In Situ H2 Reduction of Al2O3-Supported Ni- and Mo-Based Catalysts
Abstract
:1. Introduction
2. Results
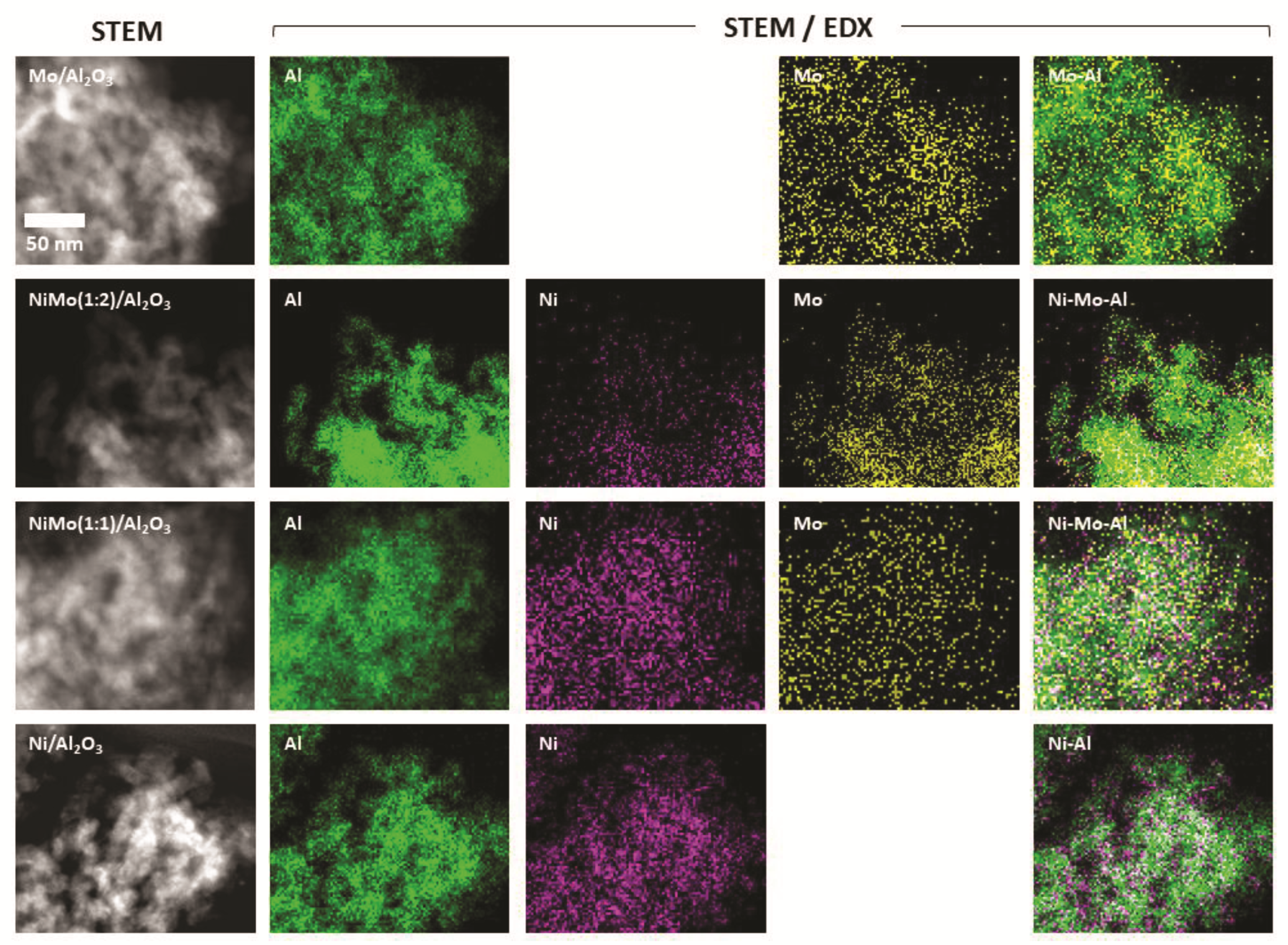
2.1. Characterization of Al2O3-Supported Catalysts before Reduction Experiments
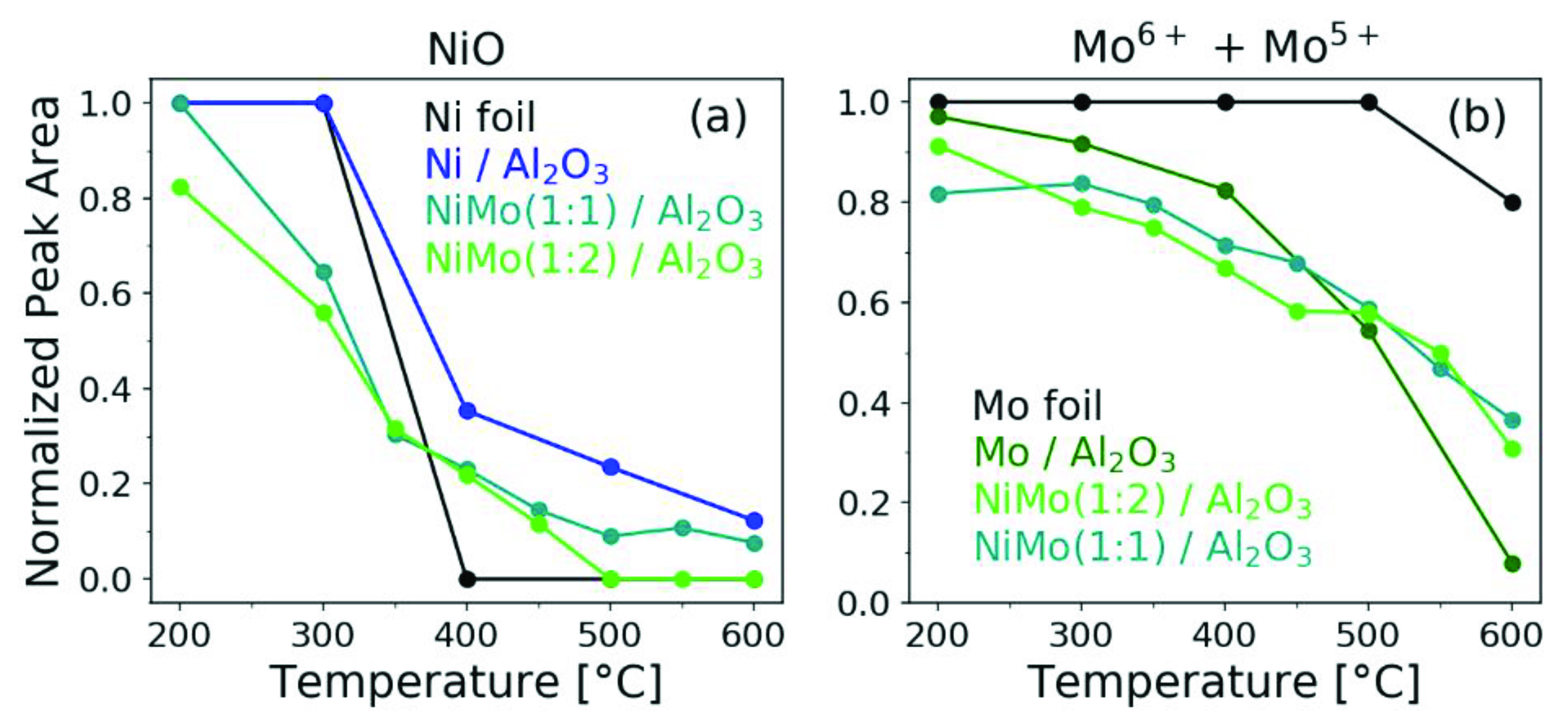
2.2. In Situ H2 Reduction Followed with Ambient-Pressure X-ray Photoelectron Spectroscopy
2.3. In Situ H2 Reduction Followed by XANES
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.1.1. Synthesis of Al2O3-Supported Catalysts
4.1.2. Metal Foils
4.2. Experimental Techniques
4.2.1. Nitrogen Physisorption
4.2.2. Transmission Electron Microscopy and Energy-Dispersive X-Ray Spectroscopy
4.2.3. Ambient-Pressure X-ray Photoelectron Spectroscopy
4.2.4. X-ray Absorption Near-Edge Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grange, P.; Vanhaeren, X. Hydrotreating Catalysts, an Old Story with New Challenges. Catal. Today 1997, 36, 375–391. [Google Scholar] [CrossRef]
- Breysse, M.; Afanasiev, P.; Geantet, C.; Vrinat, M. Overview of Support Effects in Hydrotreating Catalysts. Catal. Today 2003, 86, 5–16. [Google Scholar] [CrossRef]
- Topsøe, H.; Clausen, B.S.; Topsøe, N.Y.; Zeuthen, P. Progress in the Design of Hydrotreating Catalysts Based on Fundamental Molecular Insight. Stud. Surf. Sci. Catal. 1989, 53, 77–102. [Google Scholar] [CrossRef]
- Koklyukhin, A.S.; Mozhaev, A.V.; Sal’nikov, V.A.; Nikul’shin, P.A. Promoter Nature Effect on the Sensitivity of Ni–Mo/Al2O3, Co–Mo/Al2O3, and Ni–Co–Mo/Al2O3 Catalysts to Dodecanoic Acid in the Co-Hydrotreating of Dibenzothiophene and Naphthalene. Kinet. Catal. 2017, 58, 463–470. [Google Scholar] [CrossRef]
- Erhan Aksoylu, A.; Önsan, Z.I. Interaction between Nickel and Molybdenum in Ni-Mo/Al2O3 Catalysts: II CO Hydrogenation. Appl. Catal. A Gen. 1998, 168, 399–407. [Google Scholar] [CrossRef]
- Kumar, P.; Yenumala, S.R.; Maity, S.K.; Shee, D. Kinetics of Hydrodeoxygenation of Stearic Acid Using Supported Nickel Catalysts: Effects of Supports. Appl. Catal. A Gen. 2014, 471, 28–38. [Google Scholar] [CrossRef]
- Sotelo-Boyás, R.; Liu, Y.; Minowa, T. Renewable Diesel Production from the Hydrotreating of Rapeseed Oil with Pt/Zeolite and NiMo/Al2O3 Catalysts. Ind. Eng. Chem. Res. 2011, 50, 2791–2799. [Google Scholar] [CrossRef]
- Shetty, M.; Murugappan, K.; Prasomsri, T.; Green, W.H.; Román-Leshkov, Y. Reactivity and Stability Investigation of Supported Molybdenum Oxide Catalysts for the Hydrodeoxygenation (HDO) of m-Cresol. J. Catal. 2015, 331, 86–97. [Google Scholar] [CrossRef]
- Dupont, C.; Lemeur, R.; Daudin, A.; Raybaud, P. Hydrodeoxygenation Pathways Catalyzed by MoS2 and NiMoS Active Phases: A DFT Study. J. Catal. 2011, 279, 276–286. [Google Scholar] [CrossRef]
- Tsurov, M.A.; Afanasiev, P.V.; Lunin, V.V. Composition and Catalytic Properties of Products from the Reduction of NiMoO4. Appl. Catal. A Gen. 1993, 105, 205–221. [Google Scholar] [CrossRef]
- Abdel-Dayem, H.M. Dynamic Phenomena during Reduction of α-NiMoO4 in Different Atmospheres: In-Situ Thermo-Raman Spectroscopy Study. Ind. Eng. Chem. Res. 2007, 46, 2466–2472. [Google Scholar] [CrossRef]
- Brito, J.L.; Laine, J.; Pratt, K.C. Temperature-Programmed Reduction of Ni-Mo Oxides. J. Mater. Sci. 1989, 24, 425–431. [Google Scholar] [CrossRef]
- Burch, R.; Collins, A. Temperature-Programmed Reduction of Ni/Mo Hydrotreating Catalysts. Appl. Catal. 1985, 18, 389–400. [Google Scholar] [CrossRef]
- Dufresne, P.; Payen, E.; Grimbolt, J.; Bonnelle, J.P. Study of Ni-Mo-γ-Al2O3 Catalysts by X-Ray Photoelectron and Raman Spectroscopy. Comparison with Co-Mo-γ-Al2O3. J. Phys. Chem. 1981, 85, 2344–2351. [Google Scholar] [CrossRef]
- Brito, J.; Laine, J. Characterization of Supported MoO3 by Temperature-Programmed Reduction. Polyhedron 1986, 5, 179–182. [Google Scholar] [CrossRef]
- Brito, J.L.; Laine, J. Reducibility of Ni-Mo/Al2O3-Catalysts: A TPR Study. J. Catal. 1993, 139, 540–550. [Google Scholar] [CrossRef]
- Merte, L.R.; Gustafson, J.; Shipilin, M.; Zhang, C.; Lundgren, E. Redox Behavior of Iron at the Surface of an Fe0.01Mg0.99O(100) Single Crystal Studied by Ambient-Pressure Photoelectron Spectroscopy. Catal. Struct. React. 2017, 3, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Brunauer, S.; Emmett, P.H.; Teller, E. In Multimolecular. J. Am.Chem.Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Yu, W.; Yang, B.; Chen, X.; Jiang, W.; Yu, Q.; Xu, B. Thermodynamic Calculation and Experimental Investigation on the Products of Carbothermal Reduction of Al2O3 under Vacuum. Vacuum 2012, 86, 2005–2009. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray Photoelectron Spectroscopic Chemical State Quantification of Mixed Nickel Metal, Oxide and Hydroxide Systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Salagre, P.; Fierro, J.L.G.; Medina, F.; Sueiras, J.E. Characterization of Nickel Species on Several γ-Alumina Supported Nickel Samples. J. Mol. Catal. A Chem. 1996, 106, 125–134. [Google Scholar] [CrossRef]
- Liu, H.; Yin, C.; Li, X.; Chai, Y.; Li, Y.; Liu, C. Effect of NiMo Phases on the Hydrodesulfurization Activities of Dibenzothiophene. Catal. Today 2017, 282, 222–229. [Google Scholar] [CrossRef]
- Hernández-Huesca, R.; Mérida-Robles, J.; Maireles-Torres, P.; Rodríguez-Castellón, E.; Jiménez-López, A. Hydrogenation and Ring-Opening of Tetralin on Ni and NiMo Supported on Alumina-Pillared α-Zirconium Phosphate Catalysts. A Thiotolerance Study. J. Catal. 2001, 203, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Katrib, A.; Sobczak, J.W.; Krawczyk, M.; Zommer, L.; Benadda, A.; Jablonski, A.; Maire, G. Surface Studies and Catalytic Properties of the Bifunctional Bulk MoO2 System. Surf. Interface Anal. 2002, 34, 225–229. [Google Scholar] [CrossRef]
- Murugappan, K.; Anderson, E.M.; Teschner, D.; Jones, T.E.; Skorupska, K.; Román-Leshkov, Y. Operando NAP-XPS Unveils Differences in MoO3 and Mo2C during Hydrodeoxygenation. Nat. Catal. 2018, 1, 960–967. [Google Scholar] [CrossRef] [Green Version]
- Scanlon, D.O.; Watson, G.W.; Payne, D.J.; Atkinson, G.R.; Egdell, R.G.; Law, D.S.L. Theoretical and Experimental Study of the Electronic Structures of MoO3 and MoO2. J. Phys. Chem. C 2010, 114, 4636–4645. [Google Scholar] [CrossRef]
- van Veenendaal, M.A.; Sawatzky, G.A. Nonlocal Screening Effects in 2p X-Ray Photoemission Spectroscopy Core-Level Line Shapes of Transition Metal Compounds. Phys. Rev. Lett. 1993, 70, 2459–2462. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New Interpretations of XPS Spectra of Nickel Metal and Oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Liu, F.; Xu, S.; Cao, L.; Chi, Y.; Zhang, T.; Xue, D. A Comparison of NiMo/Al2O3 Catalysts Prepared by Impregnation and Coprecipitation Methods for Hydrodesulfurization of Dibenzothiophene. J. Phys. Chem. C 2007, 111, 7396–7402. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, G.; Liu, X.; Yu, X.; Liu, L.; Wang, J.; Feng, Z.; Li, C. Structure and Reducibility of NiO-MoO3/γ-Al2O3 Catalysts: Effects of Loading and Molar Ratio. J. Phys. Chem. C 2008, 112, 17265–17271. [Google Scholar] [CrossRef]
- Prasomsri, T.; Shetty, M.; Murugappan, K.; Román-Leshkov, Y. Insights into the Catalytic Activity and Surface Modification of MoO3 during the Hydrodeoxygenation of Lignin-Derived Model Compounds into Aromatic Hydrocarbons under Low Hydrogen Pressures. Energy Environ. Sci. 2014, 7, 2660–2669. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Liu, C.M.; Guo, L.; Hu, R.; Abbas, M.I.; Hu, T.D.; Xu, H.B. Structural Characterization of Nickel Oxide Nanowires by X-Ray Absorption near-Edge Structure Spectroscopy. J. Phys. Chem. B 2005, 109, 2512–2515. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Jian, J.K.; Ablat, A.; Li, J.; Sun, Y.F.; Wu, R. Structure and Magnetic Properties of Ni-Doped AlN Films. J. Appl. Phys. 2012, 112, 053911. [Google Scholar] [CrossRef]
- Zhang, R.; Li, P.; Wang, F.; Ye, L.; Gaur, A.; Huang, Z.; Zhao, Z.; Bai, Y.; Zhou, Y. Atomically Dispersed Mo Atoms on Amorphous G-C3N4 Promotes Visible-Light Absorption and Charge Carriers Transfer. Appl. Catal. B Environ. 2019, 250, 273–279. [Google Scholar] [CrossRef]
- He, C.; Tao, J. Exploration of the Electrochemical Mechanism of Ultrasmall Multiple Phases Molybdenum Carbides Nanocrystals for Hydrogen Evolution Reaction. RSC Adv. 2016, 6, 9240–9246. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Blomberg, S.; Johansson, N.; Kokkonen, E.; Rissler, J.; Kollberg, L.; Preger, C.; Franzén, S.M.; Messing, M.E.; Hulteberg, C. Bimetallic Nanoparticles as a Model System for an Industrial NiMo Catalyst. Materials 2019, 12, 3727. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Scardamaglia, M.; Kundsen, J.; Sankari, R.; Tarawneh, H.; Temperton, R.; Pickworth, L.; Cavalca, F.; Wang, C.; Tissot, H.; et al. HIPPIE: A New Platform for Ambient-Pressure X-ray Photoelectron Spectroscopy at the MAX IV Laboratory. J. Synchrotron Radiat. 2021, 28, 624–636. [Google Scholar] [CrossRef]
- Fairely, N. CasaXPS Manual 2.3. 15 Getting Started with CasaXPS; Casa Software Ltd.: London, UK, 2009; pp. 1–177. [Google Scholar]
- Head, A.R.; Tsyshevsky, R.; Trotochaud, L.; Yu, Y.; Karsliolu, O.; Eichhorn, B.; Kuklja, M.M.; Bluhm, H. Dimethyl Methylphosphonate Adsorption and Decomposition on MoO2 as Studied by Ambient Pressure X-Ray Photoelectron Spectroscopy and DFT Calculations. J. Phys. Condens. Matter 2018, 30, 134005. [Google Scholar] [CrossRef] [Green Version]
- Klementiev, K.; Norén, K.; Carlson, S.; Sigfridsson Clauss, K.G.V.; Persson, I. The BALDER Beamline at the MAX IV Laboratory. J. Phys. Conf. Ser. 2016, 712, 12023. [Google Scholar] [CrossRef] [Green Version]
- Tröger, L.; Arvanitis, D.; Baberschke, K.; Michaelis, H.; Grimm, U.; Zschech, E. Full Correction of the Self-Absorption in Soft-Fluorescence Extended X-ray-Absorption Fine Structure. Phys. Rev. B 1992, 46, 3283–3289. [Google Scholar] [CrossRef] [PubMed]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data Analysis for X-Ray Absorption Spectroscopy Using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangaletti, L.; Depero, L.E.; Parmigiani, F. On the Non-Local Screening Mechanisms in the 2p Photoelectron Spectra of NiO and La2NiO4. Solid State Commun. 1997, 103, 421–424. [Google Scholar] [CrossRef]
- Carley, A.F.; Jackson, S.D.; O’Shea, J.N.; Roberts, M.W. The Formation and Characterisation of Ni3+—An X-ray Photoelectron Spectroscopic Investigation of Potassium-Doped Ni(1 1 0)-O. Surf. Sci. 1999, 440, L868–L874. [Google Scholar] [CrossRef]
- Furstenau, R.P.; McDougall, G.; Langell, M.A. Initial Stages of Hydrogen Reduction of NiO(100). Surf. Sci. 1985, 150, 55–79. [Google Scholar] [CrossRef]
- Jiménez, J.A.; Padilla, I.; Lõpez-Delgado, A.; Fillali, L.; Lõpez-Andrés, S. Characterization of the Aluminas Formed during the Thermal Decomposition of Boehmite by the Rietveld Refinement Method. Int. J. Appl. Ceram. Technol. 2015, 12, E178–E186. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.; Khanna, A.; Jain, R.K. Hirdesh Structure-Property Correlations in Molybdenum Trioxide Thin Films and Nanoparticles. Mater. Res. Express 2019, 6, 186409. [Google Scholar] [CrossRef]
- Walls, B.; Mazilkin, A.A.; Mukhamedov, B.O.; Ionov, A.; Smirnova, I.A.; Ponomareva, A.V.; Fleischer, K.; Kozlovskaya, N.A.; Shulyatev, D.A.; Abrikosov, I.A.; et al. Nanodomain Structure of Single Crystalline Nickel Oxide. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]



| Catalyst | BET Area in m2/g |
|---|---|
| Mo/Al2O3 | 107 |
| NiMo(1:2)/Al2O3 | 109 |
| NiMo(1:1)/Al2O3 | 109 |
| Ni/Al2O3 | 114 |
| Al2O3 | 121 |
| Catalyst | Ni [at. %] | Mo [at. %] | Ni: Mo |
|---|---|---|---|
| Mo/Al2O3 | - | 2.95 ± 0.09 | - |
| NiMo(1:2)/Al2O3 | 0.98 ± 0.17 | 2.03 ± 0.39 | 0.48 ± 0.17 |
| NiMo(1:1)/Al2O3 | 1.37 ± 0.67 | 1.42 ± 0.33 | 0.96 ± 0.68 |
| Ni/Al2O3 | 2.95 ± 1.47 | - | - |
| Catalyst | Peak 1 | Peak 2 | Peak 3 | Peak 4 | Peak 5 | Peak 6 | Ref. |
|---|---|---|---|---|---|---|---|
| NiO | 853.7 | 855.4 | 860.9 | 864.0 | 866.3 | [20] | |
| NiOH | 854.9 | 855.7 | 857.7 | 860.5 | 861.5 | 866.5 | [20] |
| Ni | 852.6 | 856.3 | 858.7 | [20] | |||
| NiO/Al2O3 | 855.5–855.0 | ~862.2 | [21] | ||||
| Ni/Al2O3 | 853.0–852. | 856.6–856.0 | [21] | ||||
| NiMoO4/Al2O3 | 858.3 | 864.3 | [22] | ||||
| 856.2 | 862.0 | [23] |
| Catalyst | 3d5/2 | 3d3/2 | Ref. |
|---|---|---|---|
| MoO3 (Mo6+) | 232.5 | 235.8 | [24,25] |
| Mo5O10 (Mo5+) | 231.2 | 234.4 | [24,25] |
| MoO2 (Mo4+) | 229.3 | 230.6 | [24,25] |
| Mo2C (Mo2+) | 228.9 | 231.9 | [25] |
| Mo (Mo0) | 228.3 | 231.5 | [26] |
| Catalyst | Ni [at. %] | Mo [at. %] | Ni [at. %]:Mo [at. %] |
|---|---|---|---|
| Mo/Al2O3 | - | 3.3 | - |
| NiMo(1:2)/Al2O3 | 1.3 | 1.8 | 0.7 |
| NiMo(1:1)/Al2O3 | 2.2 | 1.1 | 2.2 |
| Ni/Al2O3 | 3.1 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gericke, S.M.; Rissler, J.; Bermeo, M.; Wallander, H.; Karlsson, H.; Kollberg, L.; Scardamaglia, M.; Temperton, R.; Zhu, S.; Sigfridsson Clauss, K.G.V.; et al. In Situ H2 Reduction of Al2O3-Supported Ni- and Mo-Based Catalysts. Catalysts 2022, 12, 755. https://doi.org/10.3390/catal12070755
Gericke SM, Rissler J, Bermeo M, Wallander H, Karlsson H, Kollberg L, Scardamaglia M, Temperton R, Zhu S, Sigfridsson Clauss KGV, et al. In Situ H2 Reduction of Al2O3-Supported Ni- and Mo-Based Catalysts. Catalysts. 2022; 12(7):755. https://doi.org/10.3390/catal12070755
Chicago/Turabian StyleGericke, Sabrina Maria, Jenny Rissler, Marie Bermeo, Harald Wallander, Hanna Karlsson, Linnéa Kollberg, Mattia Scardamaglia, Robert Temperton, Suyun Zhu, Kajsa G. V. Sigfridsson Clauss, and et al. 2022. "In Situ H2 Reduction of Al2O3-Supported Ni- and Mo-Based Catalysts" Catalysts 12, no. 7: 755. https://doi.org/10.3390/catal12070755
APA StyleGericke, S. M., Rissler, J., Bermeo, M., Wallander, H., Karlsson, H., Kollberg, L., Scardamaglia, M., Temperton, R., Zhu, S., Sigfridsson Clauss, K. G. V., Hulteberg, C., Shavorskiy, A., Merte, L. R., Messing, M. E., Zetterberg, J., & Blomberg, S. (2022). In Situ H2 Reduction of Al2O3-Supported Ni- and Mo-Based Catalysts. Catalysts, 12(7), 755. https://doi.org/10.3390/catal12070755








