]
- Jia, L.; Shi, J.; Xing, B. VOCs adsorption on activated carbon with initial water vapor contents: Adsorption mechanism and modified characteristic curves. Sci. Total Environ. 2020, 731, 139184. [Google Scholar] [CrossRef]
- Liang, Q.; Bao, X.; Chu, Y. Imaging VOC distribution in cities and tracing VOC emission sources with a novel mobile proton transfer reaction mass spectrometer. Environ. Pollut. 2020, 265, 114628. [Google Scholar] [CrossRef]
- Arı, A.; Arı, P.E.; Gaga, E.O. Source characterization and risk assessment of occupational exposure to volatile organic compounds (VOCs) in a barbecue restaurant. Build. Environ. 2020, 174, 106791. [Google Scholar] [CrossRef]
- Wantz, E.; Kane, A.; Couvert, A. A mathematical model for VOCs removal in a treatment process coupling absorption and biodegradation. Chem. Eng. J. 2021, 423, 130106. [Google Scholar] [CrossRef]
- Sansotera, M.; Kheyli, S.G.M.; Navarrini, W. Absorption and photocatalytic degradation of VOCs by perfluorinated ionomeric coating with TiO2 nanopowders for air purification. Chem. Eng. J. 2019, 361, 885–896. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Yu, C.; Ji, H. Recent advances in VOCs and CO removal via photothermal synergistic catalysis. Chin. J. Catal. 2021, 42, 1078–1095. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, X.; Sun, J. Difference of photodegradation characteristics between single and mixed VOC pollutants under simulated sunlight irradiation. J. Photochem. Photobiol. A 2019, 384, 112029. [Google Scholar] [CrossRef]
- Truc, N.T.T.; Pham, T.D.; Trang, H.T. Superior activity of Cu-NiWO4/g-C3N4 Z direct system for photocatalytic decomposition of VOCs in aerosol under visible light. J. Alloys Compd. 2019, 798, 12–18. [Google Scholar] [CrossRef]
- Vikrant, K.; Park, C.M.; Jeon, E.C. Recent advancements in photocatalyst-based platforms for the destruction of gaseous benzene: Performance evaluation of different modes of photocatalytic operations and against adsorption techniques. J. Photochem. Photobiol. C 2019, 41, 100316. [Google Scholar] [CrossRef]
- Heydari, G.; Hollman, J.; Achari, G.; Langford, C.H. Comparative Study of Four TiO2-Based Photocatalysts to Degrade 2,4-D in a Semi-Passive System. Water 2019, 11, 621. [Google Scholar] [CrossRef] [Green Version]
- Ehlert, M.; Radtke, A.; Topolski, A.; Śmigiel, J.; Piszczek, P. The Photocatalytic Activity of Titania Coatings Produced by Electrochemical and Chemical Oxidation of Ti6Al4V Substrate, Estimated According to ISO 10678:2010. Materials 2020, 13, 2649. [Google Scholar] [CrossRef]
- Dudita, M.; Bogatu, C.; Enesca, A.; Duta, A. The influence of the additives composition and concentration on the properties of SnOx thin films used in photocatalysis. Mater. Lett. 2011, 65, 2185–2189. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, L.; Ma, J.; Alam, N.; Zhou, X.; Ni, Y. Nano-Cellulose/MOF Derived Carbon Doped CuO/Fe3O4 Nanocomposite as High Efficient Catalyst for Organic Pollutant Remedy. Nanomaterials 2019, 9, 277. [Google Scholar] [CrossRef] [Green Version]
- Pu, S.; Wang, H.; Zhu, J.; Li, L.; Long, D.; Jian, Y.; Zeng, Y. Heterostructure Cu2O/(001)TiO2 Effected on Photocatalytic Degradation of Ammonia of Livestock Houses. Catalysts 2019, 9, 267. [Google Scholar] [CrossRef] [Green Version]
- Pang, C.; Mackevica, A.; Tian, J.; Feng, H.; Li, Z.; Baun, A. Release of Ag/ZnO Nanomaterials and Associated Risks of a Novel Water Sterilization Technology. Water 2019, 11, 2276. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; El-Shishtawy, R.M.; Barakat, M.A. Synthesis and Characterization of Ag-Ag2O/TiO2@polypyrrole Heterojunction for Enhanced Photocatalytic Degradation of Methylene Blue. Catalysts 2016, 6, 76. [Google Scholar] [CrossRef]
- Chen, H.; Liu, X.Y.; Zhang, Y.X. Facile biphasic synthesis of TiO2–MnO2 nanocomposites for photocatalysis. Ceram. Int. 2016, 42, 19425–19428. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, X.; Li, L. Synergic effects of CuxO electron transfer co-catalyst and valence band edge control over TiO2 for efficient visible-light photocatalysis. Chin. J. Catal. 2017, 38, 2120–2131. [Google Scholar] [CrossRef]
- Huang, F.; Hao, H.; Lang, X. Dye-TiO2/SiO2 assembly photocatalysis for blue light-initiated selective aerobic oxidation of organic sulfides. Chem. Eng. J. 2021, 423, 129419. [Google Scholar] [CrossRef]
- Theil, F.; Dellith, A.; Dietzek, B. Ru dye functionalized Au–SiO2@TiO2 and Au/Pt–SiO2@TiO2 nanoassemblies for surface-plasmon-induced visible light photocatalysis. J. Colloid Interface Sci. 2014, 421, 114–121. [Google Scholar] [CrossRef]
- Xu, W.; Tang, H.; Shen, Y. Enhanced photocatalytic activity of TiO2/Ag2O heterostructures by optimizing the separation of electric charges. Vacuum 2021, 190, 110283. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H. Photocatalysis of sprayed nitrogen-containing Fe2O3–ZnO and WO3–ZnO composite powders in gas-phase acetaldehyde decomposition. J. Photochem. Photobiol. A Chem. 2003, 160, 203–212. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, X.; Liu, C.; Qian, X.; Wen, Y.; Yang, Q.; Sun, T.; Chang, W.; Liu, X.; Chen, Z. Facile Construction of All-Solid-State Z-Scheme g-C3N4/TiO2 Thin Film for the Efficient Visible-Light Degradation of Organic Pollutant. Nanomaterials 2020, 10, 600. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Meng, X.; Zhang, Z. An Effective Approach to Improve the Photocatalytic Activity of Graphitic Carbon Nitride via Hydroxyl Surface Modification. Catalysts 2019, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Z.; Wang, Y.; Huang, W. Effect of the activator on the performance of alkali-activated slag mortars with pottery sand as fine aggregate. Constr. Build. Mater. 2019, 197, 83–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.; Lu, X. Experimental study of hysteretic behaviour for concrete-filled square thin-walled steel tubular columns. J. Constr. Steel Res. 2007, 63, 317–325. [Google Scholar] [CrossRef]
- Imran, M.; Alam, M.M.; Irshad, K. Highly photocatalytic active r-GO/Fe3O4 nanocomposites development for enhanced photocatalysis application: A facile low-cost preparation and characterization. Ceram. Int. 2021, 47, 31973–31982. [Google Scholar] [CrossRef]
- Noor, S.; Sajjad, S.; Long, M. Energy harvesting for electrochemical OER and solar photocatalysis via dual functional GO/TiO2-NiO nanocomposite. J. Clean. Prod. 2020, 277, 123280. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wang, B.K.; Hou, W.C. Graphitic carbon nitride embedded with graphene materials towards photocatalysis of bisphenol A: The role of graphene and mediation of superoxide and singlet oxygen. Chemosphere 2021, 278, 130334. [Google Scholar] [CrossRef]
- Enesca, A.; Duta, A. Tailoring WO3 thin layers using spray pyrolysis technique. Phys. Status Solidi C 2008, 5, 3499–3502. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Zheng, W. One-step synthesized CuS and MWCNTs composite as a highly efficient counter electrode for quantum dot sensitized solar cells. Mater. Des. 2018, 160, 870–875. [Google Scholar] [CrossRef]
- Yu, J.; Lee, T.I.; Misra, M. Synergetic impact of surface plasmon hot electron and CuS nanolayer of CuS/Au/ZnO core-shell nanorods for the degradation of toxic pollutant. J. Ind. Eng. Chem. 2018, 66, 468–477. [Google Scholar] [CrossRef]
- Peng, J.; Lu, X.; Yao, G. Degradation of atrazine by persulfate activation with copper sulfide (CuS): Kinetics study, degradation pathways and mechanism. Chem. Eng. J. 2018, 354, 740–752. [Google Scholar] [CrossRef]
- Kovacı, H.; Akaltun, Y.; Çelik, A. Investigation of the usage possibility of CuO and CuS thin films produced by successive ionic layer adsorption and reaction (SILAR) as solid lubricant. Surf. Coat. Technol. 2018, 344, 522–527. [Google Scholar] [CrossRef]
- Jilani, A.; Ansari, M.O.; Rehman, G.; Shakoor, M.B.; Hussain, S.Z.; Othman, M.H.D.; Ahmad, S.R.; Dustgeer, M.R.; Alshahri, A. Phenol removal and hydrogen production from water: Silver nanoparticles decorated on polyaniline wrapped zinc oxide nanorods. J. Ind. Eng. Chem. 2022, 109, 347–358. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, X.; Yang, Z. Biotemplate synthesis and photocatalysis performance of multilayer porous ZnWO4 nano-photocatalyst with rose petals as template. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127459. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, B.B.; Li, D.W. Carbon dots induced in-situ formation of porous europium micro-networks with enhanced photocatalysis. J. Colloid Interface Sci. 2022, 606, 600–606. [Google Scholar] [CrossRef]
- Mouchaal, Y.; Enesca, A.; Mihoreanu, C.; Khelil, A.; Duta, A. Tuning the opto-electrical properties of SnO2 thin films by Ag+1 and In+3 co-doping. Mater. Sci. Eng. B 2015, 199, 22–29. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Liu, S. Piezotronic effect and oxygen vacancies boosted photocatalysis C–N coupling of benzylamine. Nano Energy 2021, 83, 105831. [Google Scholar] [CrossRef]
- Feng, C.; Tang, L.; Wang, J. Maintaining stable LSPR performance of W18O49 by protecting its oxygen vacancy: A novel strategy for achieving durable sunlight driven photocatalysis. Appl. Catal. B Environ. 2020, 276, 119167. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Salaspuro, M. ALDH2-deficiency as genetic epidemiologic and biochemical model for the carcinogenicity of acetaldehyde. Regul. Toxicol. Pharm. 2017, 86, 128–136. [Google Scholar] [CrossRef]
- Kanjanasiranont, N.; Prueksasit, T.; Morknoy, D. Inhalation exposure and health risk levels to BTEX and carbonyl compounds of traffic policeman working in the inner city of Bangkok, Thailand. Atmos. Environ. 2017, 152, 111–120. [Google Scholar] [CrossRef]
- Gong, Y.; Wei, Y.; Xu, B. Health risk assessment and personal exposure to Volatile Organic Compounds (VOCs) in metro carriages—A case study in Shanghai, China. Sci. Total Environ. 2017, 574, 1432–1438. [Google Scholar] [CrossRef]
- Bari, M.A.; Kindzierski, W.B. Ambient volatile organic compounds (VOCs) in communities of the Athabasca oil sands region: Sources and screening health risk assessment. Environ. Pollut. 2018, 235, 602–614. [Google Scholar] [CrossRef] [Green Version]
- Dai, W.; Zhong, H.; Ho, K.F. Characterization and health risk assessment of airborne pollutants in commercial restaurants in northwestern China: Under a low ventilation condition in wintertime. Sci. Total Environ. 2018, 633, 308–316. [Google Scholar] [CrossRef]
- Enesca, A.; Isac, L.; Duta, A. Charge carriers injection in tandem semiconductors for dyes mineralization. App. Catal. B 2015, 162, 352–363. [Google Scholar] [CrossRef]
- Wang, H.; Cui, W.; Dong, F. Interfacial activation of reactants and intermediates on CaSO4 insulator-based heterostructure for efficient photocatalytic NO removal. Chem. Eng. J. 2020, 390, 124609. [Google Scholar] [CrossRef]
- Ji, H.; Du, P.; Liu, W. 2D/1D graphitic carbon nitride/titanate nanotubes heterostructure for efficient photocatalysis of sulfamethazine under solar light: Catalytic “hot spots” at the rutile–anatase–titanate interfaces. Appl. Catal. B Environ. 2020, 263, 118357. [Google Scholar] [CrossRef]
- Su, J.; Yu, S.; Zhao, W. Enhanced visible light photocatalytic performances of few-layer MoS2@TiO2 hollow spheres heterostructures. Mater. Res. Bull. 2020, 130, 110936. [Google Scholar] [CrossRef]
- Lin, W.; Xie, X.; Wang, X.; Wang, Y.; Segets, D.; Sun, J. Efficient adsorption and sustainable degradation of gaseous acetaldehyde and o-xylene using rGO-TiO2 photocatalyst. Chem. Eng. J. 2018, 349, 708–718. [Google Scholar] [CrossRef]
- Zeng, Q.; Xie, X.; Wang, X.; Wang, Y.; Lu, G.; Pui, D.Y.H.; Sun, J. Enhanced photocatalytic performance of Ag@TiO2 for the gaseous acetaldehyde photodegradation under fluorescent lamp. Chem. Eng. J. 2018, 341, 83–92. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, X.; Wang, X.; Wang, Y.; Zeng, Y.; Pui, D.Y.H.; Sun, J. Visible-Light Upconversion Carbon Quantum Dots Decorated TiO2 for the Photodegradation of Flowing Gaseous Acetaldehyde. Appl. Surf. Sci. 2018, 440, 266–274. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, X.; Xie, X.; Mahmood, A.; Lu, G.; Wang, Y.; Sun, J. Band bending of TiO2 induced by O-xylene and acetaldehyde adsorption and its effect on the generation of active radicals. J. Colloid Interface Sci. 2020, 572, 374–383. [Google Scholar] [CrossRef]
- Wang, H.; Dong, X.; Tang, R.; Li, J.; Sun, Y.; Wanga, Z.; Kimb, K.H.; Dong, F. Selective breakage of CH bonds in the key oxidation intermediates of gaseous formaldehyde on self-doped CaSn(OH)6 cubes for safe and efficient photocatalysis. Appl. Catal. B 2020, 277, 119214. [Google Scholar] [CrossRef]
- Laciste, M.T.; Luna, M.D.G.; Tolosa, N.C.; Lu, M.C. Degradation of gaseous formaldehyde via visible light photocatalysis using multi-element doped titania nanoparticles. Chemosphere 2017, 182, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Yang, R.; Li, C.; Zhang, X.; Sun, Z. Enhanced visible-light properties of TiO2/diatomite composite over varied bismuth semiconductors modification for formaldehyde photodegradation: A comparative study. Sep. Purif. Technol. 2022, 297, 121477. [Google Scholar] [CrossRef]
- Jilani, A.; Hussain, S.Z.; Melaibari, A.A.; Abu-Hamdeh, N.H. Development and Mechanistic Studies of Ternary Nanocomposites for Hydrogen Production from Water Splitting to Yield Sustainable/Green Energy and Environmental Remediation. Polymers 2022, 14, 1290. [Google Scholar] [CrossRef]
- Baneto, M.; Enesca, A.; Mihoreanu, C.; Lare, Y.; Jondo, K.; Napo, K.; Duta, A. Effects of the growth temperature on the properties of spray deposited CuInS2 thin films for photovoltaic applications. Ceram. Int. 2015, 41, 4742–4749. [Google Scholar] [CrossRef]
- Gao, C.; Li, J.; Shan, Z.; Huang, F.; Shen, H. Preparation and visible-light photocatalytic activity of In2S3/TiO2 composite. Mater. Chem. Phys. 2010, 122, 183–187. [Google Scholar] [CrossRef]
- Mise, T.; Nakada, T. Low temperature growth and properties of Cu–In–Te based 433 thin films for narrow bandgap solar cells. Thin Solid Films 2010, 518, 5604–5609. [Google Scholar] [CrossRef]
- Wu, J.; Wang, W.; Xue, H. Piezotronic effect boosted photocatalytic performance of heterostructured BaTiO3/TiO2 nanofibers for degradation of organic pollutants. Nano Energy 2020, 77, 105122. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Tang, W. Designing strained C2N/GaTe(InTe) heterostructures for photovoltaic and photocatalytic application. J. Alloys Compd. 2020, 816, 152559. [Google Scholar] [CrossRef]
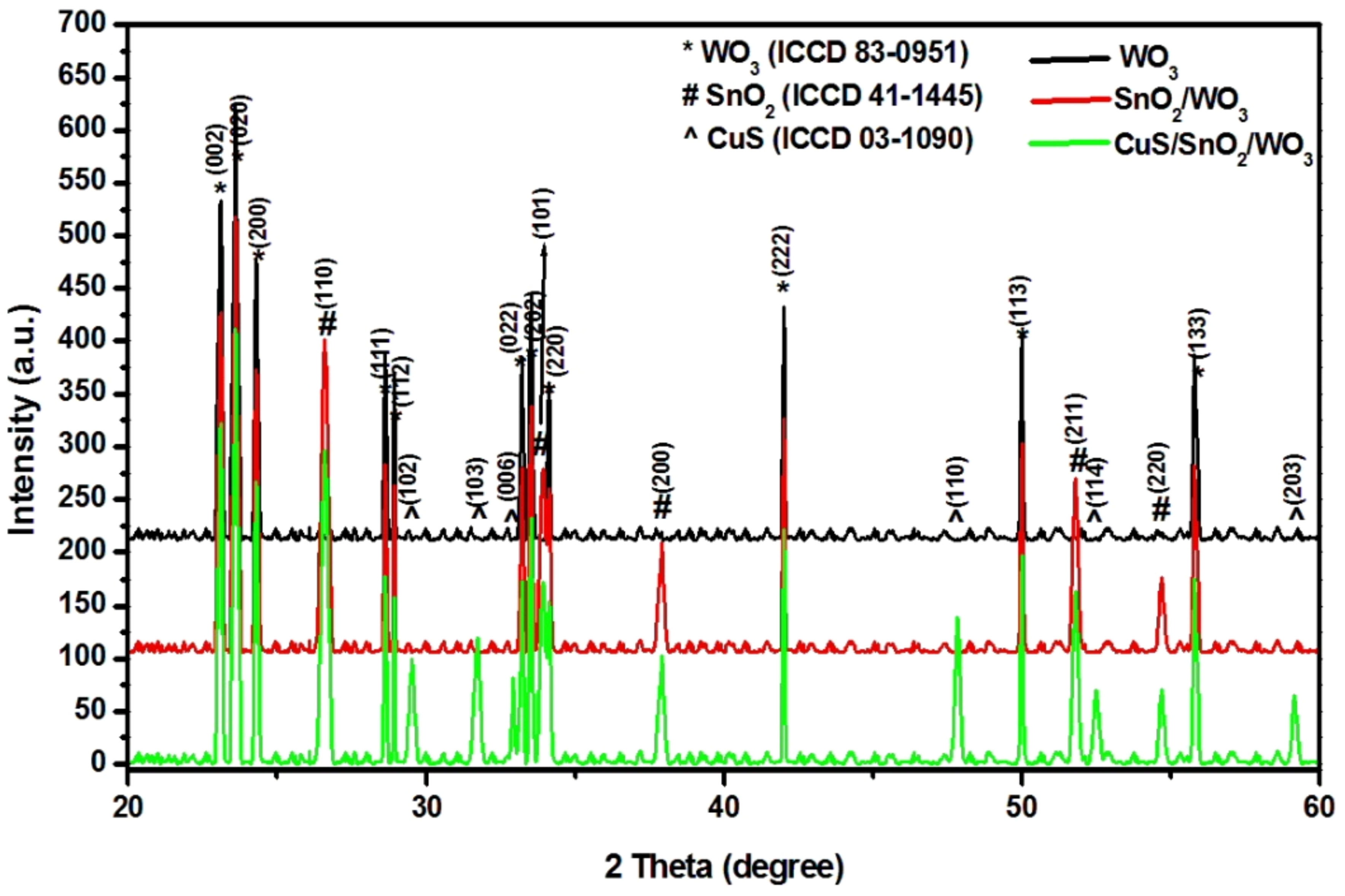

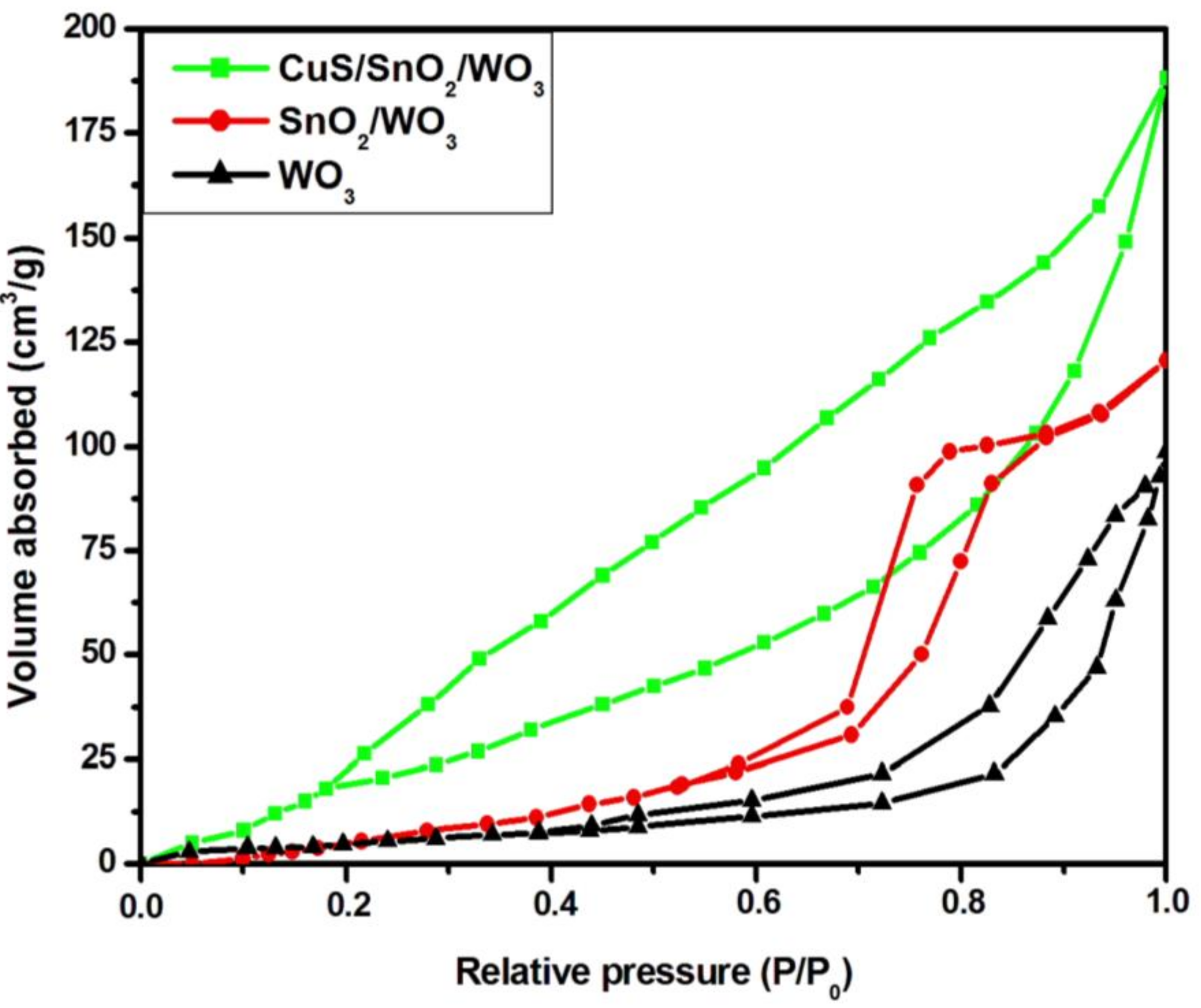


| Samples | Crystallite Size (Å) | ||
|---|---|---|---|
| WO3 | SnO2 | CuS | |
| WO3 | 84.2 | - | - |
| SnO2/WO3 | 83.7 | 78.1 | - |
| CuS/SnO2/WO3 | 83.4 | 77.7 | 51.5 |
| Samples | Elemental Composition [% at.] | ||||||
|---|---|---|---|---|---|---|---|
| W | Sn | Cu | O | Oth 1 | S | Sth 1 | |
| WO3 | 24.6 | 75.4 | 73.8 | ||||
| SnO2/WO3 | 11.2 | 16.4 | 72.4 | 66.2 | |||
| CuS/SnO2/WO3 | 9.7 | 11.8 | 13.5 | 54.8 | 52.7 | 10.2 | 13.5 |
| Materials | Pollutant Type and Concentration | Radiation Type and Intensity | Photocatalytic Efficiency and Degradation Time | Ref. |
|---|---|---|---|---|
| (rGO)-TiO2 | Acetaldehyde, 25 ppm | Vis, 200 W | 42%, 160 min | [57] |
| o-xylene, 25 ppm | 54%, 160 min | |||
| Ag@TiO2 | Acetaldehyde, 500 ppm | UV, 260 W | 72%, 4.8 min | [58] |
| Carbon quantum dots/TiO2 | Acetaldehyde, 500 ppm | Vis, 400 W | 30%, 120 min | [59] |
| Rutile TiO2 | Acetaldehyde, 50 ppm | Vis, 260 W | 65%, 65 min | [60] |
| Sn-CaSn(OH)6(m) | Formaldehyde, 100 ppm | UV, 300 W | 30%, 60 min | [61] |
| Doped TiO2 | Formaldehyde, 37% | LED, 25.7 W/m2 | 43%, 120 min | [62] |
| Bi2MoO6-TiO/diatomite | Formaldehyde, 35 mg/m3 | Vis, 300 W | 50%, 300 min | [63] |
| CuS/SnO2/WO3 | Acetaldehyde, 170 ppm | UV–Vis, 300W | 69.2%, 720 min | This work |
| Formaldehyde, 170 ppm | 78.5%, 720 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enesca, A.; Sisman, V. Indoor Air Photocatalytic Decontamination by UV–Vis Activated CuS/SnO2/WO3 Heterostructure. Catalysts 2022, 12, 728. https://doi.org/10.3390/catal12070728
Enesca A, Sisman V. Indoor Air Photocatalytic Decontamination by UV–Vis Activated CuS/SnO2/WO3 Heterostructure. Catalysts. 2022; 12(7):728. https://doi.org/10.3390/catal12070728
Chicago/Turabian StyleEnesca, Alexandru, and Viorel Sisman. 2022. "Indoor Air Photocatalytic Decontamination by UV–Vis Activated CuS/SnO2/WO3 Heterostructure" Catalysts 12, no. 7: 728. https://doi.org/10.3390/catal12070728
APA StyleEnesca, A., & Sisman, V. (2022). Indoor Air Photocatalytic Decontamination by UV–Vis Activated CuS/SnO2/WO3 Heterostructure. Catalysts, 12(7), 728. https://doi.org/10.3390/catal12070728






