Elucidating Evidence for the In Situ Reduction of Graphene Oxide by Magnesium Hydride and the Consequence of Reduction on Hydrogen Storage
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
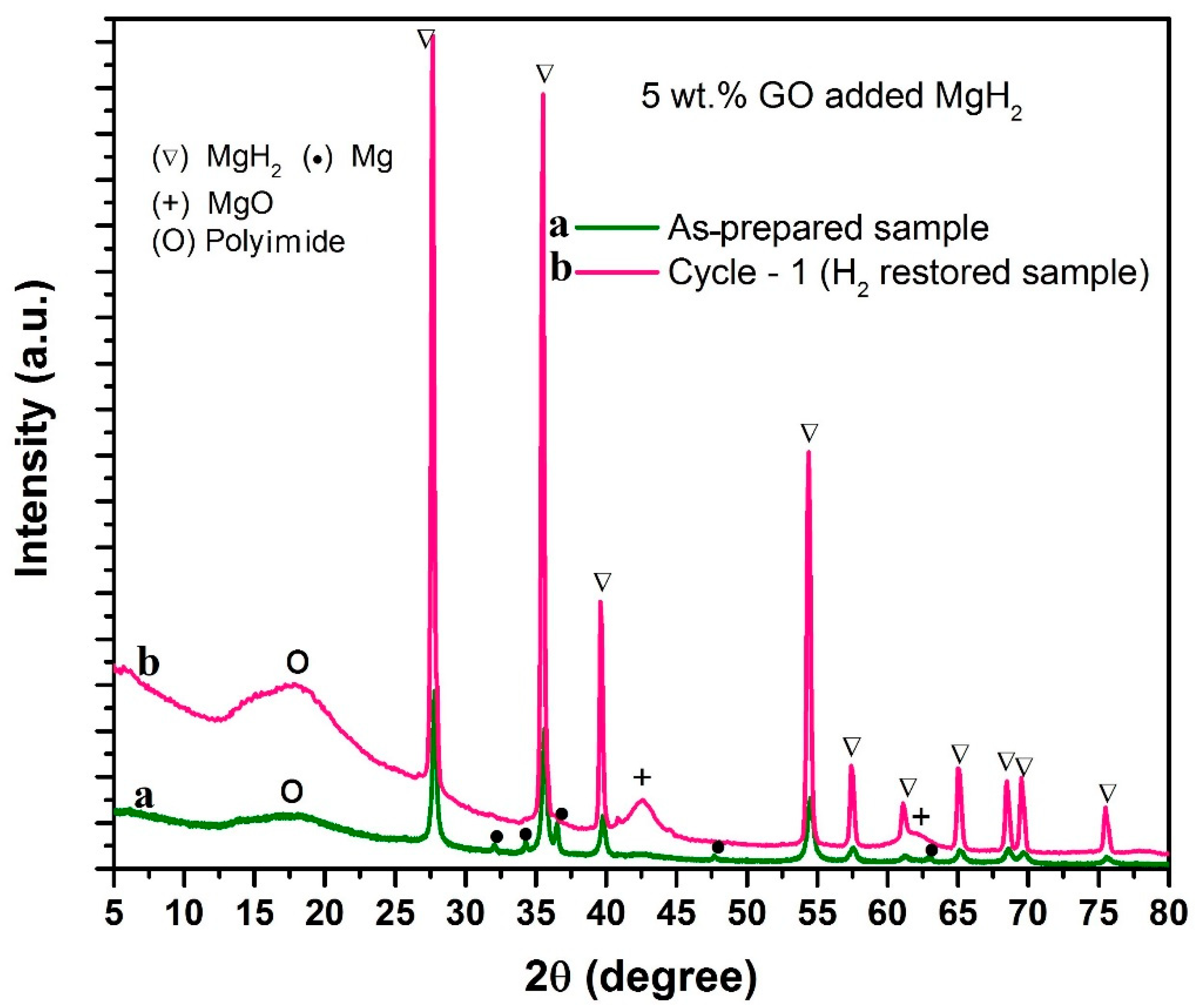
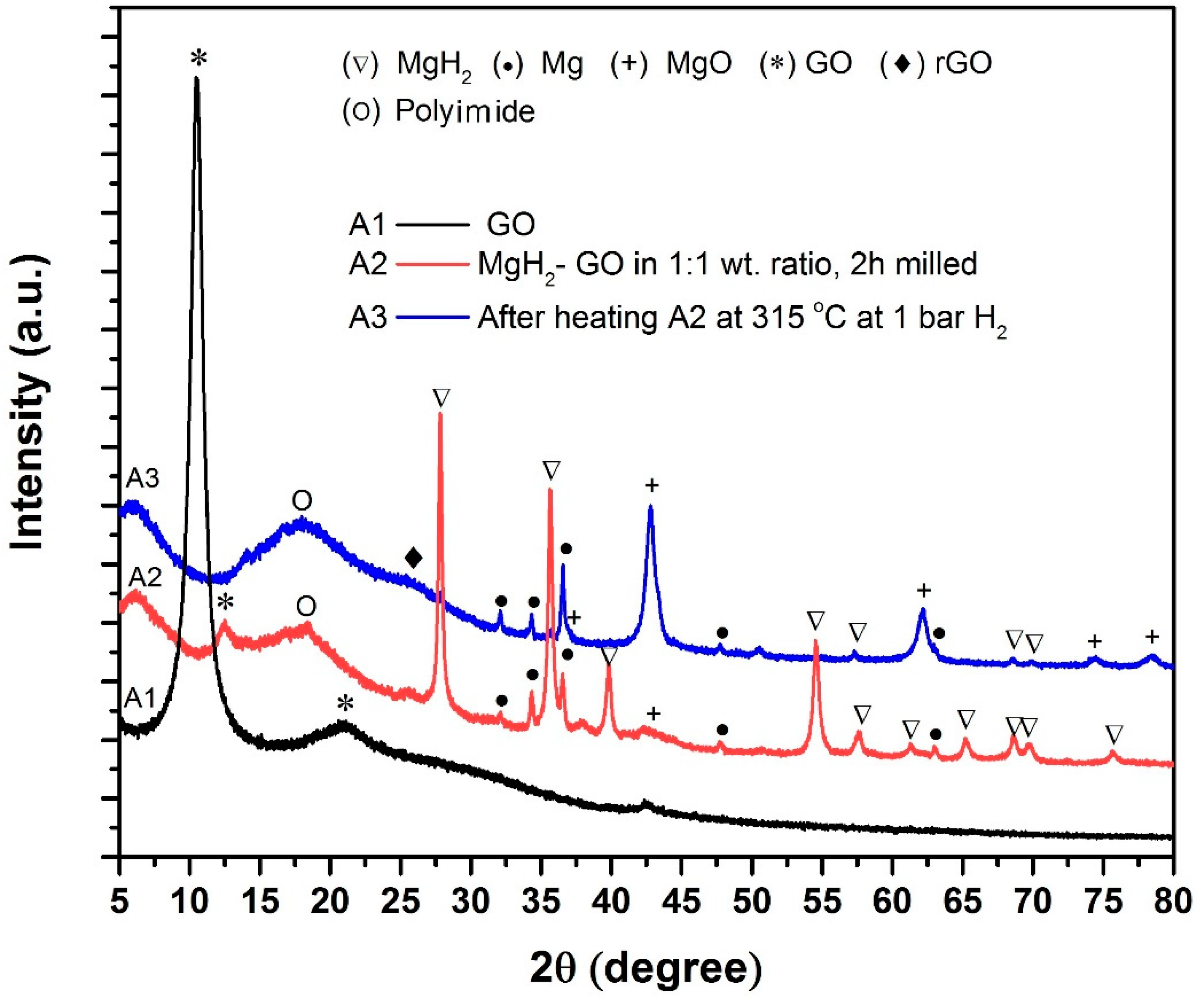
- As per XRD observations, GO and MgH2 react during ball-milling itself (reaction time 2 h, milling speed 150 rpm). However, substantial transformation from MgH2/GO to MgO/rGO is witnessed after heating the sample at 315 °C.
- DSC study proves that peak dehydrogenation in the case of rGO containing MgH2 occurs by about 58 °C lower temperature as compared to that of GO-containing MgH2.
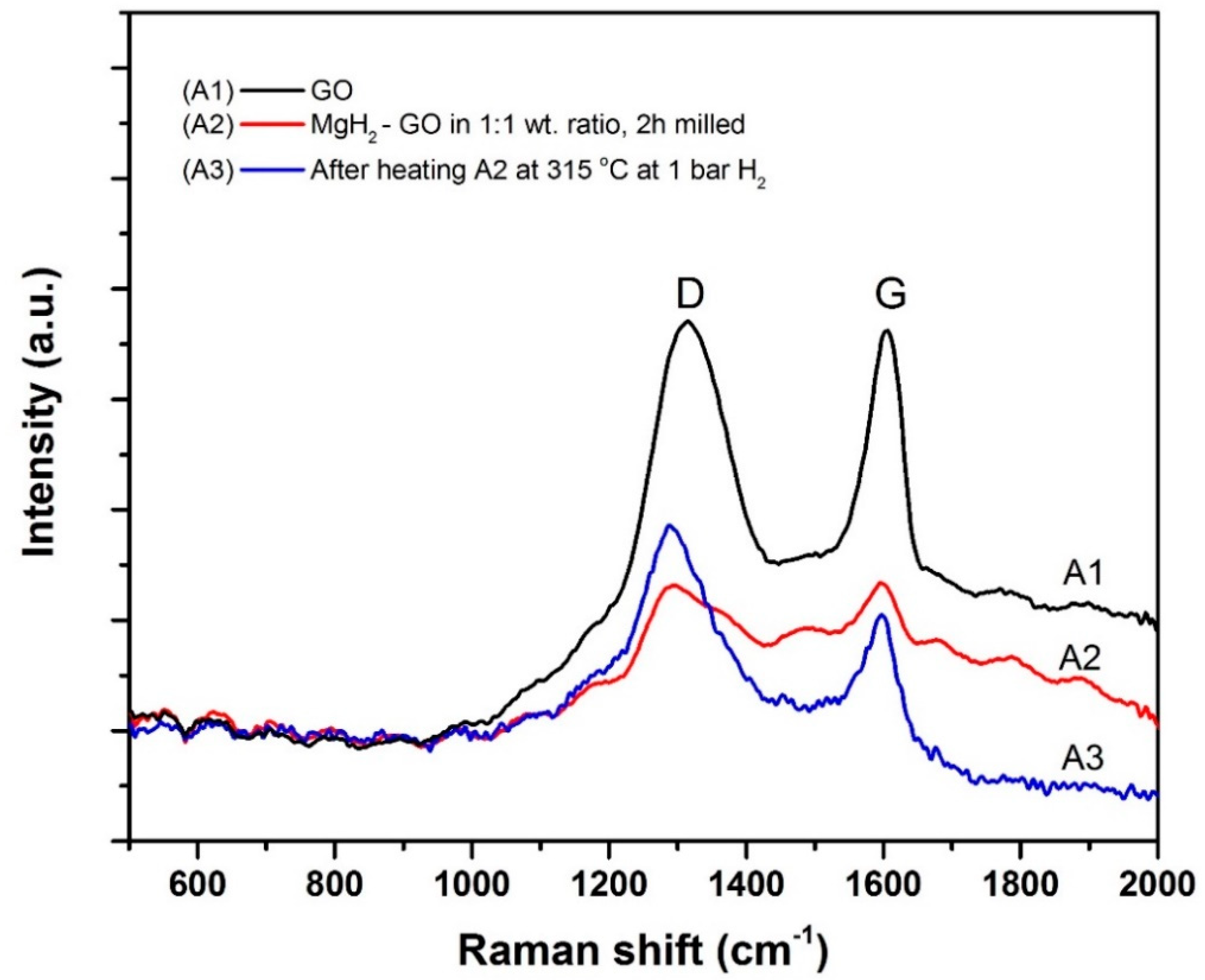
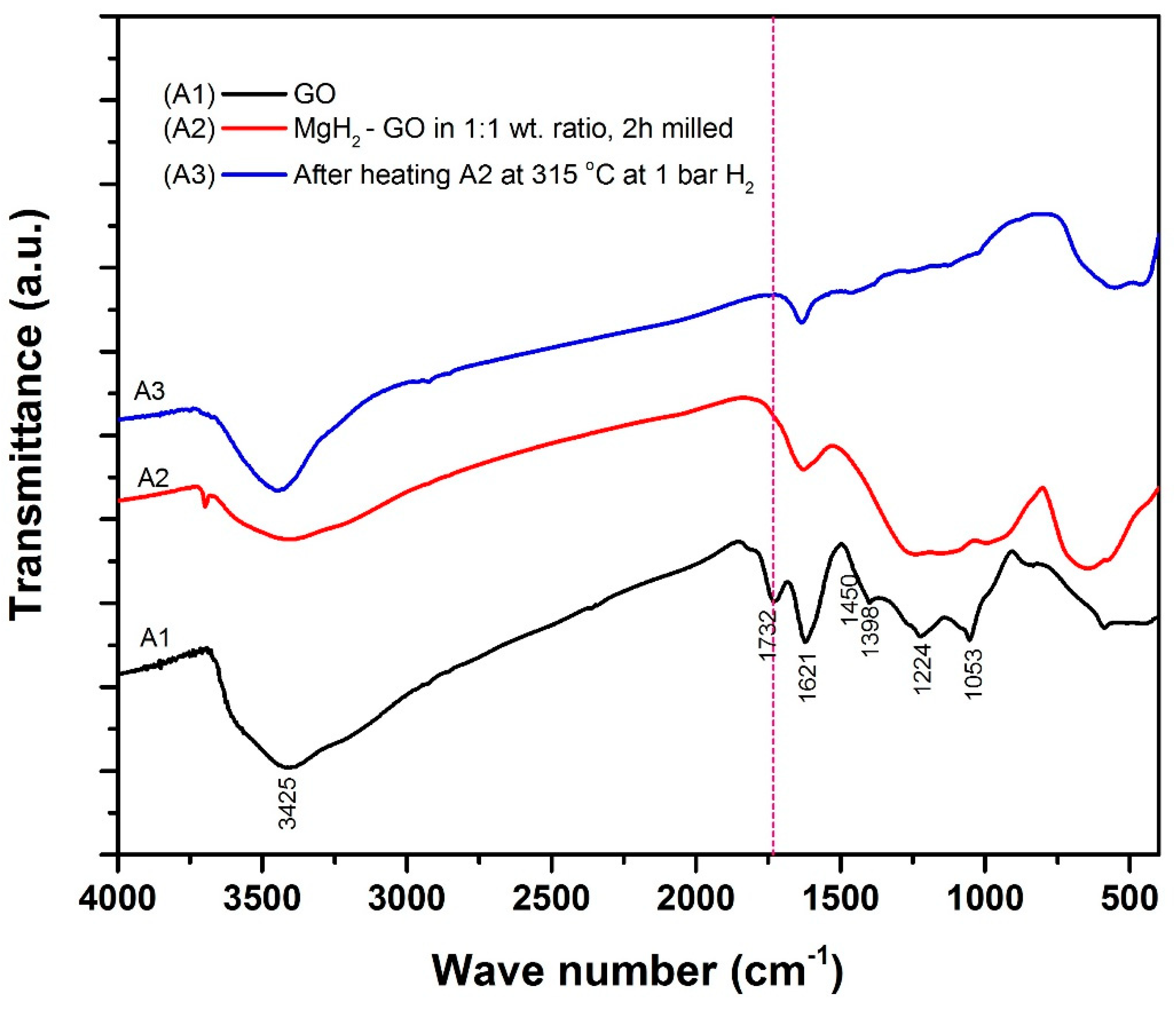
- FTIR and Raman spectroscopy studies further prove that, with mild mechanical milling itself, there is GO to rGO transformation in the powder. On the other hand, significant transformation occurs after heating the powder at 315 °C.
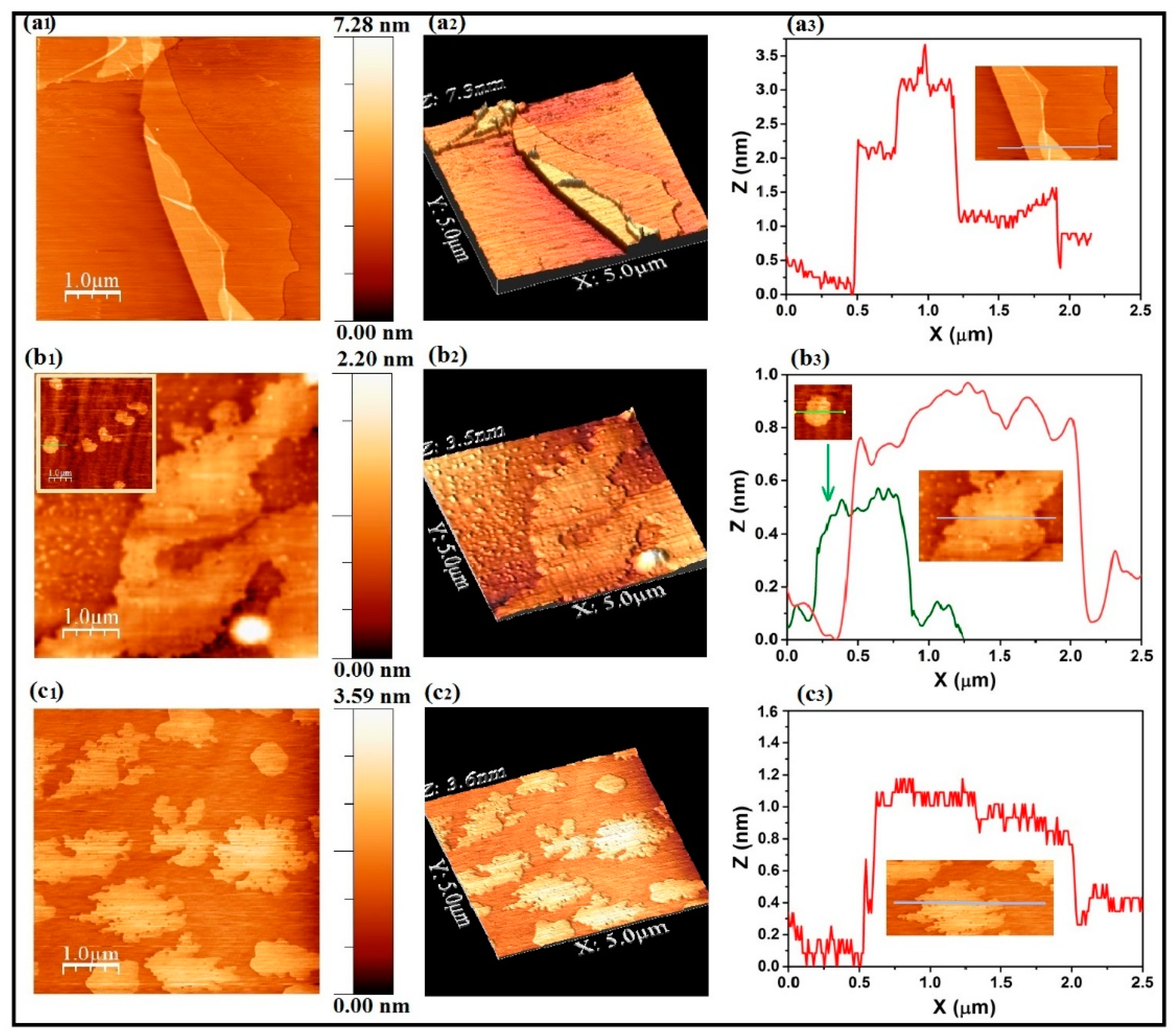
- AFM studies prove that the length and thickness of rGO flakes is smaller than that of the starting GO sample. Evidence for monolayer rGO is observed in the case of heat-treated sample.
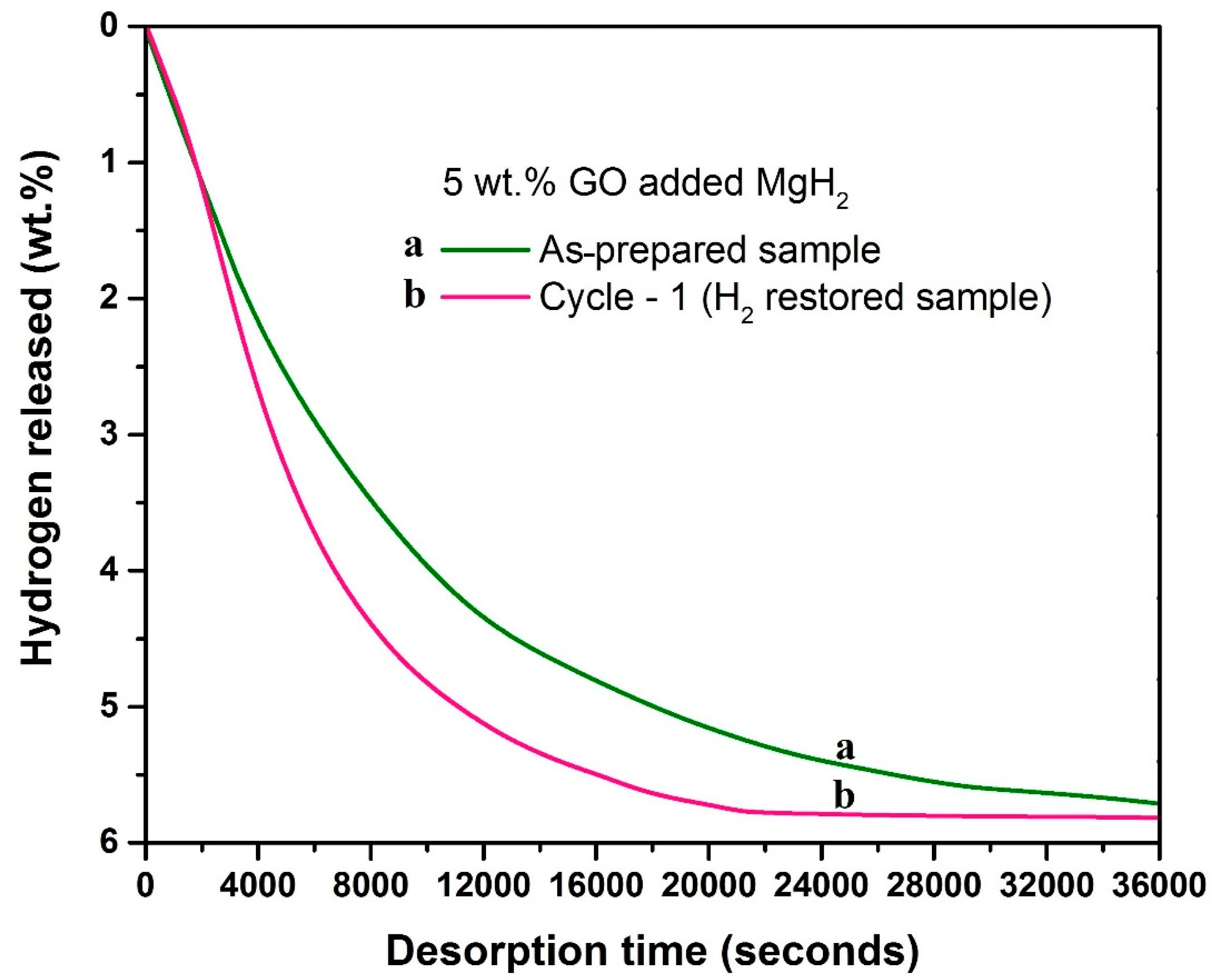
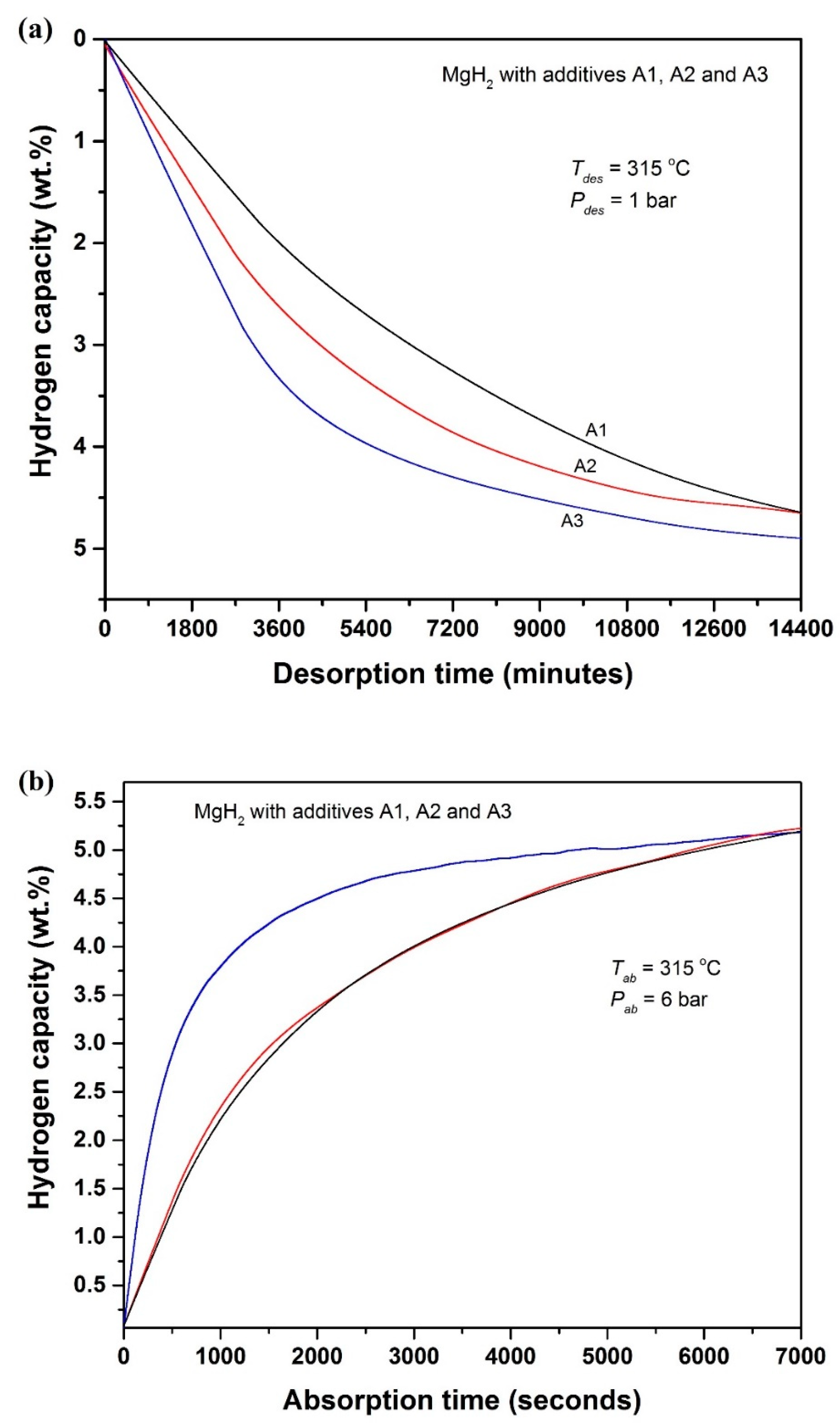
- The in situ GO to rGO transformation also improves the dehydrogenation of MgH2. Nearly two times improved dehydrogenation kinetics is observed during the isothermal dehydrogenation at 315 °C. On the other hand, during H2 absorption (315 °C/6 bar H2) the kinetics improves about three times with respect to the starting sample.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- U.S. DRIVE Partnership. Target Explanation Document: Onboard Hydrogen Storage for Light-Duty Fuel Cell Vehicles. 2017; pp. 1–19. Available online: https://www.energy.gov/sites/prod/files/2017/05/f34/fcto_targets_onboard_hydro_storage_explanation.pdf (accessed on 5 June 2022).
- Pukazhselvan, D.; Kumar, D.; Singh, S.K. High capacity hydrogen storage: Basic aspects, new developments and milestones. Nano Energy 2012, 1, 566–589. [Google Scholar] [CrossRef]
- Oelerich, W.; Klassen, T.; Bormann, R. Metal oxides as catalysts for improved hydrogen sorption in nanocrystalline Mg-based materials. J. Alloys Compd. 2001, 315, 237–242. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Fujii, H. Catalytic effect of nanoparticle 3d-transition metals on hydrogen storage properties in magnesium hydride MgH2 prepared by mechanical milling. J. Phys. Chem. B 2005, 109, 7188–7194. [Google Scholar] [CrossRef] [PubMed]
- Pukazhselvan, D.; Sandhya, K.S.; Ramasamy, D.; Shaula, A.; Fagg, D.P. Transformation of metallic Ti to TiH2 phase in the Ti/MgH2 composite and its influence on the hydrogen storage behavior of MgH2. ChemPhysChem 2020, 21, 1195–1201. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Bdikin, I.; Perez, J.; Carbo-Argibay, E.; Antunes, I.; Stroppa, D.G.; Fagg, D.P. Formation of Mg-Nb-O rock salt structures in a series of mechanochemically activated MgH2 + nNb2O5 (n = 0.083–1.50) mixtures. Int. J. Hydrogen Energy 2016, 41, 2677–2688. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Nasani, N.; Correia, P.; Carbo-Argibay, E.; Otero-Irurueta, G.; Stroppa, D.G.; Fagg, D.P. Evolution of reduced Ti containing phase(s) in MgH2/TiO2 system and its effect on the hydrogen storage behavior of MgH2. J. Power Sources 2017, 362, 174–183. [Google Scholar] [CrossRef]
- Zhou, C.; Fang, Z.Z.; Ren, C.; Li, J.; Lu, J. Effect of Ti intermetallic catalysts on hydrogen storage properties of magnesium hydride. J. Phys. Chem. C 2013, 117, 12973–12980. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Zang, L.; Chang, X.; Jiao, L.; Yuan, H.; Wang, Y. Core-Shell Ni3N@nitrogen-doped carbon: Synthesis and application in MgH2. J. Alloys Compd. 2017, 703, 381–388. [Google Scholar] [CrossRef]
- Ismail, M. Effect of LaCl3 addition on the hydrogen storage properties of MgH2. Energy 2015, 79, 177–182. [Google Scholar] [CrossRef]
- Lin, H.J.; Matsuda, J.; Li, H.W.; Zhu, M.; Akiba, E. Enhanced hydrogen desorption property of MgH2 with the addition of cerium fluorides. J. Alloys Compd. 2015, 645, S392–S396. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Yu, H.; Lu, Z.; He, J.; Zheng, J.; Wu, F.; Chen, L. Achieving superior hydrogen storage properties of MgH2 by the effect of TiFe and carbon nanotubes. Chem. Eng. J. 2021, 422, 130101. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, X.F.; Mao, C.; Long, C.G.; Chen, J.; Zhou, D.W. Influences and mechanisms of graphene-doping on dehydrogenation properties of MgH2: Experimental and first-principles studies. Energy 2015, 89, 957–964. [Google Scholar] [CrossRef]
- Lillo-Rodenas, M.A.; Guo, Z.X.; Aguey-Zinsou, K.F.; Cazorla-Amoros, D.; Linares-Solano, A. Effects of different carbon materials on MgH2 decomposition. Carbon 2008, 46, 126–137. [Google Scholar] [CrossRef]
- Wu, C.Z.; Wang, P.; Yao, X.; Liu, C.; Chen, D.M.; Lu, G.Q.; Cheng, H.M. Hydrogen storage properties of MgH2/SWNT composite prepared by ball milling. J. Alloys Compd. 2006, 420, 278–282. [Google Scholar] [CrossRef]
- Liu, J.; Ma, Z.; Liu, Z.; Tang, Q.; Zhu, Y.; Lin, H.; Zhang, Y.; Zhang, J.; Liu, Y.; Li, L. Synergistic effect of RGO supported Ni3Fe on hydrogen storage performance of MgH2. Int. J. Hydrogen Energy 2020, 45, 16622–16633. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, Z.; Ma, Z.; Ding, Y.; Zhu, Y.; Zhang, Y.; Zhang, J.; Li, L. Effect of RGO Supported NiCu derived from layered double hydroxide on hydrogen sorption kinetics of MgH2. J. Alloys Compd. 2019, 789, 768–776. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Hu, Y.; Sun, C.; Leng, H.; Li, Q.; Wu, C. Enhanced catalytic effect of TiO2@RGO synthesized by one-pot ethylene glycol-assisted solvothermal method for MgH2. J. Alloys Compd. 2021, 881, 160644. [Google Scholar] [CrossRef]
- Wang, K.; Wu, G.; Cao, H.; Li, H.; Zhao, X. Improved reversible dehydrogenation properties of MgH2 by the synergetic effects of graphene oxide-based porous carbon and TiCl3. Int. J. Hydrogen Energy 2018, 43, 7440–7446. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, Q.; Guo, X.; Yang, X. Achieve high-efficiency hydrogen storage of MgH2 catalyzed by nanosheets CoMoO4 and RGO. J. Alloys Compd. 2022, 911, 165153. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, L.; Yang, X.; Zhu, X.; Chen, L. The remarkably improved hydrogen storage performance of MgH2 by the synergetic effect of an FeNi/RGO nanocomposite. Dalton Trans. 2020, 49, 4146–4154. [Google Scholar] [CrossRef]
- Yao, P.; Jiang, Y.; Liu, Y.; Wu, C.; Chou, K.C.; Lyu, T.; Li, Q. Catalytic effect of Ni@RGO on the hydrogen storage properties of MgH2. J. Magnes. Alloy. 2020, 8, 461–471. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Narendar, N.; Singh, S.K.; Fagg, D.P. Metal oxide additives incorporated hydrogen storage systems: Formation of in situ catalysts and mechanistic understanding. In Emerging Nanostructured Materials for Energy and Environmental Science; Rajendran, S., Naushad, M., Raju, K., Boukherroub, R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 215–245. [Google Scholar]
- Pukazhselvan, D.; Otero-Irurueta, G.; Pérez, J.; Singh, B.; Bdikin, I.; Singh, M.K.; Fagg, D.P. Crystal structure, phase stoichiometry and chemical environment of MgxNbyOx+y nanoparticles and their impact on hydrogen storage in MgH2. Int. J. Hydrogen Energy 2016, 41, 11709–11715. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Narendar, N.; Sandhya, K.S.; Singh, B.; Bdikin, I.; Koga, N.; Fagg, D.P. Role of chemical interaction between MgH2 and TiO2 additive on the hydrogen storage behavior of MgH2. Appl. Surf. Sci. 2017, 420, 740–745. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Sandhya, K.S.; Ramasamy, D.; Shaula, A.; Bdikin, I.; Fagg, D.P. Active catalytic species generated in situ in zirconia incorporated hydrogen storage material magnesium hydride. J. Magnes. Alloy. 2022, 10, 786–796. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Silva, D.A.R.; Sandhya, K.S.; Fateixa, S.; Shaula, A.; Nogueira, H.; Bdikin, I.; Fagg, D.P. Interaction of zirconia with magnesium hydride and its Influence on the hydrogen storage behavior of magnesium hydride. Int. J. Hydrogen Energy 2022, 47, 21760–21771. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Sandhya, K.S.; Narendar, N.; Fagg, D.P. Chemical transformation of additive phase in MgH2/CeO2 hydrogen storage system and its effect on catalytic performance. Appl. Surf. Sci. 2021, 561, 150062. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Lavin-Lopez, M.P.; Paton-Carrero, A.; Sanchez-Silva, L.; Valverde, J.L.; Romero, A. Influence of the reduction strategy in the synthesis of reduced graphene oxide. Adv. Powder Technol. 2017, 28, 3195–3203. [Google Scholar] [CrossRef] [Green Version]
- Lesiak, B.; Trykowski, G.; Toth, J.; Biniak, S.; Kover, L.; Rangam, L.; Stobinski, L.; Malolepszy, A. Chemical and structural properties of reduced graphene oxide—Dependence on the reducing agent. J. Mater. Sci. 2021, 56, 3738–3754. [Google Scholar] [CrossRef]
- Guex, L.G.; Sacchi, B.; Peuvot, K.F.; Andersson, R.L.; Pourrahimi, A.M.; Strom, V.; Farris, S.; Olsson, R.T. Experimental review: Chemical reduction of graphene oxide (GO) to reduced graphene oxide (RGO) by aqueous chemistry. Nanoscale 2017, 9, 9562–9571. [Google Scholar] [CrossRef] [Green Version]
- Johra, F.T.; Jung, W.G. Effect of pH on the synthesis and characteristics of RGO-CdS nanocomposites. Appl. Surf. Sci. 2014, 317, 1015–1021. [Google Scholar] [CrossRef]
- Szabo, T.; Berkesi, O.; Forgo, P.; Josepovits, K.; Sanakis, Y.; Petridis, D.; Dekany, I. Evolution of surface functional groups in a series of progressively oxidized graphite oxides. Chem. Mater. 2006, 18, 2740–2749. [Google Scholar] [CrossRef]
- Johra, F.T.; Jung, W.G. Hydrothermally reduced graphene oxide as a supercapacitor. Appl. Surf. Sci. 2015, 357, 1911–1914. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Perez, J.; Nasani, N.; Bdikin, I.; Kovalevsky, A.V.; Fagg, D.P. Formation of MgxNbyOx+y through the mechanochemical reaction of MgH2 and Nb2O5, and its effect on the hydrogen-storage behavior of MgH2. ChemPhysChem 2016, 17, 178–183. [Google Scholar] [CrossRef]
- Nyquist, R.A.; Kagel, R.O. Handbook of Infrared and Raman Spectra of Inorganic Compounds and Organic Salts: Infrared Spectra of Inorganic Compounds; Academic Press: Cambridge, MA, USA, 2012; Volume 4. [Google Scholar]
- Yanwu, Z.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar]
- Alhadhrami, A.; Salgado, S.; Maheshwari, V. Thermal reduction to control the spacing in graphene oxide membranes: Effect on ion diffusion and electrical conduction. RSC Adv. 2016, 6, 70012–70017. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.F.; Ares-Fernandez, J.R.; Klassen, T.; Bormann, R. Using MgO to improve the (de)hydriding properties of magnesium. Mater. Res. Bull. 2006, 41, 1118–1126. [Google Scholar] [CrossRef]
- Ares-Fernández, J.R.; Kondo-Francois, A.Z. Superior MgH2 kinetics with MgO addition: A tribological effect. Catalysts 2012, 2, 330–343. [Google Scholar] [CrossRef] [Green Version]
- Tarcan, R.; Todor-Boer, O.; Petrovai, I.; Leordean, C.; Astilean, S.; Botiz, I. Reduced graphene oxide today. J. Mater. Chem. C 2020, 8, 1198–1224. [Google Scholar] [CrossRef]
- Peng, K.; Zhu, L.; Li, F.; Xu, G. Self-supporting electrode composed of SnSe nanosheets, thermally treated protein, and reduced graphene oxide with enhanced pseudocapacitance for advanced sodium-ion batteries. ChemElectroChem 2019, 6, 5642–5650. [Google Scholar]
- Li, J.; Jiang, J.; Zhao, D.; Xu, Z.; Liu, M.; Liu, X.; Tong, H.; Qian, D. Novel hierarchical sea urchin-like prussian blue@palladium core-shell heterostructures supported on nitrogen-doped reduced graphene oxide: Facile synthesis and excellent guanine sensing performance. Electrochim. Acta 2020, 330, 135196. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Yang, H.; Wen, L.; Yi, Z.; Zhou, Z.; Dai, B.; Zhang, J.; Wu, X.; Wu, P. Multi-mode surface plasmon resonance absorber based on dart-type single-layer graphene. RSC Adv. 2022, 12, 7821–7829. [Google Scholar] [CrossRef]
- Li, J.; Jiang, J.; Xu, Z.; Liu, M.; Tang, S.; Yang, C.; Qian, D. Facile synthesis of Ag@Cu2O heterogeneous nanocrystals decorated N-doped reduced graphene oxide with enhanced electrocatalytic activity for ultrasensitive detection of H2O2. Sens. Actuators B Chem. 2018, 260, 529–540. [Google Scholar] [CrossRef]
- Cheng, Z.; Liao, J.; He, B.; Zhang, F.; Zhang, F.; Huang, X.; Zhou, L. One-step fabrication of graphene oxide enhanced magnetic composite gel for highly efficient dye adsorption and catalysis. ACS Sustain. Chem. Eng. 2015, 3, 1677–1685. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Feng, J.; Ye, Y.; Xiao, M.; Wu, G.; Ke, Y. Synthetic routes of the reduced graphene oxide. Chem. Pap. 2020, 74, 3767–3783. [Google Scholar] [CrossRef]
- Blaine, R.L.; Kissinger, H.E. Homer Kissinger and the Kissinger Equation. Thermochim. Acta 2012, 540, 1–6. [Google Scholar] [CrossRef]

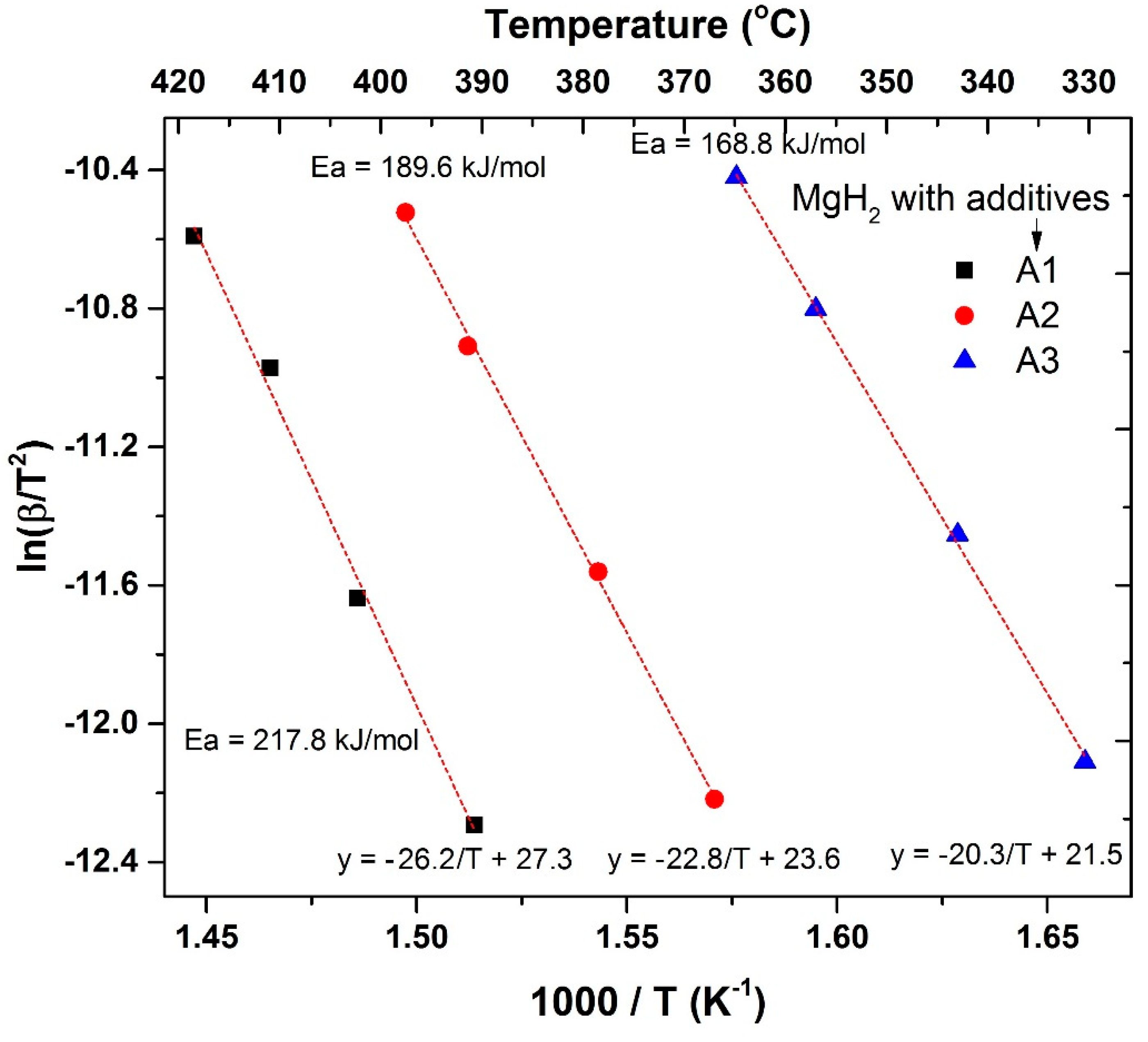
| Additive to MgH2 | Straight Line Equation | R2 Value | Activation Energy (Ea), kJ/mol | Standard Deviation of Ea Value, kJ/mol |
|---|---|---|---|---|
| A1 | −26.2/T + 27.3 | 0.992 | 217.8 | 16.2 |
| A2 | −22.8/T + 23.6 | 0.998 | 189.6 | 4.5 |
| A3 | −20.3/T + 21.5 | 0.999 | 168.8 | 3.0 |
| Sample Info | Desorption Temperature (from DSC, ΔT = 2 °C/min) | Max Kinetics (wt.%/Minute) | |||
|---|---|---|---|---|---|
| Hydride and additive | Additive Composition (as per XRD) | Onset temperate | Peak temperature | desorption at 315 °C/1 bar H2 | absorption at 315 °C/6 bar H2 |
| MgH2 with A1 | Pure GO | 355 °C | 388 °C | 0.033 wt.%/min | 0.150 wt.%/min |
| MgH2 with A2 | Partially reduced GO, MgO | 300 °C | 363 °C | 0.047 wt.%/min | 0.161 wt.%/min |
| MgH2 with A3 | rGO and MgO | 285 °C | 330 °C | 0.060 wt.%/min | 0.439 wt.%/min |
| Pure MgH2 | - | 380 °C | 415 °C | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pukazhselvan, D.; Shaula, A.L.; Mikhalev, S.M.; Bdikin, I.; Fagg, D.P. Elucidating Evidence for the In Situ Reduction of Graphene Oxide by Magnesium Hydride and the Consequence of Reduction on Hydrogen Storage. Catalysts 2022, 12, 735. https://doi.org/10.3390/catal12070735
Pukazhselvan D, Shaula AL, Mikhalev SM, Bdikin I, Fagg DP. Elucidating Evidence for the In Situ Reduction of Graphene Oxide by Magnesium Hydride and the Consequence of Reduction on Hydrogen Storage. Catalysts. 2022; 12(7):735. https://doi.org/10.3390/catal12070735
Chicago/Turabian StylePukazhselvan, D, Aliaksandr L. Shaula, Sergey M. Mikhalev, Igor Bdikin, and Duncan P. Fagg. 2022. "Elucidating Evidence for the In Situ Reduction of Graphene Oxide by Magnesium Hydride and the Consequence of Reduction on Hydrogen Storage" Catalysts 12, no. 7: 735. https://doi.org/10.3390/catal12070735
APA StylePukazhselvan, D., Shaula, A. L., Mikhalev, S. M., Bdikin, I., & Fagg, D. P. (2022). Elucidating Evidence for the In Situ Reduction of Graphene Oxide by Magnesium Hydride and the Consequence of Reduction on Hydrogen Storage. Catalysts, 12(7), 735. https://doi.org/10.3390/catal12070735







