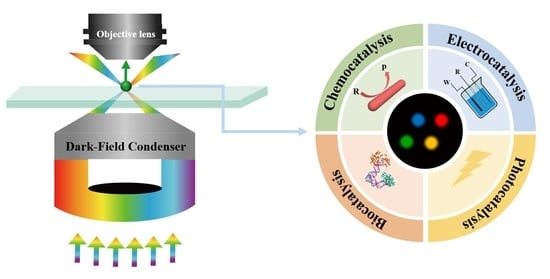
Single-Particle Measurements of Nanocatalysis with Dark-Field Microscopy
Abstract
:1. Introduction
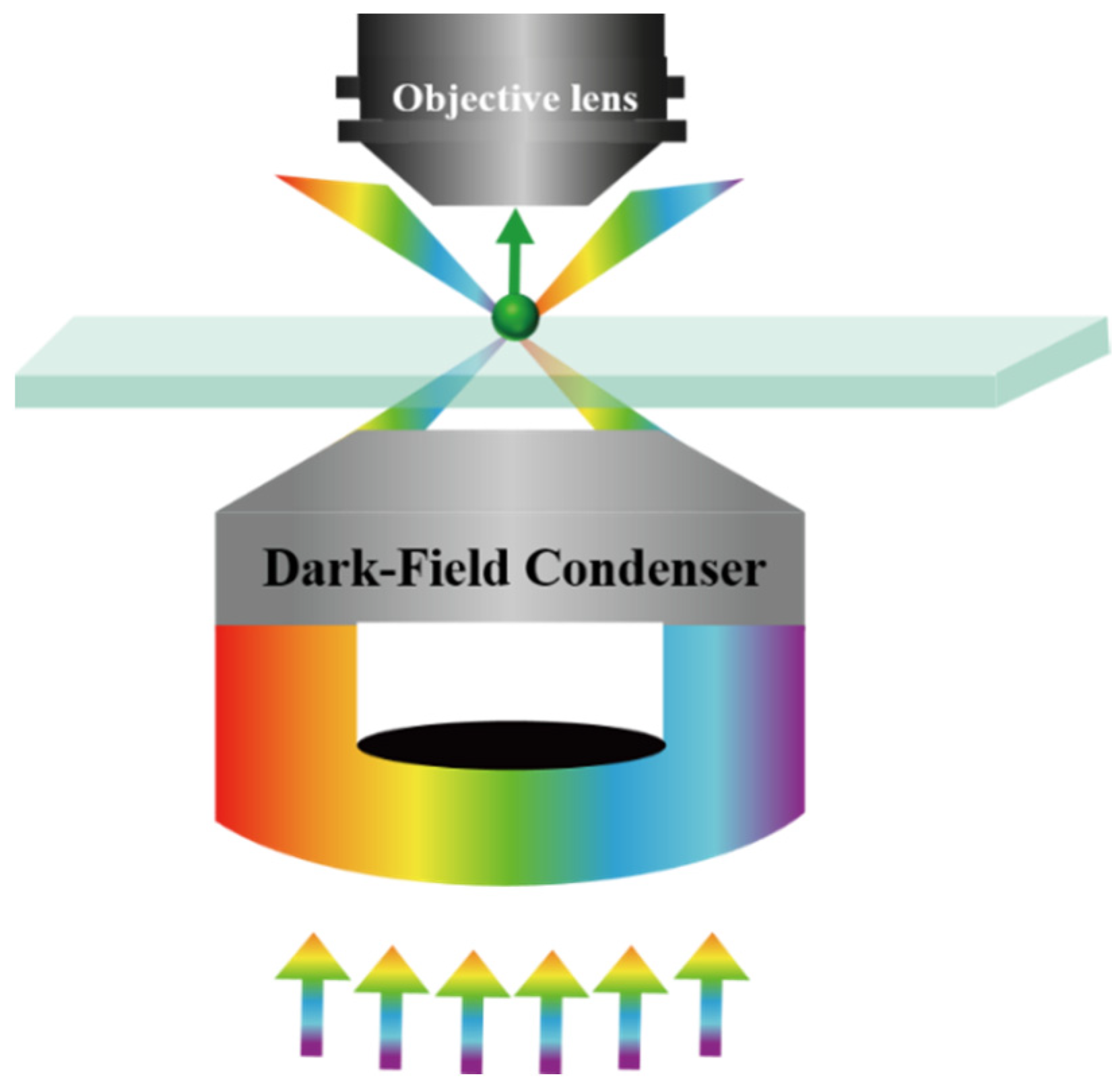
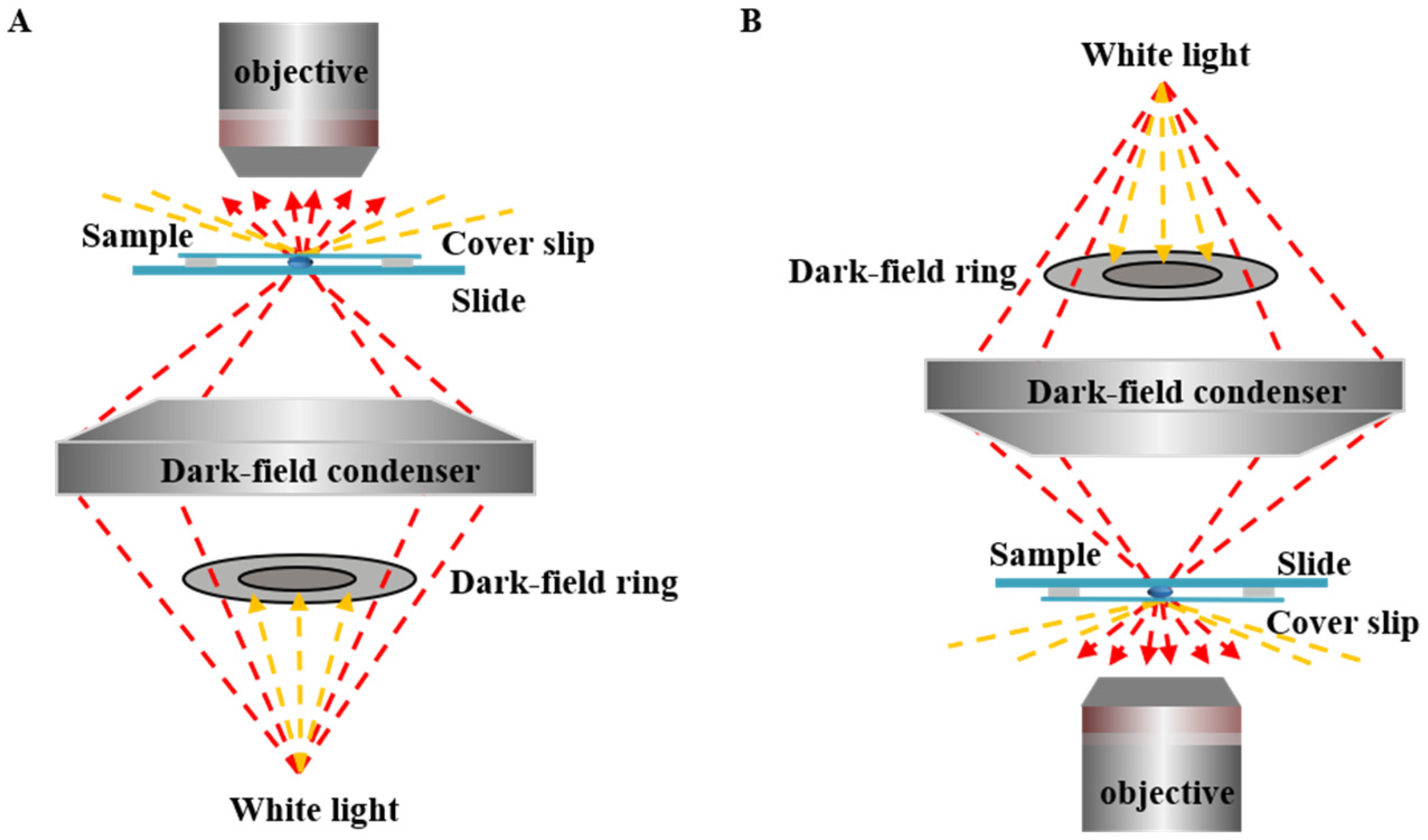
2. Dark-Field Microscopy (DFM)
2.1. Working Principle
2.2. Data Analysis

2.3. Technology Combination
3. Single-Particle Measurements of Catalytic Reactions
3.1. Chemocatalysis
3.2. Electrocatalysis
3.3. Photocatalysis
3.4. Biocatalysis
4. Perspective and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vedrine, J.C. Heterogeneous catalysis on metal oxides. Catalysts 2017, 7, 341. [Google Scholar] [CrossRef] [Green Version]
- Tachikawa, T.; Yamashita, S.; Majima, T. Evidence for crystal-face-dependent TiO2 photocatalysis from single-molecule imaging and kinetic analysis. J. Am. Chem. Soc. 2011, 133, 7197–7204. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Z.; Li, C.; Zhang, C. Advances in single quantum dot-based nanosensors. Chem. Comm. 2017, 53, 13284–13295. [Google Scholar] [CrossRef]
- Vendelbo, S.B.; Elkjaer, C.F.; Falsig, H.; Puspitasari, I.; Dona, P.; Mele, L.; Morana, B.; Nelissen, B.J.; van Rijn, R.; Creemer, J.F.; et al. Visualization of oscillatory behaviour of Pt nanoparticles catalysing CO oxidation. Nat. Mater. 2014, 13, 884–890. [Google Scholar] [CrossRef]
- Zhou, X.; Andoy, N.M.; Liu, G.; Choudhary, E.; Han, K.; Shen, H.; Chen, P. Quantitative super-resolution imaging uncovers reactivity patterns on single nanocatalysts. Nat. Nanotechnol. 2012, 7, 237–241. [Google Scholar] [CrossRef]
- Hartman, T.; Wondergem, C.S.; Kumar, N.; van den Berg, A.; Weckhuysen, B.M. Surface- and Tip-enhanced Raman spectroscopy in catalysis. J. Phys. Chem. Lett. 2016, 7, 1570–1584. [Google Scholar] [CrossRef] [Green Version]
- van Schrojenstein Lantman, E.M.; Deckert-Gaudig, T.; Mank, A.J.G.; Deckert, V.; Weckhuysen, B.M. Catalytic processes monitored at the nanoscale with tip-enhanced Raman spectroscopy. Nat. Nanotechnol. 2012, 7, 583–586. [Google Scholar] [CrossRef]
- Karim, W.; Spreafico, C.; Kleibert, A.; Gobrecht, J.; VandeVondele, J.; Ekinci, Y.; van Bokhoven, J.A. Catalyst support effects on hydrogen spillover. Nature 2017, 541, 68–71. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Xu, S.; Fu, C.; Ji, S.; Luo, F.; Lin, C.; Qiu, B.; Lin, Z. Single nanoparticle identification coupled with auto-identify algorithm for rapid and accurate detection of L-histidine. Anal. Chim. Acta 2021, 1187, 339162. [Google Scholar] [CrossRef]
- Garcia, M.A. Surface plasmons in metallic nanoparticles: Fundamentals and applications. J. Phys. D Appl. Phys. 2012, 45, 389501. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Chen, M.; Qin, H.; Peng, X. Ag nanocrystals with nearly ideal optical quality: Synthesis, growth mechanism, and characterizations. J. Am. Chem. Soc. 2018, 140, 17734–17742. [Google Scholar] [CrossRef]
- Ahmed, A.; Pelton, M.; Guest, J.R. Understanding how acoustic vibrations modulate the optical response of plasmonic metal nanoparticles. ACS Nano 2017, 11, 9360–9369. [Google Scholar] [CrossRef]
- Guisbiers, G.; Mendoza-Cruz, R.; Bazan-Diaz, L.; Velazquez-Salazar, J.J.; Mendoza-Perez, R.; Robledo-Torres, J.A.; Rodriguez-Lopez, J.L.; Montejano-Carrizales, J.M.; Whetten, R.L.; Jose-Yacaman, M. Electrum, the gold-silver alloy, from the bulk scale to the nanoscale: Synthesis, properties, and segregation Rules. ACS Nano 2016, 10, 188–198. [Google Scholar] [CrossRef]
- Ha, J.W. Chemical interface damping of single gold nanorods with low sensitivity to the medium dielectric constant. Chem. Phys. Lett. 2017, 676, 65–69. [Google Scholar] [CrossRef]
- Jackman, J.A.; Ferhan, A.R.; Cho, N.J. Nanoplasmonic sensors for biointerfacial science. Chem. Soc. Rev. 2017, 46, 3615–3660. [Google Scholar] [CrossRef]
- Li, T.; Wu, X.; Liu, F.; Li, N. Analytical methods based on the light-scattering of plasmonic nanoparticles at the single particle level with dark-field microscopy imaging. Analyst 2017, 142, 248–256. [Google Scholar] [CrossRef]
- Li, K.; Qin, W.; Xu, Y.; Peng, T.; Li, D. Optical approaches in study of nanocatalysis with single-molecule and single-particle resolution. Front. Optoelectron. 2014, 8, 379–393. [Google Scholar] [CrossRef]
- Schultz, S.; Smith, D.R.; Mock, J.J.; Schultz, D.A. Single-target molecule detection with nonbleaching multicolor optical immunolabels. Proc. Natl. Acad. Sci. USA 2000, 97, 996–1001. [Google Scholar] [CrossRef] [Green Version]
- Wang, W. Imaging the chemical activity of single nanoparticles with optical microscopy. Chem. Soc. Rev. 2018, 47, 2485–2508. [Google Scholar] [CrossRef]
- Weigel, A.; Sebesta, A.; Kukura, P. Dark field microspectroscopy with single molecule fluorescence sensitivity. ACS Photonics 2014, 1, 848–856. [Google Scholar] [CrossRef]
- Gao, P.; Lei, G.; Huang, C. Dark-field microscopy: Recent advances in accurate analysis and emerging applications. Anal. Chem. 2021, 93, 4707–4726. [Google Scholar] [CrossRef]
- Peng, Y.; Xiong, B.; Peng, L.; Li, H.; He, Y.; Yeung, E.S. Recent advances in optical imaging with anisotropic plasmonic nanoparticles. Anal. Chem. 2015, 87, 200–215. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, T.; Zhou, X. Dark-field spectroscopy: Development, applications and perspectives in single nanoparticle catalysis. J. Phys. Condens. Matter 2019, 31, 473001. [Google Scholar] [CrossRef]
- Sriram, M.; Markhali, B.P.; Nicovich, P.R.; Bennett, D.T.; Reece, P.J.; Hibbert, D.B.; Tilley, R.D.; Gaus, K.; Vivekchand, S.R.C.; Gooding, J.J. A rapid readout for many single plasmonic nanoparticles using dark-field microscopy and digital color analysis. Biosens. Bioelectron. 2018, 117, 530–536. [Google Scholar] [CrossRef]
- Jing, C.; Gu, Z.; Ying, Y.; Li, D.; Zhang, L.; Long, Y. Chrominance to dimension: A real-time method for measuring the size of single gold nanoparticles. Anal. Chem. 2012, 84, 4284–4291. [Google Scholar] [CrossRef]
- Zhou, J.; Lei, G.; Zheng, L.; Gao, P.; Huang, C. HSI colour-coded analysis of scattered light of single plasmonic nanoparticles. Nanoscale 2016, 8, 11467–11471. [Google Scholar] [CrossRef]
- Le, N.H.; Cathcart, N.; Kitaev, V.; Chen, J.I.L. Core-satellite assembly of gold nanoshells on solid gold nanoparticles for a color coding plasmonic nanosensor. Analyst 2022, 147, 155–164. [Google Scholar] [CrossRef]
- Song, M.; Chen, S.; Hu, P.; Huang, C.; Zhou, J. Automated plasmonic resonance scattering imaging analysis via deep learning. Anal. Chem. 2021, 93, 2619–2626. [Google Scholar] [CrossRef]
- Liu, M.; Wang, F.; Zhang, X.; Mao, X.; Wang, L.; Tian, Y.; Fan, C.; Li, Q. Tracking endocytosis and intracellular distribution of spherical nucleic acids with correlative single-cell imaging. Nat. Protoc. 2021, 16, 383–404. [Google Scholar] [CrossRef]
- Chaudhari, K.; Ahuja, T.; Murugesan, V.; Subramanian, V.; Ganayee, M.A.; Thundat, T.; Pradeep, T. Appearance of SERS activity in single silver nanoparticles by laser-induced reshaping. Nanoscale 2018, 11, 321–330. [Google Scholar] [CrossRef]
- Hao, J.; Xiong, B.; Cheng, X.; He, Y.; Yeung, E.S. High-throughput sulfide sensing with colorimetric analysis of single Au-Ag core-shell nanoparticles. Anal. Chem. 2014, 86, 4663–4667. [Google Scholar] [CrossRef]
- Yuan, J.; Shen, J.; Chen, M.; Lou, Z.; Zhang, S.; Song, Z.; Li, W.; Zhou, X. Artificial intelligence-assisted enumeration of ultra-small viruses with dual dark-field plasmon resonance probes. Biosens. Bioelectron. 2022, 199, 113893. [Google Scholar] [CrossRef]
- Byers, C.P.; Hoener, B.S.; Chang, W.; Yorulmaz, M.; Link, S.; Landes, C.F. Single-particle spectroscopy reveals heterogeneity in electrochemical tuning of the localized surface plasmon. J. Phys. Chem. B 2014, 118, 14047–14055. [Google Scholar] [CrossRef] [Green Version]
- Tachikawa, T.; Yonezawa, T.; Majima, T. Super-resolution mapping of reactive sites on titania-based nanoparticles with water-soluble fluorogenic probes. ACS Nano 2013, 7, 263–275. [Google Scholar] [CrossRef]
- Larsson, E.M.; Langhammer, C.; Zoric, I.; Kasemo, B. Nanoplasmonic probes of catalytic reactions. Science 2009, 326, 1091–1094. [Google Scholar] [CrossRef]
- Liu, N.; Tang, M.; Hentschel, M.; Giessen, H.; Alivisatos, A.P. Nanoantenna-enhanced gas sensing in a single tailored nanofocus. Nat. Mater. 2011, 10, 631–636. [Google Scholar] [CrossRef]
- Tang, M.; Liu, N.; Dionne, J.A.; Alivisatos, A.P. Observations of shape-dependent hydrogen uptake trajectories from single nanocrystals. J. Am. Chem. Soc. 2011, 133, 13220–13223. [Google Scholar] [CrossRef]
- Wang, P.; Lin, Z.; Su, X.; Tang, Z. Application of Au based nanomaterials in analytical science. Nano Today 2017, 12, 64–97. [Google Scholar] [CrossRef]
- Tao, A.R.; Habas, S.; Yang, P. Shape control of colloidal metal nanocrystals. Small 2008, 4, 310–325. [Google Scholar] [CrossRef]
- Huang, J.; Yang, X.; Zeng, Q.; Wang, J. A simple green route to prepare stable silver nanoparticles with pear juice and a new selective colorimetric method for detection of cysteine. Analyst 2013, 138, 5296–5302. [Google Scholar] [CrossRef]
- Liz-Marzan, L.M.; Murphy, C.J.; Wang, J.F. Nanoplasmonics. Chem. Soc. Rev. 2014, 43, 3820–3822. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Meng, L.; Zhang, W.; Zheng, Y.; Wang, S.; Ren, B.; Yang, Z.; Yan, X. High-throughput single-particle analysis of metal-enhanced fluorescence in free solution using Ag@SiO2 core-shell nanoparticles. ACS Sens. 2017, 2, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhuo, X.; Wang, J. Active plasmonics: Principles, structures, and applications. Chem. Rev. 2018, 118, 3054–3099. [Google Scholar] [CrossRef] [PubMed]
- Langhammer, C.; Larsson, E.M. Nanoplasmonic in situ spectroscopy for catalysis applications. ACS Catal. 2012, 2, 2036–2045. [Google Scholar] [CrossRef]
- Novo, C.; Funston, A.M.; Mulvaney, P. Direct observation of chemical reactions on single gold nanocrystals using surface plasmon spectroscopy. Nat. Nanotechnol. 2008, 3, 598–602. [Google Scholar] [CrossRef]
- Wang, J.; Fossey, J.S.; Li, M.; Xie, T.; Long, Y. Real-time plasmonic monitoring of single gold amalgam nanoalloy electrochemical formation and stripping. ACS Appl. Mater. Interfaces 2016, 8, 8305–8314. [Google Scholar] [CrossRef]
- Wang, J.; Hua, X.; Li, M.; Long, Y. Mussel-inspired polydopamine functionalized plasmonic nanocomposites for single-particle catalysis. ACS Appl. Mater. Interfaces 2017, 9, 3016–3023. [Google Scholar] [CrossRef]
- Li, K.; Wang, K.; Qin, W.; Deng, S.; Li, D.; Shi, J.; Huang, Q.; Fan, C. DNA-directed assembly of gold nanohalo for quantitative plasmonic imaging of single-particle catalysis. J. Am. Chem. Soc. 2015, 137, 4292–4295. [Google Scholar] [CrossRef]
- Qi, F.; Han, Y.; Ye, Z.; Liu, H.; Wei, L.; Xiao, L. Color-coded single-particle pyrophosphate assay with dark-field optical microscopy. Anal. Chem. 2018, 90, 11146–11153. [Google Scholar] [CrossRef]
- Kong, C.; Liu, Q.; Li, W.; Chen, Z. Single particle-based colorimetric assay of pyrophosphate ions and pyrophosphatase with dark-field microscope. Sens. Actuators B Chem. 2019, 299, 126999. [Google Scholar] [CrossRef]
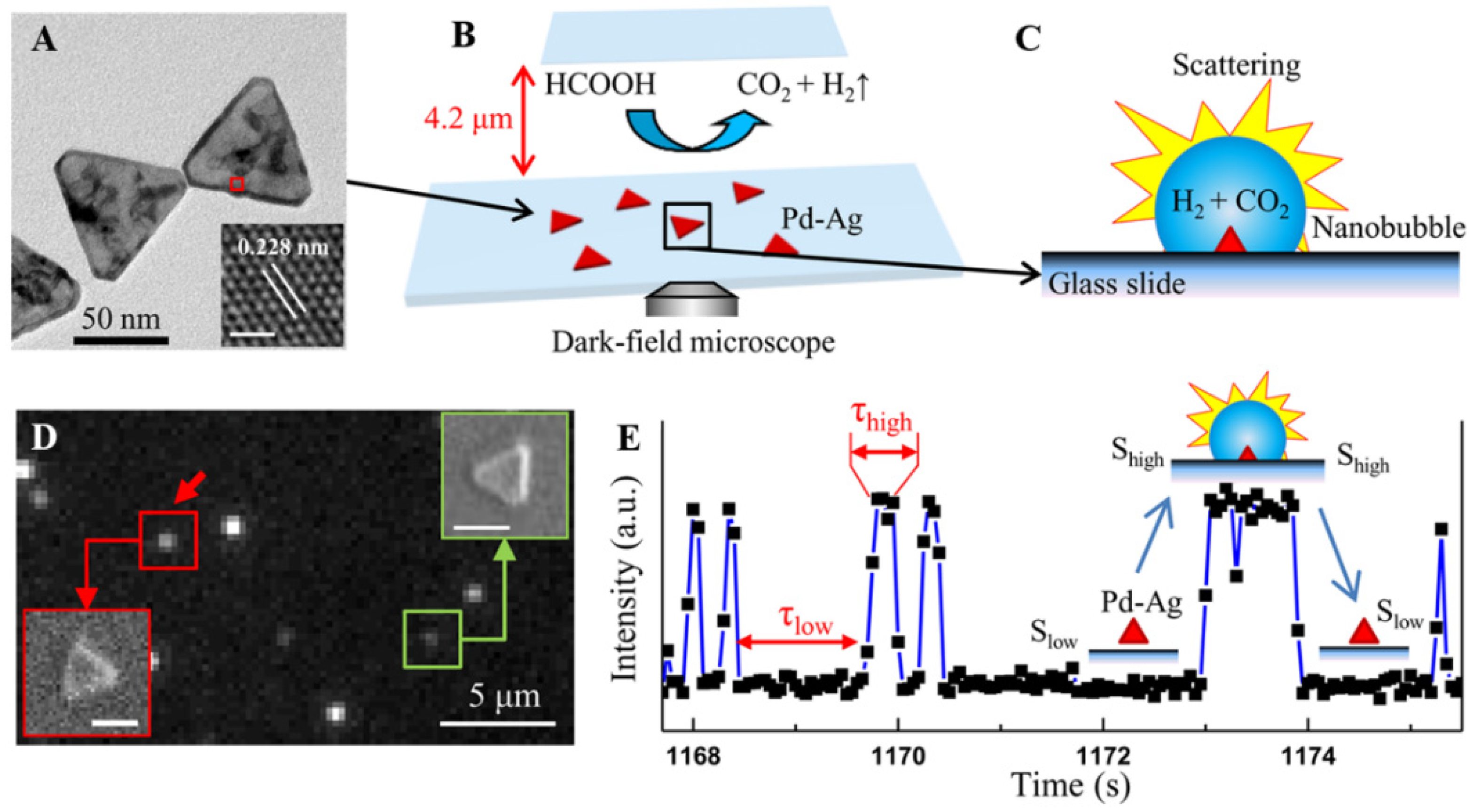
- Li, S.; Du, Y.; He, T.; Shen, Y.; Bai, C.; Ning, F.; Hu, X.; Wang, W.; Xi, S.; Zhou, X. Nanobubbles: An effective way to study gas-generating catalysis on a single nanoparticle. J. Am. Chem. Soc. 2017, 139, 14277–14284. [Google Scholar] [CrossRef]
- Wang, H.; He, T.; Du, Y.; Wang, W.; Shen, Y.; Li, S.; Zhou, X.; Yang, F. Evolution of single nanobubbles through multi-state dynamics. Chin. Chem. Lett. 2020, 31, 2442–2446. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Chirea, M.; Collins, S.S.E.; Wei, X.; Mulvaney, P. Spectroelectrochemistry of silver deposition on single gold nanocrystals. J. Phys. Chem. Lett. 2014, 5, 4331–4335. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, X.; Andoy, N.M.; Han, K.S.; Choudhary, E.; Zou, N.; Chen, G.; Shen, H. Spatiotemporal catalytic dynamics within single nanocatalysts revealed by single-molecule microscopy. Chem. Soc. Rev. 2014, 43, 1107–1117. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Govorov, A.O. Optical generation of hot plasmonic carriers in metal nanocrystals: The effects of shape and field enhancement. J. Phys. Chem. C 2014, 118, 7606–7614. [Google Scholar] [CrossRef]
- Wang, W.; Tao, N. Detection, counting, and imaging of single nanoparticles. Anal. Chem. 2014, 86, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Brasiliense, V.; Patel, A.N.; Martinez-Marrades, A.; Shi, J.; Chen, Y.; Combellas, C.; Tessier, G.; Kanoufi, F. Correlated electrochemical and optical detection reveals the chemical reactivity of individual silver nanoparticles. J. Am. Chem. Soc. 2016, 138, 3478–3483. [Google Scholar] [CrossRef]
- Patrice, F.T.; Qiu, K.; Ying, Y.; Long, Y. Single nanoparticle electrochemistry. Annu. Rev. Anal. Chem. 2019, 12, 347–370. [Google Scholar] [CrossRef] [Green Version]
- Jing, C.; Rawson, F.J.; Zhou, H.; Shi, X.; Li, W.; Li, D.; Long, Y. New insights into electrocatalysis based on plasmon resonance for the real-time monitoring of catalytic events on single gold nanorods. Anal. Chem. 2014, 86, 5513–5518. [Google Scholar] [CrossRef]
- Xu, S.; Yu, X.; Chen, Z.; Zeng, Y.; Guo, L.; Li, L.; Luo, F.; Wang, J.; Qiu, B.; Lin, Z. Real-time visualization of the single-nanoparticle electrocatalytic hydrogen generation process and activity under dark field microscopy. Anal. Chem. 2020, 92, 9016–9023. [Google Scholar] [CrossRef]
- Lin, M.; Zhou, Y.; Bu, L.; Bai, C.; Tariq, M.; Wang, H.; Han, J.L.; Huang, X.; Zhou, X. Single-nanoparticle coulometry method with high sensitivity and high throughput to study the electrochemical activity and oscillation of single nanocatalysts. Small 2021, 17, 2007302. [Google Scholar] [CrossRef]
- Brasiliense, V.; Clausmeyer, J.; Dauphin, A.L.; Noel, J.M.; Berto, P.; Tessier, G.; Schuhmann, W.; Kanoufi, F. Opto-electrochemical in situ monitoring of the cathodic formation of single cobalt nanoparticles. Angew. Chem. Int. Ed. 2017, 56, 10598–10601. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor composites: Strategies for enhancing charge carrier separation to improve photocatalytic activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Mulvaney, P.; Perez-Juste, J.; Giersig, M.; Liz-Marzan, L.M.; Pecharroman, C. Drastic surface plasmon mode shifts in gold nanorods due to electron charging. Plasmonics 2006, 1, 61–66. [Google Scholar] [CrossRef]
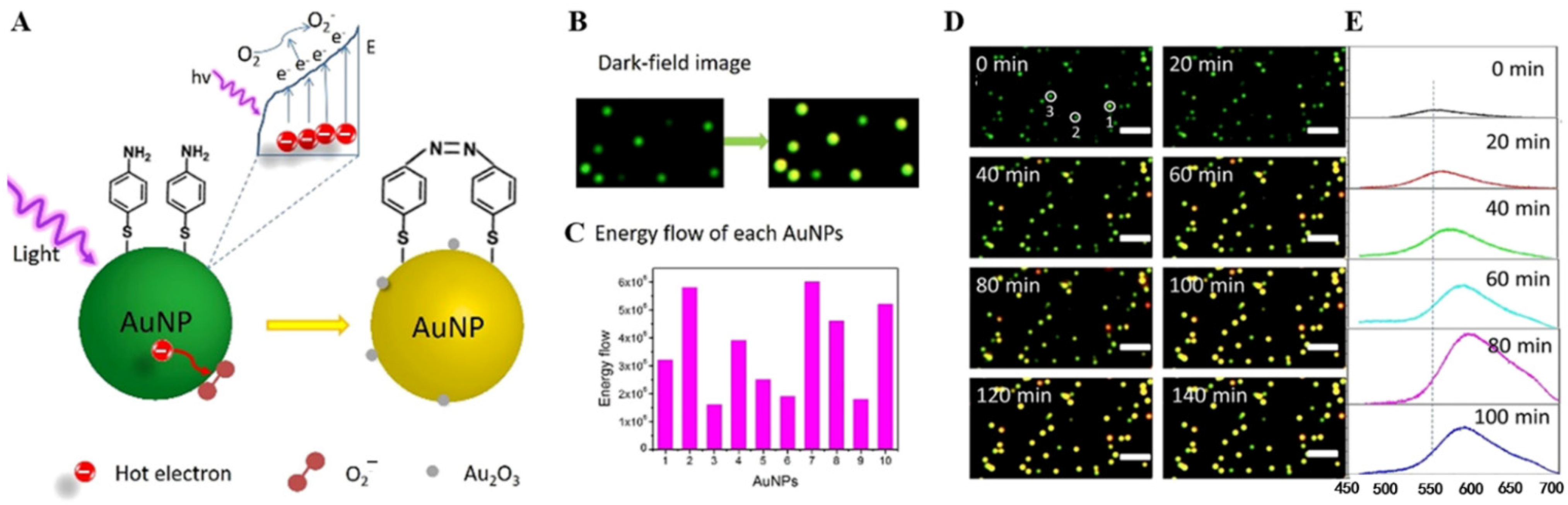
- Lei, G.; Gao, P.; Yang, T.; Zhou, J.; Zhang, H.; Sun, S.; Gao, M.; Huang, C. Photoinduced electron transfer process visualized on single silver nanoparticles. ACS Nano 2017, 11, 2085–2093. [Google Scholar] [CrossRef]
- Zhou, J.; He, W.; Liu, H.; Huang, C. Energy flow during the plasmon resonance-driven photocatalytic reactions on single nanoparticles. ACS Catal. 2022, 12, 847–853. [Google Scholar] [CrossRef]
- Lee, J.; Mubeen, S.; Ji, X.; Stucky, G.D.; Moskovits, M. Plasmonic photoanodes for solar water splitting with visible light. Nano Lett. 2012, 12, 5014–5019. [Google Scholar] [CrossRef]
- Hou, W.; Hung, W.; Pavaskar, P.; Goeppert, A.; Aykol, M.; Cronin, S.B. Photocatalytic conversion of CO2 to hydrocarbon fuels via plasmon-enhanced absorption and metallic interband transitions. ACS Catal. 2011, 1, 929–936. [Google Scholar] [CrossRef]
- Govorov, A.O.; Zhang, H.; Gun’ko, Y.K. Theory of photoinjection of hot plasmonic carriers from metal nanostructures into semiconductors and surface molecules. J. Phys. Chem. C 2013, 117, 16616–16631. [Google Scholar] [CrossRef]
- Sundararaman, R.; Narang, P.; Jermyn, A.S.; Goddard, W.A.; Atwater, H.A. Theoretical predictions for hot-carrier generation from surface plasmon decay. Nat. Commun. 2014, 5, 5788. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef]
- Yan, J.; Lin, Z.; Ma, C.; Zheng, Z.; Liu, P.; Yang, G. Plasmon resonances in semiconductor materials for detecting photocatalysis at the single-particle level. Nanoscale 2016, 8, 15001–15007. [Google Scholar] [CrossRef]
- Lim, S.; Lo, W.; Yang, P.; Lu, S.; Joplin, A.; Link, S.; Chang, W.; Tuan, H. Au@CdSe heteroepitaxial nanorods: An example of metal nanorods fully covered by a semiconductor shell with strong photo-induced interfacial charge transfer effects. Colloid Interface Sci. Commun. 2018, 532, 143–152. [Google Scholar] [CrossRef]
- Luo, W.; Zhu, C.; Su, S.; Li, D.; He, Y.; Huang, Q.; Fan, C. Self-catalyzed, self-limiting growth of glucose oxidase-mimicking gold nanoparticles. ACS Nano 2010, 4, 7451–7458. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Q.; Jing, C.; Li, Y.; Li, D.; Luo, W.; Wen, Y.; He, Y.; Huang, Q.; Long, Y.; et al. Catalytic gold nanoparticles for nanoplasmonic detection of DNA hybridization. Angew. Chem. Int. Ed. 2011, 50, 11994–11998. [Google Scholar] [CrossRef]
- Liu, Q.; Jing, C.; Zheng, X.; Gu, Z.; Li, D.; Li, D.; Huang, Q.; Long, Y.; Fan, C. Nanoplasmonic detection of adenosine triphosphate by aptamer regulated self-catalytic growth of single gold nanoparticles. Chem. Comm. 2012, 48, 9574–9576. [Google Scholar] [CrossRef]
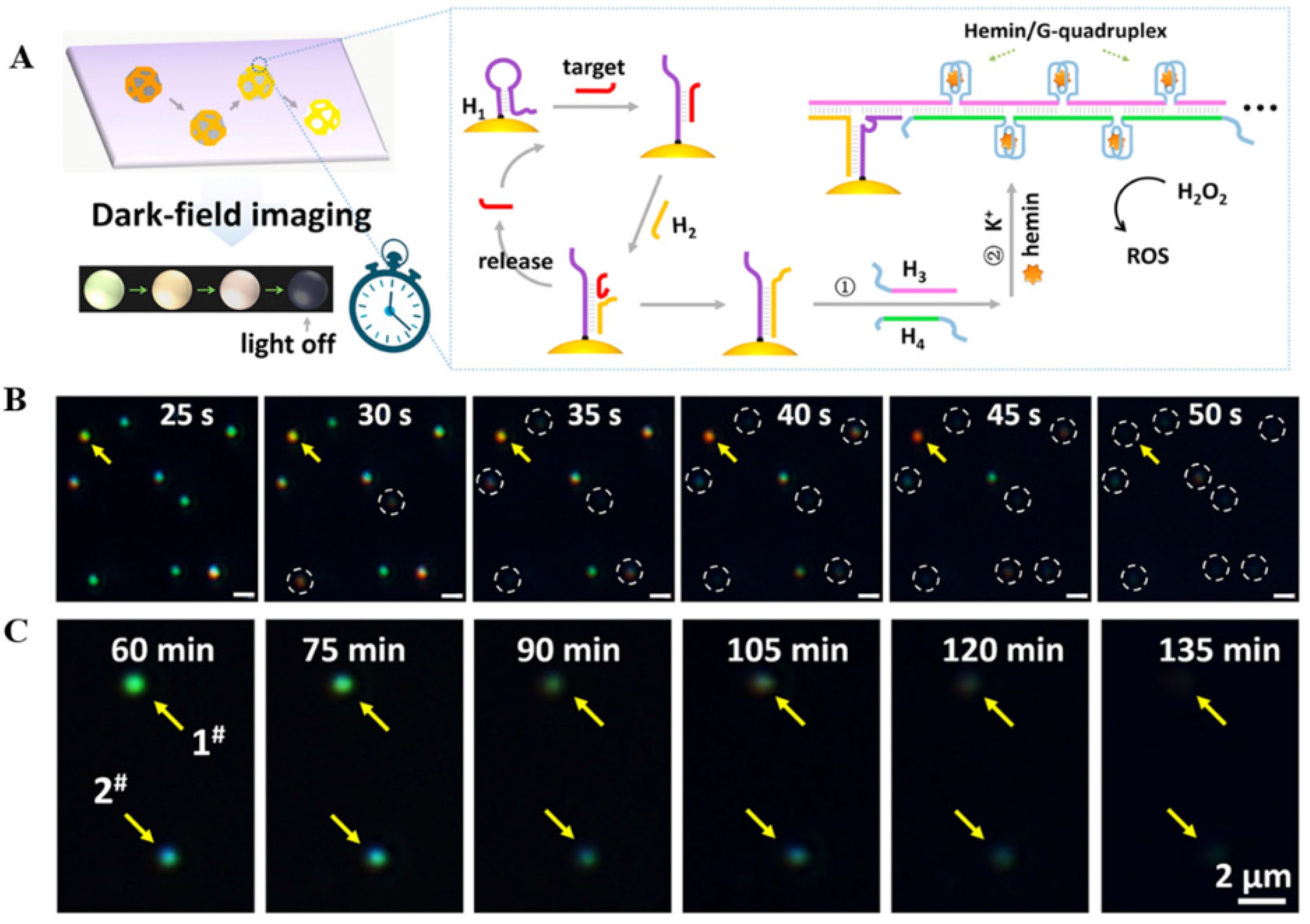
- Zhao, Y.; He, Y.; Zhang, J.; Wang, F.; Wang, K.; Xia, X. Conformational change and biocatalysis-triggered spectral shift of single Au nanoparticles. Chem. Comm. 2014, 50, 5480–5483. [Google Scholar] [CrossRef]
- Pang, J.; Zhao, Y.; Liu, H.; Wang, K. A single nanoparticle-based real-time monitoring of biocatalytic progress and detection of hydrogen peroxide. Talanta 2018, 185, 581–585. [Google Scholar] [CrossRef]
- Xiong, B.; Zhou, R.; Hao, J.; Jia, Y.; He, Y.; Yeung, E.S. Highly sensitive sulphide mapping in live cells by kinetic spectral analysis of single Au-Ag core-shell nanoparticles. Nat. Commun. 2013, 4, 1708. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, Y.; Li, D.; Jing, C.; Chen, X.; Lv, M.; Huang, Q.; Long, Y.; Willner, I. Single gold nanoparticles as real-time optical probes for the detection of NADH-dependent intracellular metabolic enzymatic pathways. Angew. Chem. Int. Ed. 2011, 50, 6789–6792. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Chen, X.; Cao, J.; Zhang, J.; Min, Q.; Zhu, J. Single gold@silver nanoprobes for real-time tracing the entire autophagy process at single-cell level. J. Am. Chem. Soc. 2015, 137, 1903–1908. [Google Scholar] [CrossRef]
- Li, M.; Cheng, J.; Yuan, Z.; Shen, Q.; Fan, Q. DNAzyme-catalyzed etching process of Au/Ag nanocages visualized via dark-field imaging with time elapse for ultrasensitive detection of microRNA. Sens. Actuators B Chem. 2021, 330, 129347. [Google Scholar] [CrossRef]
- Banik, S.; Melanthota, S.K.; Arbaaz; Vaz, J.M.; Kadambalithaya, V.M.; Hussain, I.; Dutta, S.; Mazumder, N. Recent trends in smartphone-based detection for biomedical applications: A review. Anal. Bioanal. Chem. 2021, 413, 2389–2406. [Google Scholar] [CrossRef]
- Sun, D.; Hu, T. A low cost mobile phone dark-field microscope for nanoparticle-based quantitative studies. Biosens. Bioelectron. 2018, 99, 513–518. [Google Scholar] [CrossRef]
- Chazot, C.A.C.; Nagelberg, S.; Rowlands, C.J.; Scherer, M.R.J.; Coropceanu, I.; Broderick, K.; Kim, Y.; Bawendi, M.G.; So, P.T.C.; Kolle, M. Luminescent surfaces with tailored angular emission for compact dark-field imaging devices. Nat. Photonics 2020, 14, 310–315. [Google Scholar] [CrossRef]
- Su, M.N.; Dongare, P.D.; Chakraborty, D.; Zhang, Y.; Yi, C.; Wen, F.; Chang, W.; Nordlander, P.; Sader, J.E.; Halas, N.J.; et al. Optomechanics of single aluminum nanodisks. Nano Lett. 2017, 17, 2575–2583. [Google Scholar] [CrossRef]
- Kim, M.; Kwon, H.; Lee, S.; Yoon, S. Effect of nanogap morphology on plasmon coupling. ACS Nano 2019, 13, 12100–12108. [Google Scholar] [CrossRef]
- Jin, C.M.; Lee, W.; Kim, D.; Kang, T.; Choi, I. Photothermal convection lithography for rapid and direct assembly of colloidal plasmonic nanoparticles on generic substrates. Small 2018, 14, 1803055. [Google Scholar] [CrossRef]
- Lee, H.E.; Ahn, H.Y.; Mun, J.; Lee, Y.Y.; Kim, M.; Cho, N.H.; Chang, K.; Kim, W.S.; Rho, J.; Nam, K.T. Amino-acid- and peptide-directed synthesis of chiral plasmonic gold nanoparticles. Nature 2018, 556, 360–365. [Google Scholar] [CrossRef]
- Zhou, K.; Yuan, T.; Su, H.; Wang, W. Accessing the spatiotemporal heterogeneities of single nanocatalysts by optically imaging gas nanobubbles. Curr. Opin. Colloid Interface Sci. 2021, 55, 101465. [Google Scholar] [CrossRef]
- Peng, T.; Li, X.; Li, K.; Nie, Z.; Tan, W. DNA-modulated plasmon resonance: Methods and optical applications. ACS Appl. Mater. Interfaces 2020, 12, 14741–14760. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, J.; Fan, J.; Qin, W.; Li, K. Single-Particle Measurements of Nanocatalysis with Dark-Field Microscopy. Catalysts 2022, 12, 764. https://doi.org/10.3390/catal12070764
Shang J, Fan J, Qin W, Li K. Single-Particle Measurements of Nanocatalysis with Dark-Field Microscopy. Catalysts. 2022; 12(7):764. https://doi.org/10.3390/catal12070764
Chicago/Turabian StyleShang, Jing, Jinsong Fan, Weiwei Qin, and Kun Li. 2022. "Single-Particle Measurements of Nanocatalysis with Dark-Field Microscopy" Catalysts 12, no. 7: 764. https://doi.org/10.3390/catal12070764