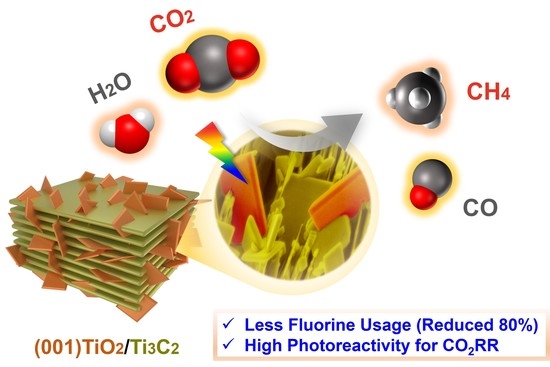
Facile Preparation of Highly Active CO2 Reduction (001)TiO2/Ti3C2Tx Photocatalyst from Ti3AlC2 with Less Fluorine
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Procedures
3.1. Chemicals
3.2. Sample Preparation
3.3. Characterization
3.4. Electrochemical Measurements
3.5. Photocatalytic CO2 Reduction and Isotope-Labelling Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Elzatahry, A.; Aldhayan, D.; Zhao, D. Core-Shell Structured Titanium Dioxide Nanomaterials for Solar Energy Utilization. Chem. Soc. Rev. 2018, 47, 8203–8237. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Wang, L.; Zhou, K.; Zhang, D.; Zhang, Y.; Adamaki, V.; Sergejevs, A.; Bowen, C. Improved Photocatalytic Performance of Gradient Reduced TiO2 Ceramics with Aligned Pore Channels. Adv. Powder Mater. 2022, 1, 100025. [Google Scholar] [CrossRef]
- Xu, F.; Meng, K.; Zhu, B.; Liu, H.; Xu, J.; Yu, J. Graphdiyne: A New Photocatalytic CO2 Reduction Cocatalyst. Adv. Funct. Mater. 2019, 29, 1904256. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual Cocatalysts in TiO2 Photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef] [PubMed]
- Sajan, C.P.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J.; Cao, S. TiO2 Nanosheets with Exposed {001} Facets for Photocatalytic Applications. Nano Res. 2016, 9, 3–27. [Google Scholar] [CrossRef]
- Xu, F.; Meng, K.; Cheng, B.; Wang, S.; Xu, J.; Yu, J. Unique S-Scheme Heterojunctions in Self-Assembled TiO2/CsPbBr3 Hybrids for CO2 Photoreduction. Nat. Commun. 2020, 11, 4613. [Google Scholar] [CrossRef]
- Low, J.; Dai, B.; Tong, T.; Jiang, C.; Yu, J. In Situ Irradiated X-ray Photoelectron Spectroscopy Investigation on a Direct Z-scheme TiO2/CdS Composite Film Photocatalyst. Adv. Mater. 2019, 31, 1802981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.; Han, X.; Guo, L.; Wang, D.; Lv, K. Assembly of CaIn2S4 on Defect-Rich BiOCl for Acceleration of Interfacial Charge Separation and Photocatalytic Phenol Degradation via S-Scheme Electron Transfer Mechanism. Catalysts 2021, 11, 1130. [Google Scholar] [CrossRef]
- Naguib, M.; Barsoum, M.W.; Gogotsi, Y. Ten Years of Progress in the Synthesis and Development of MXenes. Adv. Mater. 2021, 33, 2103393. [Google Scholar] [CrossRef]
- Amrillah, T.; Hermawan, A.; Alviani, V.N.; Seh, Z.W.; Yin, S. MXenes and Their Derivatives as Nitrogen Reduction Reaction Catalysts: Recent Progress and Perspectives. Mater. Today Energy 2021, 22, 100864. [Google Scholar] [CrossRef]
- Ran, J.; Gao, G.; Li, F.T.; Ma, T.Y.; Du, A.; Qiao, S.Z. Ti3C2 MXene Co-Catalyst on Metal Sulfide Photo-Absorbers for Enhanced Visible-Light Photocatalytic Hydrogen Production. Nat. Commun. 2017, 8, 13907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, J.; Zhang, L.; Tong, T.; Shen, B.; Yu, J. TiO2/MXene Ti3C2 Composite with Excellent Photocatalytic CO2 Reduction Activity. J. Catal. 2018, 361, 255–266. [Google Scholar] [CrossRef]
- Chen, L.; Ye, X.; Chen, S.; Ma, L.; Wang, Z.; Wang, Q.; Hua, N.; Xiao, X.; Cai, S.; Liu, X. Ti3C2 MXene Nanosheet/TiO2 Composites for Efficient Visible Light Photocatalytic Activity. Ceram. Int. 2020, 46, 25895–25904. [Google Scholar] [CrossRef]
- Li, W.; Zhuang, C.; Li, Y.; Gao, C.; Jiang, W.; Sun, Z.; Qi, K. Anchoring Ultra-Small TiO2 Quantum Dots onto Ultra-Thin and Large-Sized Mxene Nanosheets for Highly Efficient Photocatalytic Water Splitting. Ceram. Int. 2021, 47, 21769–21776. [Google Scholar] [CrossRef]
- Li, Z.; Huang, W.; Liu, J.; Lv, K.; Li, Q. Embedding CdS@Au into Ultrathin Ti3-XC2Ty to Build Dual Schottky Barriers for Photocatalytic H2 production. ACS Catal. 2021, 11, 8510–8520. [Google Scholar] [CrossRef]
- Peng, C.; Yang, X.; Li, Y.; Yu, H.; Wang, H.; Peng, F. Hybrids of Two-Dimensional Ti3C2 and TiO2 Exposing {001} Facets toward Enhanced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2016, 8, 6051–6060. [Google Scholar] [CrossRef]
- Hermawan, A.; Hasegawa, T.; Asakura, Y.; Yin, S. Enhanced Visible-Light-Induced Photocatalytic NOx Degradation over (Ti,C)-BiOBr/Ti3C2Tx MXene Nanocomposites: Role of Ti and C Doping. Sep. Purif. Technol. 2021, 270, 118815. [Google Scholar] [CrossRef]
- Cao, S.; Shen, B.; Tong, T.; Fu, J.; Yu, J. 2D/2D Heterojunction of Ultrathin MXene/Bi2WO6 Nanosheets for Improved Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Sreedhar, A.; Noh, J.S. Recent Advances in Partially and Completely Derived 2D Ti3C2 MXene Based TiO2 Nanocomposites towards Photocatalytic Applications. Sol. Energy 2021, 222, 48–73. [Google Scholar] [CrossRef]
- Li, Y.; Deng, X.; Tian, J.; Liang, Z.; Cui, H. Ti3C2 MXene-Derived Ti3C2/TiO2 Nanoflowers for Noble-Metal-Free Photocatalytic Overall Water Splitting. Appl. Mater. Today 2018, 13, 217–227. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Feng, X.; Liao, Y.; Wen, Q.; Xiang, Q. Truncated Octahedral Bipyramidal TiO2/MXene Ti3C2 Hybrids with Enhanced Photocatalytic H2 Production Activity. Nanoscale Adv. 2019, 1, 1812–1818. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, A.; Rasool, K.; Nawaz, M.; Miran, W.; Jang, J.; Moztahida, M.; Mahmoud, K.A.; Lee, D.S. Heterostructural TiO2/Ti3C2Tx (MXene) for Photocatalytic Degradation of Antiepileptic Drug Carbamazepine. Chem. Eng. J. 2018, 349, 748–755. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Cai, G.; Li, M.; Tang, D. In Situ Formation of (0 0 1) TiO2/Ti3C2 Heterojunctions for Enhanced Photoelectrochemical Detection of Dopamine. Electrochem. Commun. 2021, 125, 106987. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Yang, J.; Han, B.; Nie, R.; Wang, J.; Wang, J.; Jing, H. In-Situ Grown Nanocrystal TiO2 on 2D Ti3C2 Nanosheets for Artificial Photosynthesis of Chemical Fuels. Nano Energy 2018, 51, 442–450. [Google Scholar] [CrossRef]
- Hantanasirisakul, K.; Gogotsi, Y. Electronic and Optical Properties of 2D Transition Metal Carbides and Nitrides (MXenes). Adv. Mater. 2018, 30, 1804779. [Google Scholar] [CrossRef] [PubMed]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 Single Crystals with a Large Percentage of Reactive Facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Tan, Q.; Li, Q.; Zhou, J.; Fan, J.; Li, B.; Sun, J.; Lv, K. 2D/2D Ti3C2 MXene/g-C3N4 Nanosheets Heterojunction for High Efficient CO2 Reduction Photocatalyst: Dual Effects of Urea. Appl. Catal. B Environ. 2020, 268, 118738. [Google Scholar] [CrossRef]
- Zhang, C.J.; Pinilla, S.; McEvoy, N.; Cullen, C.P.; Anasori, B.; Long, E.; Park, S.H.; Seral-Ascaso, A.; Shmeliov, A.; Krishnan, D.; et al. Oxidation Stability of Colloidal Two-Dimensional Titanium Carbides (MXenes). Chem. Mater. 2017, 29, 4848–4856. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Lukatskaya, M.R.; Dyatkin, B.; Zhang, C.; Presser, V.; Gogotsi, Y.; Barsoum, M.W. One-Step Synthesis of Nanocrystalline Transition Metal Oxides on Thin Sheets of Disordered Graphitic Carbon by Oxidation of MXenes. Chem. Commun. 2014, 50, 7420–7423. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Yu, J.; Jaroniec, M. Anatase TiO2 with Dominant High-Energy {001} Facets: Synthesis, Properties, and Applications. Chem. Mater. 2011, 23, 4085–4093. [Google Scholar] [CrossRef]
- Diebold, U. The Surface Science of Titanium Dioxide. Surf. Sci. Rep. 2002, 48, 53–229. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Liu, S.; Li, Y.; Fan, J.; Lv, K. Effects of Fluorine on Photocatalysis. Chin. J. Catal. 2020, 41, 1451–1467. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Ahmed, B.; Hedhili, M.N.; Anjum, D.H.; Alshareef, H.N. Effect of Postetch Annealing Gas Composition on the Structural and Electrochemical Properties of Ti2CTx MXene Electrodes for Supercapacitor Applications. Chem. Mater. 2015, 27, 5314–5323. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Zhao, Z.; Gao, F.; Wang, X.; Qiu, J. Mesoporous Microspheres Composed of Carbon-Coated TiO2 Nanocrystals with Exposed {001} Facets for Improved Visible Light Photocatalytic Activity. Appl. Catal. B Environ. 2014, 147, 958–964. [Google Scholar] [CrossRef]
- Ye, M.; Wang, X.; Liu, E.; Ye, J.; Wang, D. Boosting the Photocatalytic Activity of P25 for Carbon Dioxide Reduction by Using a Surface-Alkalinized Titanium Carbide MXene as Cocatalyst. ChemSusChem 2018, 11, 1606–1611. [Google Scholar] [CrossRef]
- Wang, K.; Li, X.; Wang, N.; Shen, Q.; Liu, M.; Zhou, J.; Li, N. Z-Scheme Core-Shell meso-TiO2@ZnIn2S4/Ti3C2 MXene Enhances Visible Light-Driven CO2-to-CH4 Selectivity. Ind. Eng. Chem. Res. 2021, 60, 8720–8732. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Xue, W.; Sun, K.; Song, X.; Wu, C.; Nie, L.; Li, Y.; Liu, C.; Pan, Y.; et al. Self-Adaptive Dual-Metal-Site Pairs in Metal-Organic Frameworks for Selective CO2 Photoreduction to CH4. Nat. Catal. 2021, 4, 719–729. [Google Scholar] [CrossRef]
- Huo, Y.; Zhang, J.; Dai, K.; Li, Q.; Lv, J.; Zhu, G.; Liang, C. All-Solid-State Artificial Z-Scheme Porous g-C3N4/Sn2S3-DETA Heterostructure Photocatalyst with Enhanced Performance in Photocatalytic CO2 Reduction. Appl. Catal. B Environ. 2019, 241, 528–538. [Google Scholar] [CrossRef]
- Di, J.; Chen, C.; Yang, S.Z.; Chen, S.; Duan, M.; Xiong, J.; Zhu, C.; Long, R.; Hao, W.; Chi, Z.; et al. Isolated Single Atom Cobalt in Bi3O4Br Atomic Layers to Trigger Efficient CO2 Photoreduction. Nat. Commun. 2019, 10, 2840. [Google Scholar] [CrossRef] [Green Version]
- Xia, P.; Zhu, B.; Yu, J.; Cao, S.; Jaroniec, M. Ultra-Thin Nanosheet Assemblies of Graphitic Carbon Nitride for Enhanced Photocatalytic CO2 Reduction. J. Mater. Chem. A 2017, 5, 3230–3238. [Google Scholar] [CrossRef]
- Jiao, X.; Li, X.; Jin, X.; Sun, Y.; Xu, J.; Liang, L.; Ju, H.; Zhu, J.; Pan, Y.; Yan, W.; et al. Partially Oxidized SnS2 Atomic Layers Achieving Efficient Visible-Light-Driven CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 18044–18051. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Y.; Xu, J.; Shao, Y.; Wu, J.; Xu, X.; Pan, Y.; Ju, H.; Zhu, J.; Xie, Y. Selective Visible-Light-Driven Photocatalytic CO2 Reduction to CH4 Mediated by Atomically Thin CuIn5S8 Layers. Nat. Energy 2019, 4, 690–699. [Google Scholar] [CrossRef]
- Xiao, N.; Li, S.; Liu, S.; Xu, B.; Li, Y.; Gao, Y.; Ge, L.; Lu, G. Novel PtPd Alloy Nanoparticle-Decorated g-C3N4 Nanosheets with Enhanced Photocatalytic Activity for H2 Evolution under Visible Light Irradiation. Chin. J. Catal. 2019, 40, 352–361. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, K.; Wang, G.; Deng, K.; Tang, D. Study on the Shape Control and Photocatalytic Activity of High-Energy Anatase Titania. Appl. Catal. B Environ. 2010, 100, 378–385. [Google Scholar] [CrossRef]
- Xia, Y.; Cheng, B.; Fan, J.; Yu, J.; Liu, G. Unraveling Photoexcited Charge Transfer Pathway and Process of CdS/Graphene Nanoribbon Composites toward Visible-Light Photocatalytic Hydrogen Evolution. Small 2019, 15, 1902459. [Google Scholar] [CrossRef]
- Neaţu, Ş.; Maciá-Agulló, J.A.; Concepción, P.; Garcia, H. Gold-Copper Nanoalloys Supported on TiO2 as Photocatalysts for CO2 Reduction by Water. J. Am. Chem. Soc. 2014, 136, 15969–15976. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Z.; Ji, G.; Liang, Z.; Xue, Y.; Guo, Y.; Tian, J.; Wang, X.; Cui, H. 2D/2D/2D Heterojunction of Ti3C2 MXene/MoS2 Nanosheets/TiO2 Nanosheets with Exposed (001) Facets toward Enhanced Photocatalytic Hydrogen Production Activity. Appl. Catal. B Environ. 2019, 246, 12–20. [Google Scholar] [CrossRef]
- Li, Y.; Ding, L.; Liang, Z.; Xue, Y.; Cui, H.; Tian, J. Synergetic Effect of Defects Rich MoS2 and Ti3C2 MXene as Cocatalysts for Enhanced Photocatalytic H2 Production Activity of TiO2. Chem. Eng. J. 2020, 383, 123178. [Google Scholar] [CrossRef]
- Li, Y.; Ding, L.; Yin, S.; Liang, Z.; Xue, Y.; Wang, X.; Cui, H.; Tian, J. Photocatalytic H2 Evolution on TiO2 Assembled with Ti3C2 MXene and Metallic 1T-WS2 as Co-Catalysts. Nano-Micro Lett. 2020, 12, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Nie, J.; Rao, F.; Liu, H.; Wang, Y.; Qu, D.; Wu, W.; Zhong, P.; Zhu, G. Ti3C2@TiO2/g-C3N4 Heterojunction Photocatalyst with Improved Charge Transfer for Enhancing Visible-Light NO Selective Removal. Ceram. Int. 2021, 47, 31302–31310. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, K.; Tan, Q.; Li, Q.; Fan, J.; Wu, C.; Lv, K. Facile Preparation of Highly Active CO2 Reduction (001)TiO2/Ti3C2Tx Photocatalyst from Ti3AlC2 with Less Fluorine. Catalysts 2022, 12, 785. https://doi.org/10.3390/catal12070785
Li J, Li K, Tan Q, Li Q, Fan J, Wu C, Lv K. Facile Preparation of Highly Active CO2 Reduction (001)TiO2/Ti3C2Tx Photocatalyst from Ti3AlC2 with Less Fluorine. Catalysts. 2022; 12(7):785. https://doi.org/10.3390/catal12070785
Chicago/Turabian StyleLi, Jibai, Kaining Li, Qiuyan Tan, Qin Li, Jiajie Fan, Chao Wu, and Kangle Lv. 2022. "Facile Preparation of Highly Active CO2 Reduction (001)TiO2/Ti3C2Tx Photocatalyst from Ti3AlC2 with Less Fluorine" Catalysts 12, no. 7: 785. https://doi.org/10.3390/catal12070785
APA StyleLi, J., Li, K., Tan, Q., Li, Q., Fan, J., Wu, C., & Lv, K. (2022). Facile Preparation of Highly Active CO2 Reduction (001)TiO2/Ti3C2Tx Photocatalyst from Ti3AlC2 with Less Fluorine. Catalysts, 12(7), 785. https://doi.org/10.3390/catal12070785