Excellent Catalytic Performance of Ce–MOF with Abundant Oxygen Vacancies Supported Noble Metal Pt in the Oxidation of Toluene
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts Characterization
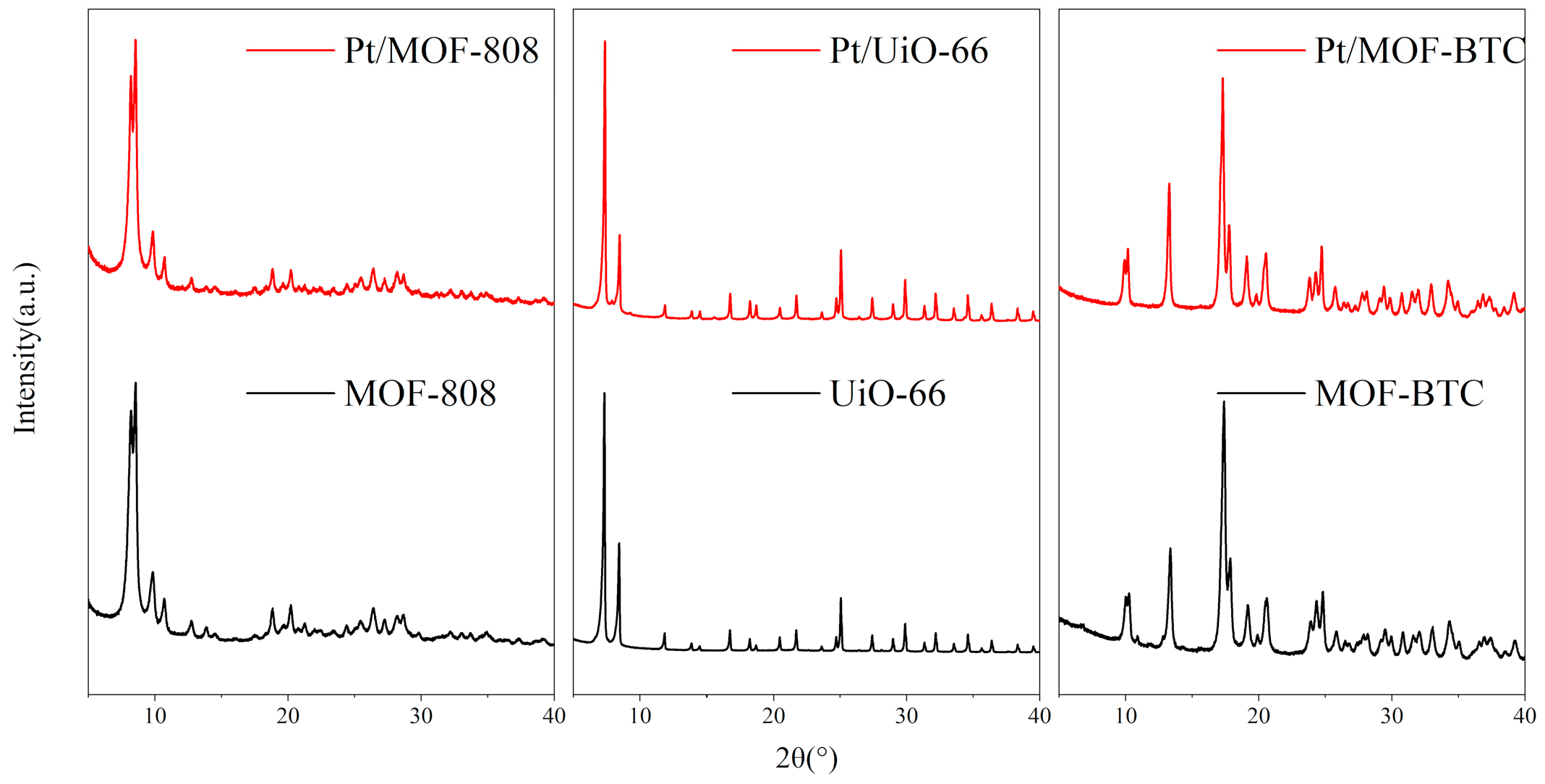
2.1.1. Phase and Stability Analysis
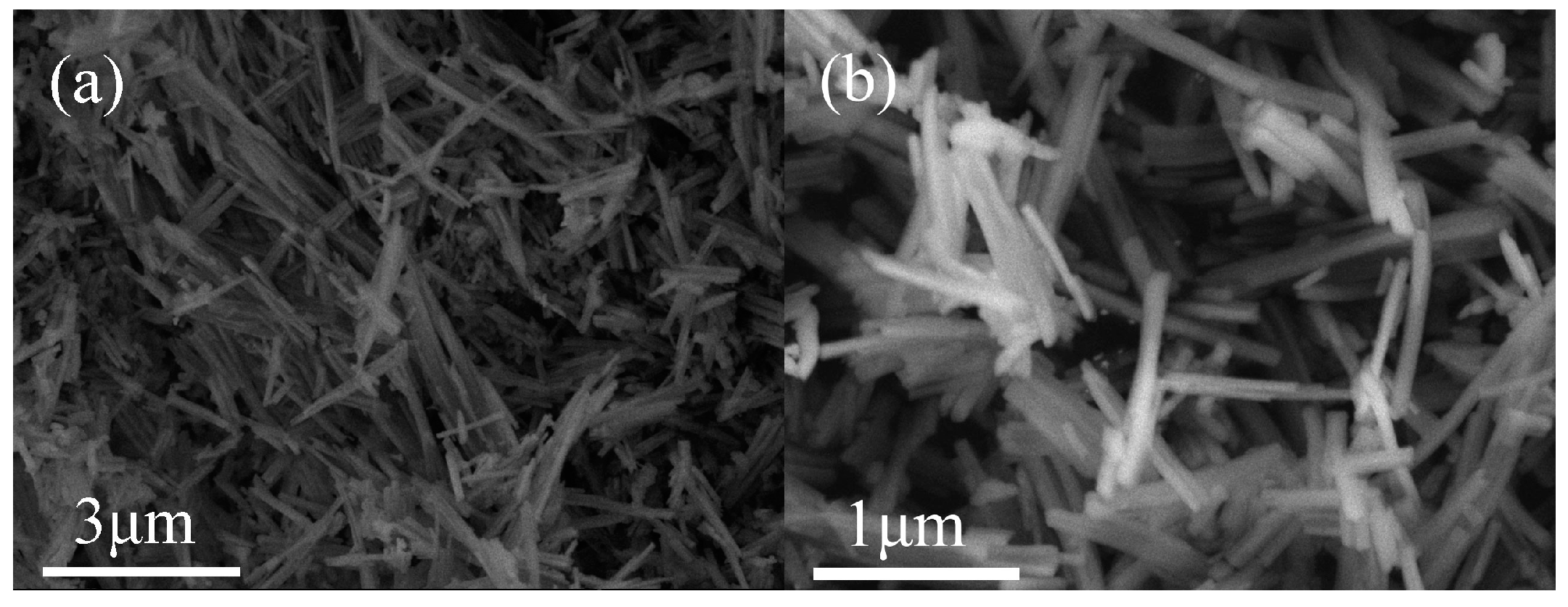
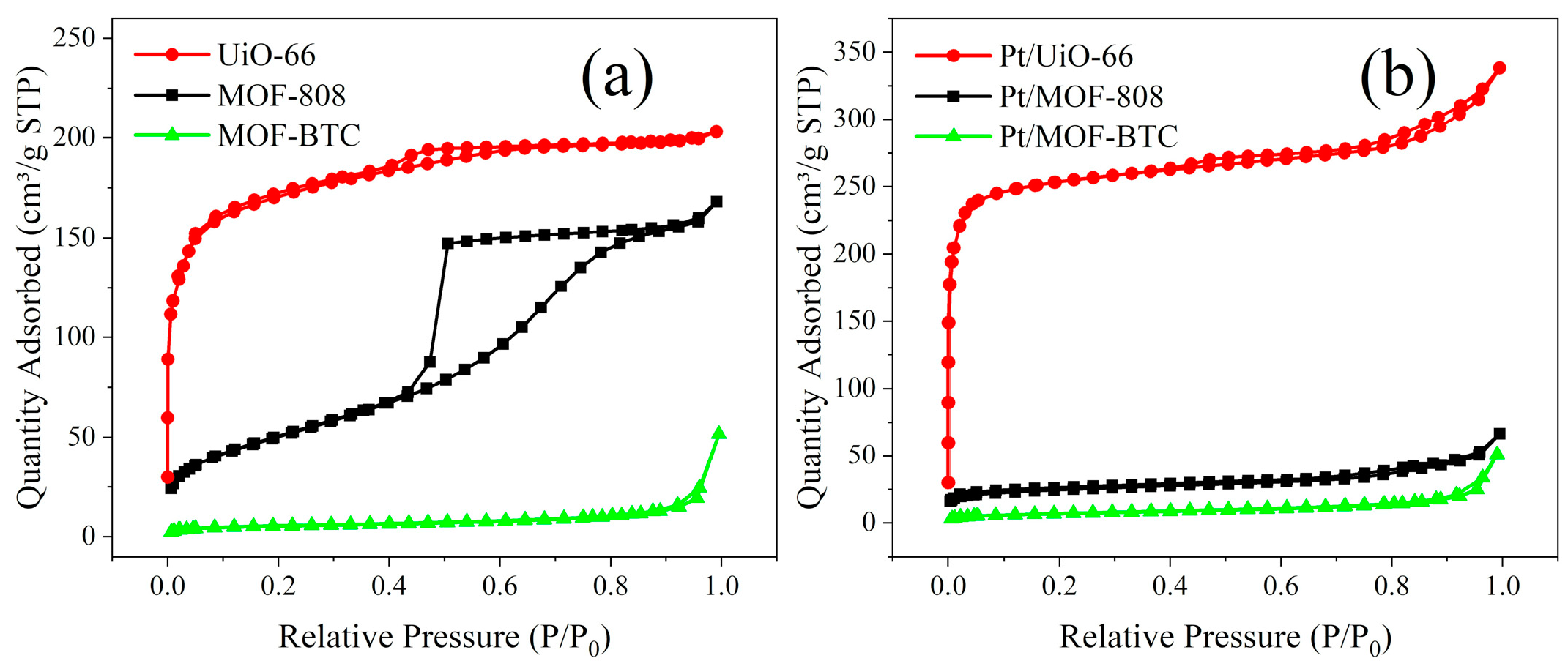
2.1.2. Morphology and Pore Structure Analysis
2.1.3. Analysis of Surface Chemical Properties
2.2. Catalyst Performance
2.3. Reusability and Stability
3. Materials and Methods
3.1. Materials
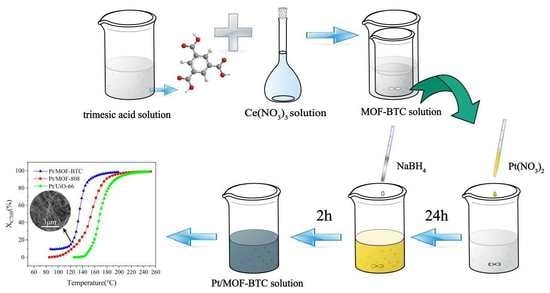
3.2. Catalysts Preparation
3.2.1. Synthesis of MOF–808
3.2.2. Synthesis of MOF–BTC
3.2.3. Synthesis of UiO–66
3.2.4. Synthesis of Pt/M (M = MOF–808, MOF–BTC, UiO–66)
3.3. Catalysts Characterization
3.4. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, B.; Lei, C.; Wei, C.; Zeng, G. Chlorinated volatile organic compounds (Cl–VOCs) in environment—Sources, potential human health impacts, and current remediation technologies. Environ. Int. 2014, 71, 118–138. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Liu, H.; Yu, Y.; Shao, Q.; Long, C. Porous polymeric resin for adsorbing low concentration of VOCs: Unveiling adsorption mechanism and effect of VOCs’ molecular properties. Sep. Purif. Technol. 2019, 228, 115755. [Google Scholar] [CrossRef]
- Wang, H.; Nie, L.; Li, J.; Wang, Y.; Wang, G.; Wang, J.; Hao, Z. Characterization and assessment of volatile organic compounds (VOCs) emissions from typical industries. Chin. Sci. Bull. 2013, 58, 724–730. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Shi, X.; Chen, J.; Yang, Y.; Lu, G. The preparation of defective UiO–66 metal–organic framework using MOF–5 as structural modifier with high sorption capacity for gaseous toluene. J. Environ. Chem. Eng. 2019, 7, 103405. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Lv, X.; Wang, Y.; Liu, N.; Chen, D.; Cui, L. Adsorption/desorption kinetics and breakthrough of gaseous toluene for modified microporous–mesoporous UiO–66 metal organic framework. J. Hazard. Mater. 2019, 366, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Y.; Song, L.; Chen, J.; Yang, Y.; Wang, Y. Enhanced adsorption performance of gaseous toluene on defective UiO–66 metal organic framework: Equilibrium and kinetic studies. J. Hazard. Mater. 2019, 365, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhen, W.; Ma, J.; Lu, G. High efficient solar hydrogen generation by modulation of Co–Ni sulfide (220) surface structure and adjusting adsorption hydrogen energy. Appl. Catal. B Environ. 2017, 206, 353–363. [Google Scholar] [CrossRef]
- Zhang, M.; Shang, Q.; Wan, Y.; Cheng, Q.; Liao, G.; Pan, Z. Self–template synthesis of double–shell TiO2@ZIF–8 hollow nanospheres via sonocrystallization with enhanced photocatalytic activities in hydrogen generation. Appl. Catal. B Environ. 2019, 241, 149–158. [Google Scholar] [CrossRef]
- Lan, Y.; Yang, Z.; Wang, P.; Yan, Y.; Zhang, L.; Ran, J. A review of microscopic seepage mechanism for shale gas extracted by supercritical CO2 flooding. Fuel 2019, 238, 412–424. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures–based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Niu, J.; Liland, S.E.; Yang, J.; Rout, K.R.; Ran, J.; Chen, D. Effect of oxide additives on the hydrotalcite derived Ni catalysts for CO2 reforming of methane. Chem. Eng. J. 2019, 377, 119763. [Google Scholar] [CrossRef]
- Ozturk, B.; Kuru, C.; Aykac, H.; Kaya, S. VOC separation using immobilized liquid membranes impregnated with oils. Sep. Purif. Technol. 2015, 153, 1–6. [Google Scholar] [CrossRef]
- Yan, Y.; Feng, S.; Huang, Z.; Zhang, L.; Pan, W.; Li, L.; Yang, Z. Thermal management and catalytic combustion stability characteristics of premixed methane/air in heat recirculation meso-combustors. Int. J. Energy Res. 2018, 42, 999–1012. [Google Scholar] [CrossRef]
- Roland, U.; Kraus, M.; Holzer, F.; Trommler, U.; Kopinke, F.-D. Selective dielectric heating for efficient adsorptive–catalytic cleaning of contaminated gas streams. Appl. Catal. A Gen. 2014, 474, 244–249. [Google Scholar] [CrossRef]
- Peng, R.; Sun, X.; Li, S.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Shape effect of Pt/CeO2 catalysts on the catalytic oxidation of toluene. Chem. Eng. J. 2016, 306, 1234–12346. [Google Scholar] [CrossRef]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.-W. Hydrogen Storage in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Huang, W.; Yang, Y.; Wang, Y.; He, C.; Liu, N.; Wu, M.; Tang, L. g–C3N4/UiO–66 nanohybrids with enhanced photocatalytic activities for the oxidation of dye under visible light irradiation. Mater. Res. Bull. 2018, 99, 349–358. [Google Scholar] [CrossRef]
- Yoon, M.; Suh, K.; Natarajan, S.; Kim, K. Proton Conduction in Metal–Organic Frameworks and Related Modularly Built Porous Solids. Angew. Chem. Int. Ed. 2013, 52, 2688–2700. [Google Scholar] [CrossRef]
- Taylor-Pashow, K.M.L.; Della Rocca, J.; Xie, Z.; Tran, S.; Lin, W. Postsynthetic Modifications of Iron–Carboxylate Nanoscale Metal–Organic Frameworks for Imaging and Drug Delivery. J. Am. Chem. Soc. 2009, 131, 14261–14263. [Google Scholar] [CrossRef] [Green Version]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–11025. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.-Y.; Hwang, Y.K.; Serre, C.; Ferey, G.; Chang, J.-S. Porous Chromium Terephthalate MIL–101 with Coordinatively Unsaturated Sites: Surface Functionalization; Encapsulation, Sorption and Catalysis. Adv. Funct. Mater. 2009, 19, 1537–1552. [Google Scholar] [CrossRef]
- Zhang, C.; Michaelides, A.; Jenkins, S.J. Theory of gold on ceria. Phys. Chem. Chem. Phys. 2011, 13, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hou, F.; Yang, Y.; Wang, Y.; Liu, N.; Chen, D.; Yang, Y. A facile synthesis for cauliflower like CeO2 catalysts from Ce–BTC precursor and their catalytic performance for CO oxidation. Appl. Surf. Sci. 2017, 423, 771–779. [Google Scholar] [CrossRef]
- Lammert, M.; Glissmann, C.; Reinsch, H.; Stock, N. Synthesis and Characterization of New Ce(IV)–MOFs Exhibiting Various Framework Topologies. Cryst. Growth Des. 2017, 17, 1125–1131. [Google Scholar] [CrossRef]
- Liu, K.; You, H.; Jia, G.; Zheng, Y.; Huang, Y.; Song, Y.; Yang, M.; Zhang, L.; Zhang, H. Hierarchically Nanostructured Coordination Polymer: Facile and Rapid Fabrication and Tunable Morphologies. Cryst. Growth Des. 2010, 10, 790–797. [Google Scholar] [CrossRef]
- Sun, W.; Li, X.; Sun, C.; Huang, Z.; Xu, H.; Shen, W. Insights into the Pyrolysis Processes of Ce–MOFs for Preparing Highly Active Catalysts of Toluene Combustion. Catalysts 2019, 9, 682. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Gao, J.; Zhang, Q.; Liu, Z.; Fu, M.; Wu, J.; Hu, Y.; Ye, D. Enhanced oxygen vacancies to improve ethyl acetate oxidation over MnOx–CeO2 catalyst derived from MOF template. Chem. Eng. J. 2019, 371, 78–87. [Google Scholar] [CrossRef]
- Almasi, M.; Zelenak, V.; Opanasenko, M.; Cisarova, I. Ce(III) and Lu(III) metal–organic frameworks with Lewis acid metal sites: Preparation, sorption properties and catalytic activity in Knoevenagel condensation. Catal. Today 2015, 243, 184–194. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Belyaeva, E.V.; Fitch, A.N.; Chernyshev, V.V.; Klyamkin, S.N.; Kustov, L.M. Synthesis and Structural Characterization of a Series of Novel Zn(II)–based MOFs with Pyridine–2,5–dicarboxylate Linkers. Cryst. Growth Des. 2013, 13, 5305–5315. [Google Scholar] [CrossRef]
- Wang, F.; Guo, H.; Chai, Y.; Li, Y.; Liu, C. The controlled regulation of morphology and size of HKUST–1 by “coordination modulation method”. Microporous Mesoporous Mater. 2013, 173, 181–188. [Google Scholar] [CrossRef]
- Fan, L.; Wang, K.; Xu, K.; Liang, Z.; Wang, H.; Zhou, S.-F.; Zhan, G. Structural Isomerism of Two Ce–BTC for Fabricating Pt/CeO(2)Nanorods toward Low–Temperature CO Oxidation. Small 2020, 16, 2003597. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Qu, Z.; Qin, Y.; Fu, Q.; Sun, H.; Duan, X. Revealing the Highly Catalytic Performance of Spinel CoMn2O4 for Toluene Oxidation: Involvement and Replenishment of Oxygen Species Using In Situ Designed–TP Techniques. ACS Catal. 2019, 9, 6698–6710. [Google Scholar] [CrossRef]
- Lammert, M.; Wharmby, M.T.; Smolders, S.; Bueken, B.; Lieb, A.; Lomachenko, K.A.; De Vos, D.; Stock, N. Cerium–based metal organic frameworks with UiO–66 architecture: Synthesis, properties and redox catalytic activity. Chem. Commun. 2015, 51, 12578–12581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, S.; Zhang, Q.; Li, J.; Sun, Y.; Ren, Q.; Zou, S.; Zhang, Q.; Lu, J.; Fu, M.; Mo, D.; et al. Highly efficient mesoporous MnO2 catalysts for the total toluene oxidation: Oxygen–Vacancy defect engineering and involved intermediates using in situ DRIFTS. Appl. Catal. B Environ. 2020, 264, 118464. [Google Scholar] [CrossRef]
- Liu, P.; Jing, P.; Xu, X.; Liu, B.; Zhang, J. Structural Reconstruction of Ce–MOF with Active Sites for Efficient Electrocatalytic N–2 Reduction. ACS Appl. Energy Mater. 2021, 4, 12128–12136. [Google Scholar] [CrossRef]
- Fonseca, J.; Gong, T.; Jiao, L.; Jiang, H.-L. Metal–organic frameworks (MOFs) beyond crystallinity: Amorphous MOFs, MOF liquids and MOF glasses. J. Mater. Chem. A 2021, 9, 10562–10611. [Google Scholar] [CrossRef]
- He, J.; Xu, Y.; Wang, W.; Hu, B.; Wang, Z.; Yang, X.; Wang, Y.; Yang, L. Ce(III) nanocomposites by partial thermal decomposition of Ce–MOF for effective phosphate adsorption in a wide pH range. Chem. Eng. J. 2020, 379, 122431. [Google Scholar] [CrossRef]
- Rojas-Buzo, S.; Concepcion, P.; Luis Olloqui-Sariego, J.; Moliner, M.; Corma, A. Metalloenzyme–Inspired Ce–MOF Catalyst for Oxidative Halogenation Reactions. ACS Appl. Mater. Interfaces 2021, 13, 31021–31030. [Google Scholar] [CrossRef]
- Huang, H.; Chen, Y.; Chen, Z.; Chen, J.; Hu, Y.; Zhu, J.-J. Electrochemical sensor based on Ce–MOF/carbon nanotube composite for the simultaneous discrimination of hydroquinone and catechol. J. Hazard. Mater. 2021, 416, 125895. [Google Scholar] [CrossRef]
- Zhang, Q.; Mo, S.; Li, J.; Sun, Y.; Zhang, M.; Chen, P.; Fu, M.; Wu, J.; Chen, L.; Ye, D. In situ DRIFT spectroscopy insights into the reaction mechanism of CO and toluene co–oxidation over Pt–based catalysts. Catal. Sci. Technol. 2019, 9, 4538–4551. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Serrano-Lotina, A.; Han, W.; Portela, R.; Wang, R.; Banares, M.A.; Yeung, K.L. Operando Investigation of Toluene Oxidation over 1D Pt@CeO2 Derived from Pt Cluster–Containing MOF. J. Am. Chem. Soc. 2021, 143, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Deng, J.; Impeng, S.; Li, S.; Yan, T.; Zhang, J.; Shi, L.; Zhang, D. Boosting Toluene Combustion by Engineering Co–O Strength in Cobalt Oxide Catalysts. Environ. Sci. Technol. 2020, 54, 10342–10350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lv, X.; Bi, F.; Lu, G.; Wang, Y. Highly efficient Mn2O3 catalysts derived from Mn–MOFs for toluene oxidation: The influence of MOFs precursors. Mol. Catal. 2020, 482, 110701. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Huang, H.; Zeng, K.; Wang, Z.; Jia, H.-p.; Li, X. Insights into the size and structural effects of zeolitic supports on gaseous toluene oxidation over MnOx/HZSM–5 catalysts. Appl. Surf. Sci. 2019, 486, 108–120. [Google Scholar] [CrossRef]
- Chen, L.W.; Liu, F.-C.; Hung, L.-F.; Huang, C.-Y.; Lien, S.-B.; Lin, L.-C.; Lai, J.-H.; Ho, L.-J. Chondroprotective Effects and Mechanisms of Dextromethorphan: Repurposing Antitussive Medication for Osteoarthritis Treatment. Int. J. Mol. Sci. 2018, 19, 825. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Wu, Z.; Song, W.; Xiao, W.; Guo, Y.; Ding, J.; Suib, S.L.; Gao, P.-X. Low temperature propane oxidation over Co3O4 based nano–array catalysts: Ni dopant effect, reaction mechanism and structural stability. Appl. Catal. B Environ. 2016, 180, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Lan, Q.; Xie, Y.; Sun, X.; Wang, Y. Label–Free Electrochemical Immunosensor for Ultrasensitive Detection of Carbohydrate Antigen 125 Based on Antibody–Immobilized Biocompatible MOF–808/CNT. ACS Appl. Mater. Interfaces 2021, 13, 3295–3302. [Google Scholar] [CrossRef]
- Nagarjun, N.; Jacob, M.; Varalakshmi, P.; Dhakshinamoorthy, A. UiO–66(Ce) metal–organic framework as a highly active and selective catalyst for the aerobic oxidation of benzyl amines. Mol. Catal. 2021, 499, 111277. [Google Scholar] [CrossRef]
- Lin, A.; Ibrahim, A.A.; Arab, P.; El-Kaderi, H.M.; El-Shall, M.S. Palladium Nanoparticles Supported on Ce–Metal–Organic Framework for Efficient CO Oxidation and Low–Temperature CO2 Capture. Acs Appl. Mater. Interfaces 2017, 9, 17961–17968. [Google Scholar] [CrossRef] [Green Version]
- Nagarjun, N.; Concepcion, P.; Dhakshinamoorthy, A. Influence of oxophilic behavior of UiO–66(Ce) metal–organic framework with superior catalytic performance in Friedel–Crafts alkylation reaction. Appl. Organomet. Chem. 2020, 34, e5578. [Google Scholar] [CrossRef]
- Lu, A.; Sun, H.; Zhang, N.; Che, L.; Shan, S.; Luo, J.; Zheng, J.; Yang, L.; Peng, D.-L.; Zhong, C.-J.; et al. Surface Partial–Charge–Tuned Enhancement of Catalytic Activity of Platinum Nanocatalysts for Toluene Oxidation. ACS Catal. 2019, 9, 7431–7442. [Google Scholar] [CrossRef]
- Zhao, S.; Li, K.; Jiang, S.; Li, J. Pd–Co based spinel oxides derived from pd nanoparticles immobilized on layered double hydroxides for toluene combustion. Appl. Catal. B Environ. 2016, 181, 236–248. [Google Scholar] [CrossRef]
- Zhang, W.; Lassen, K.; Descorme, C.; Valverde, J.L.; Giroir-Fendler, A. Effect of the precipitation pH on the characteristics and performance of Co3O4 catalysts in the total oxidation of toluene and propane. Appl. Catal. B Environ. 2021, 282, 119566. [Google Scholar] [CrossRef]
- Ma, P.; Meng, F.; Wang, N.; Zhang, J.; Xie, J.; Dai, B. Heterogeneous Amorphous Cu–MOF–74 Catalyst for C–N Coupling Reaction. Chemistryselect 2018, 3, 10694–10700. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, X.; Chen, J.; Yang, Y.; Wang, Y. Excellent catalytic activity and water resistance of UiO–66–supported highly dispersed Pd nanoparticles for toluene catalytic oxidation. Appl. Catal. B Environ. 2020, 269, 118767. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, S.; Chen, C.; He, H.; Jin, Z.; Luo, M.; Chen, J. Understanding the crucial roles of catalyst properties in ethyl acetate and toluene oxidation over Pt catalysts. New J. Chem. 2021, 45, 11352–11358. [Google Scholar] [CrossRef]
- Liotta, L.F.; Ousmane, M.; Di Carlo, G.; Pantaleo, G.; Deganello, G.; Boreave, A.; Giroir-Fendler, A. Catalytic Removal of Toluene over Co3O4–CeO2 Mixed Oxide Catalysts: Comparison with Pt/Al2O3. Catal. Lett. 2009, 127, 270–276. [Google Scholar] [CrossRef]
- Sun, Y.; Fan, J.; Cheng, H.; Mo, S.; Ke, Y.; Ren, Q.; Fu, M.; Chen, P.; Wu, J.; Ye, D. Investigation into the roles of different oxygen species in toluene oxidation over manganese–supported platinum catalysts. Mol. Catal. 2021, 507, 111569. [Google Scholar] [CrossRef]
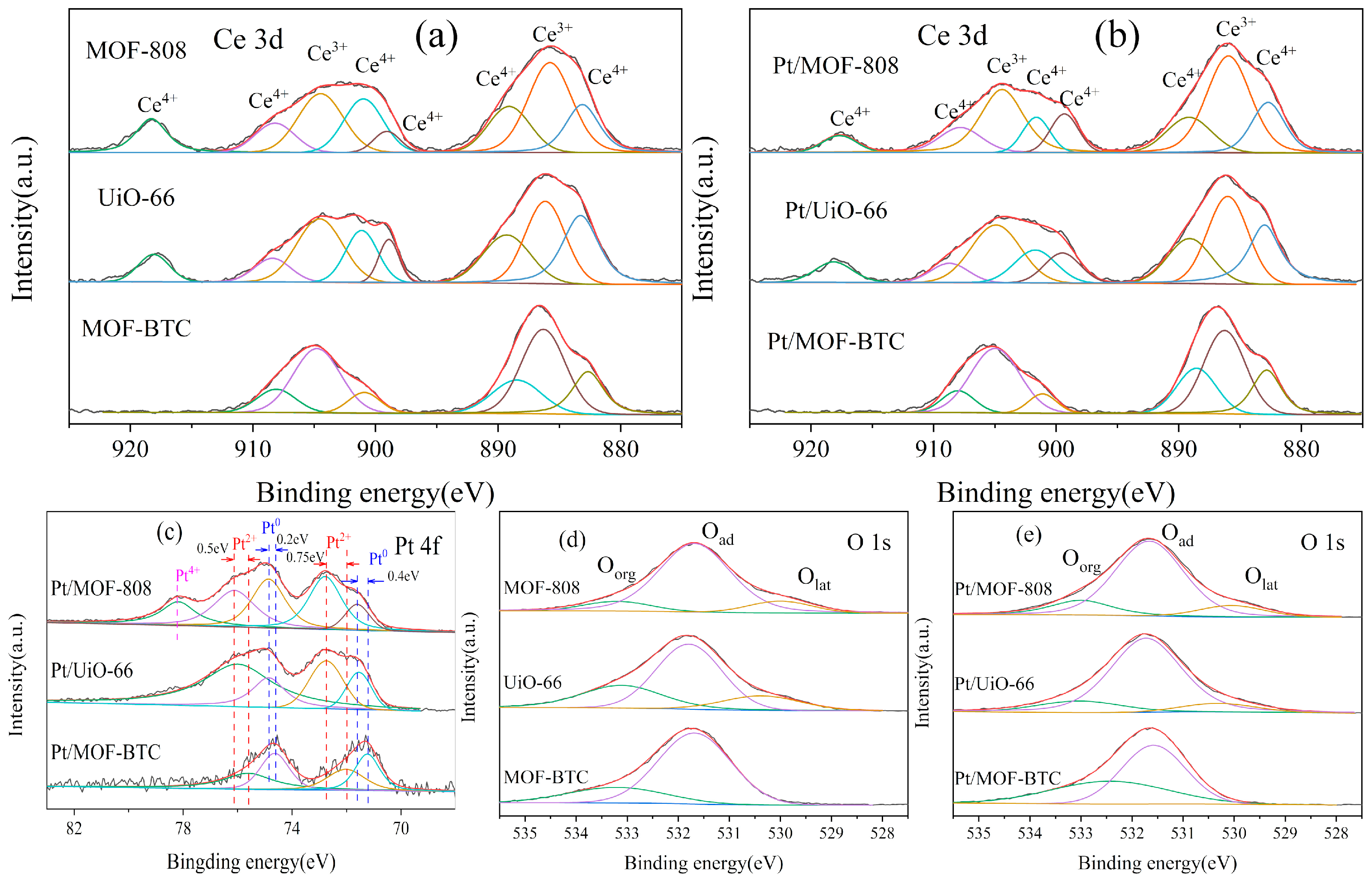
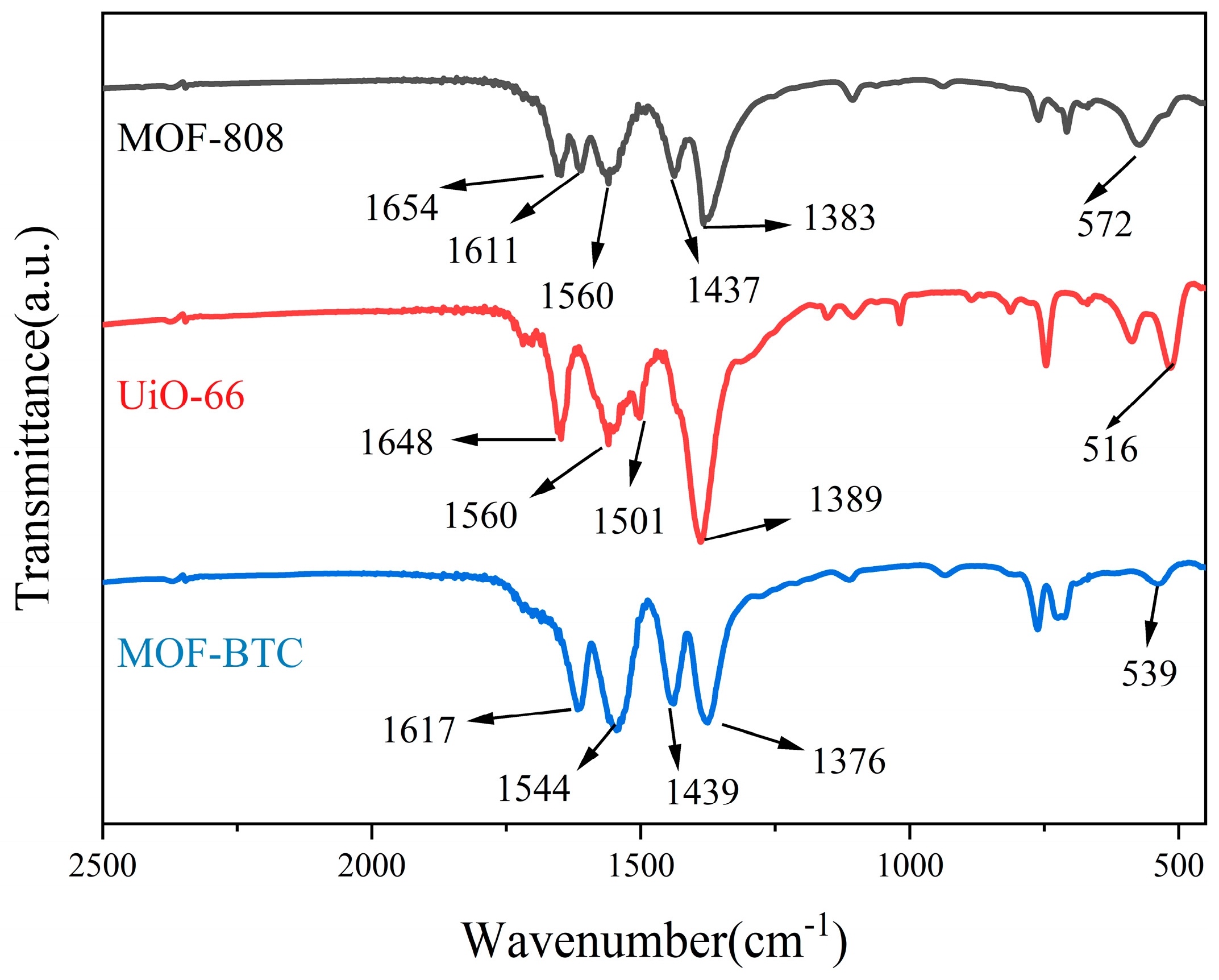
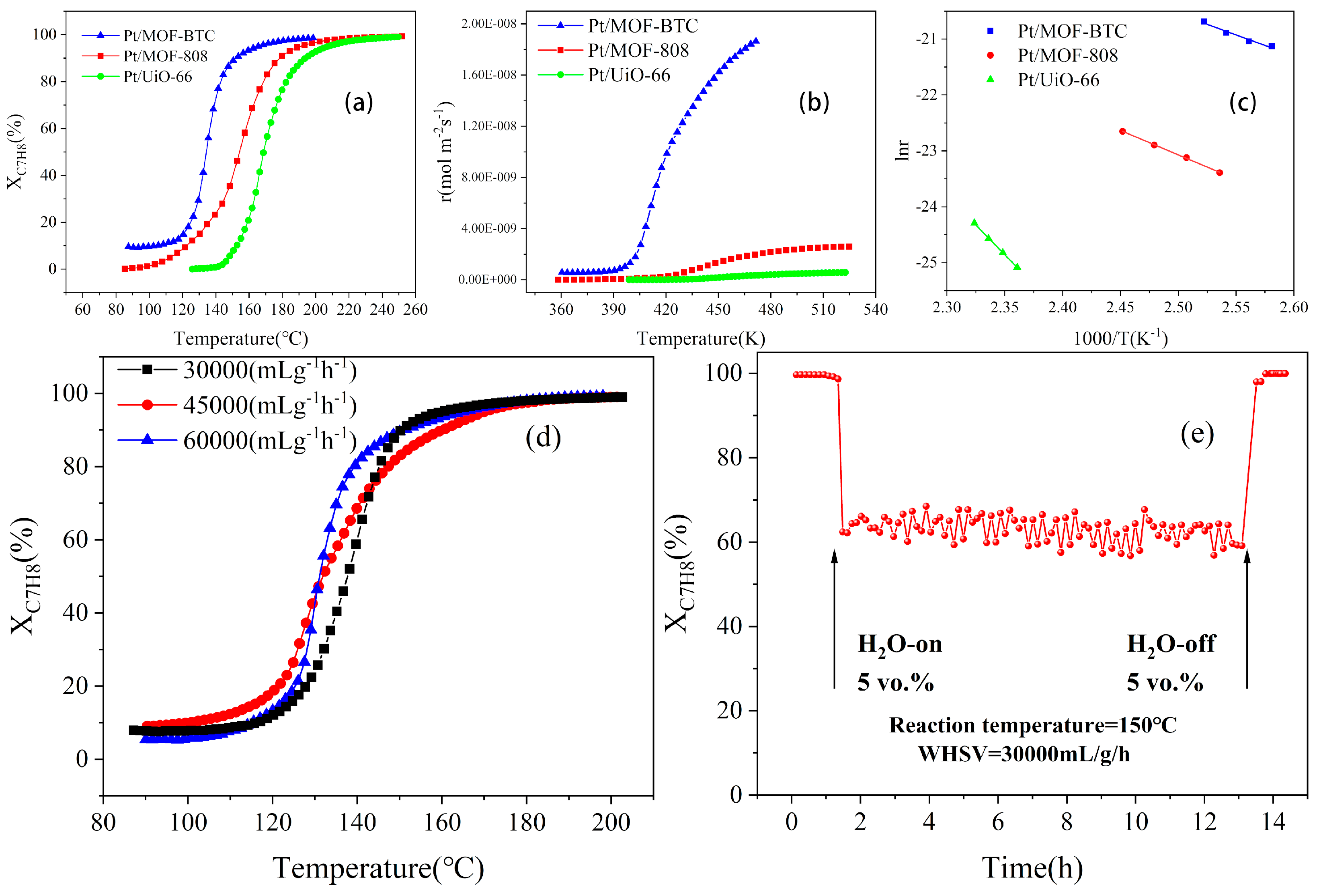
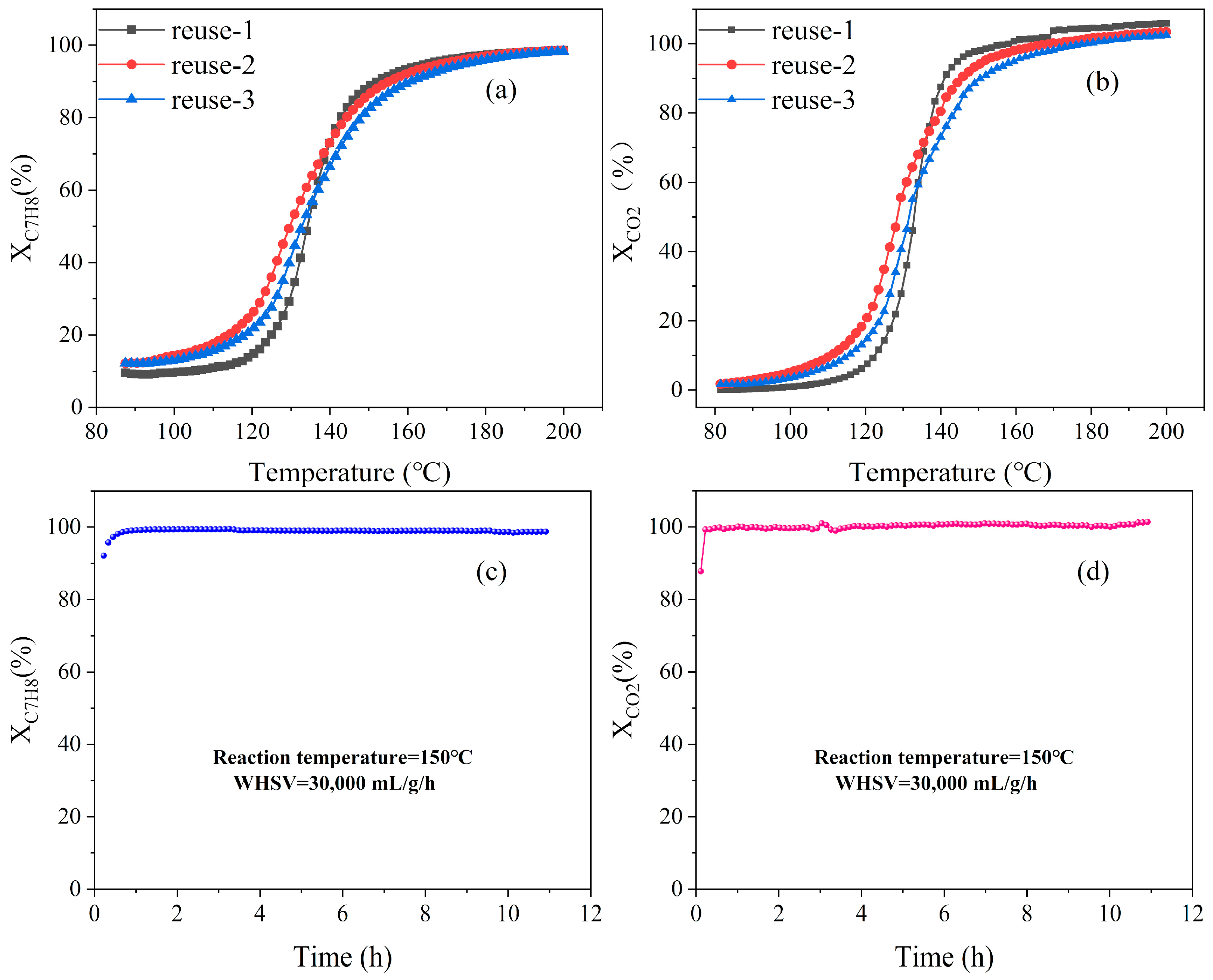
| Samples | SBET (m2/g) | Pore Volume (cm3/g) | Ce3+/(Ce3+ + Ce4+) | Pt0/Pttotal | Oad/Ototal |
|---|---|---|---|---|---|
| MOF–808 | 184.8 | 0.27 | 0.43 | * | 0.80 |
| Pt/MOF–808 | 181.8 | 0.05 | 0.52 | 0.39 | 0.57 |
| UiO–66 | 528.8 | 0.11 | 0.39 | * | 0.76 |
| Pt/UiO–66 | 778.7 | 0.18 | 0.43 | 0.27 | 0.75 |
| MOF–BTC | 18.1 | 0.03 | 0.58 | * | 0.79 |
| Pt/MOF–BTC | 24.5 | 0.08 | 0.59 | 0.52 | 0.80 |
| Samples | T50 (°C) | T90 (°C) | Ea (kJ·mol−1) |
|---|---|---|---|
| Pt/MOF–808 | 154 | 178 | 72.7 |
| Pt/UiO–66 | 168 | 193 | 176.7 |
| Pt/MOF–BTC | 140 | 149 | 62.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, G.; Chen, J.; Niu, H. Excellent Catalytic Performance of Ce–MOF with Abundant Oxygen Vacancies Supported Noble Metal Pt in the Oxidation of Toluene. Catalysts 2022, 12, 775. https://doi.org/10.3390/catal12070775
Liu Y, Chen G, Chen J, Niu H. Excellent Catalytic Performance of Ce–MOF with Abundant Oxygen Vacancies Supported Noble Metal Pt in the Oxidation of Toluene. Catalysts. 2022; 12(7):775. https://doi.org/10.3390/catal12070775
Chicago/Turabian StyleLiu, Yang, Gongda Chen, Jianjun Chen, and Hejingying Niu. 2022. "Excellent Catalytic Performance of Ce–MOF with Abundant Oxygen Vacancies Supported Noble Metal Pt in the Oxidation of Toluene" Catalysts 12, no. 7: 775. https://doi.org/10.3390/catal12070775
APA StyleLiu, Y., Chen, G., Chen, J., & Niu, H. (2022). Excellent Catalytic Performance of Ce–MOF with Abundant Oxygen Vacancies Supported Noble Metal Pt in the Oxidation of Toluene. Catalysts, 12(7), 775. https://doi.org/10.3390/catal12070775