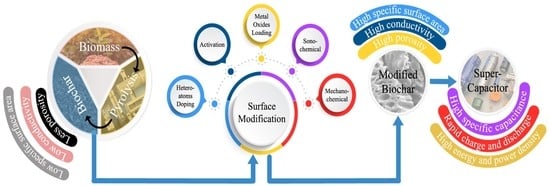
A Review on Production and Surface Modifications of Biochar Materials via Biomass Pyrolysis Process for Supercapacitor Applications
Abstract
:1. Introduction
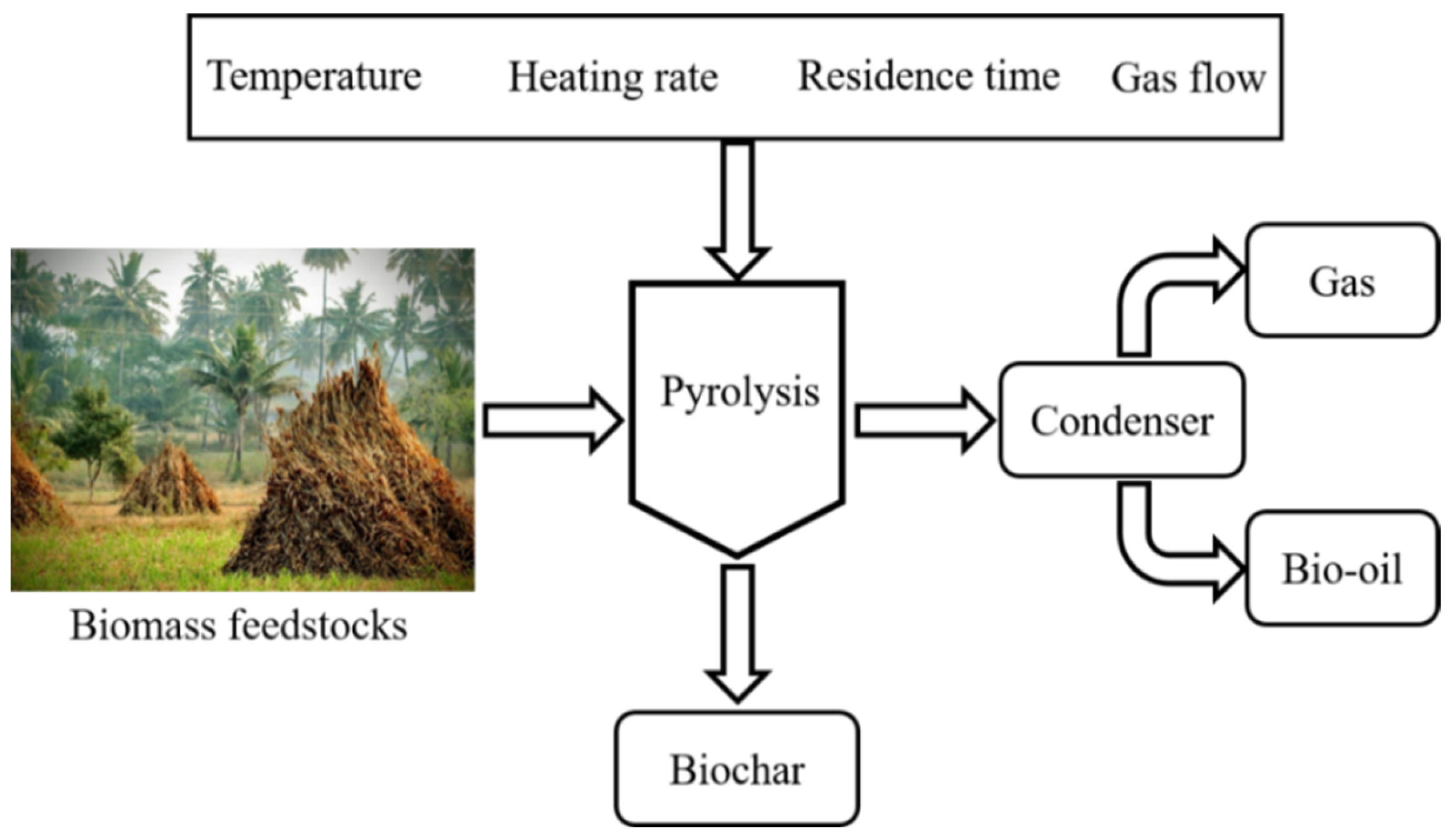
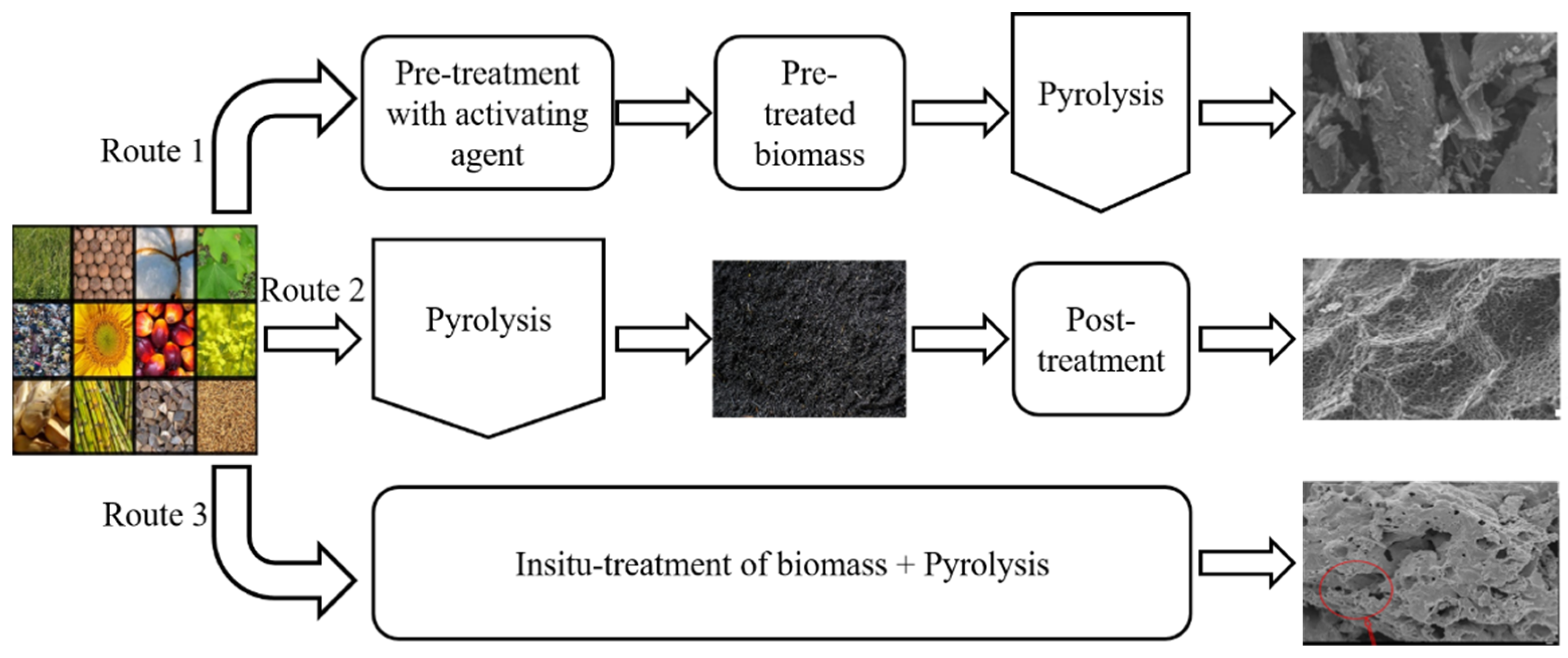
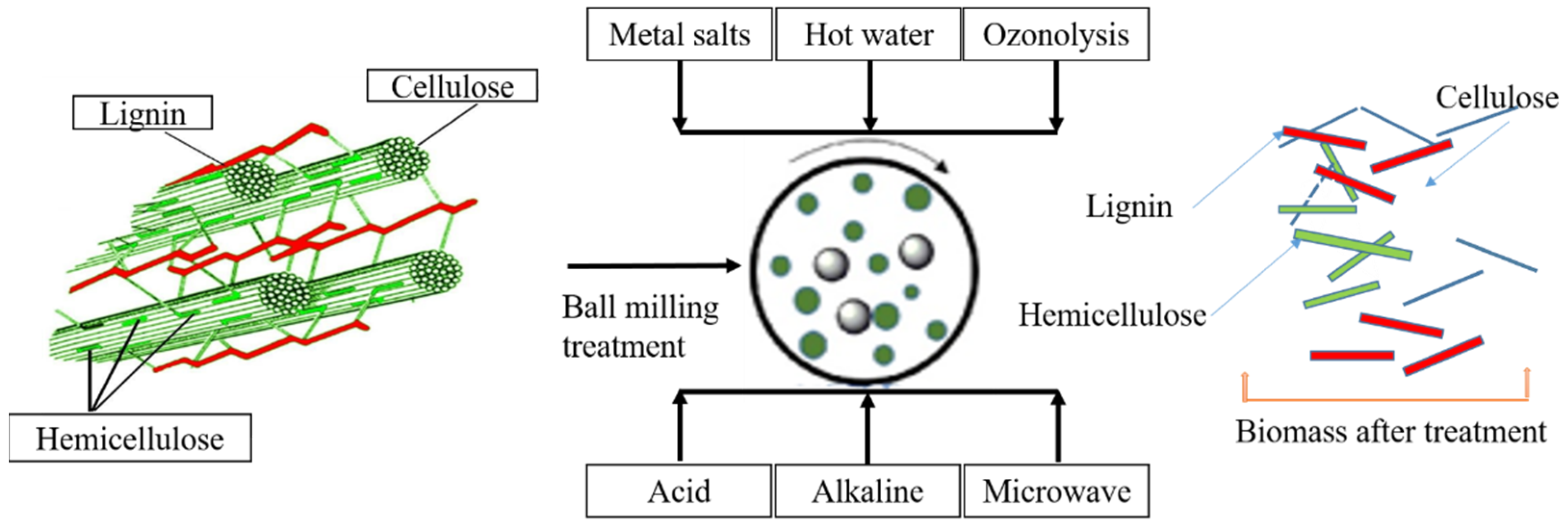
2. Overview of Biomass Pyrolysis for BC Production
3. Surface Modification of BC for Supercapacitor Applications
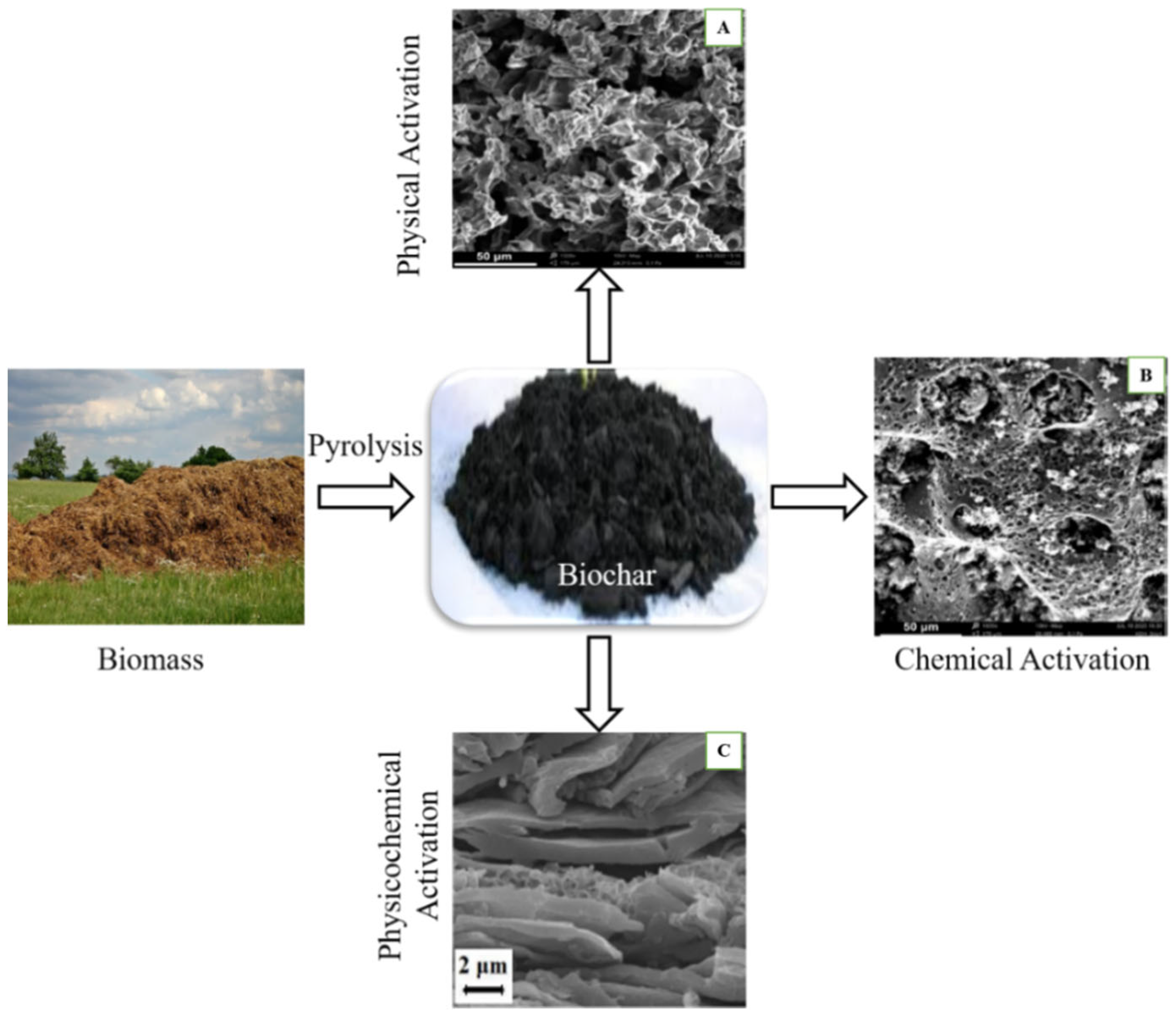
3.1. BC Activation Methods for Supercapacitor Applications
3.1.1. Physical Activation for BC Modification
3.1.2. Chemical Activation of BC
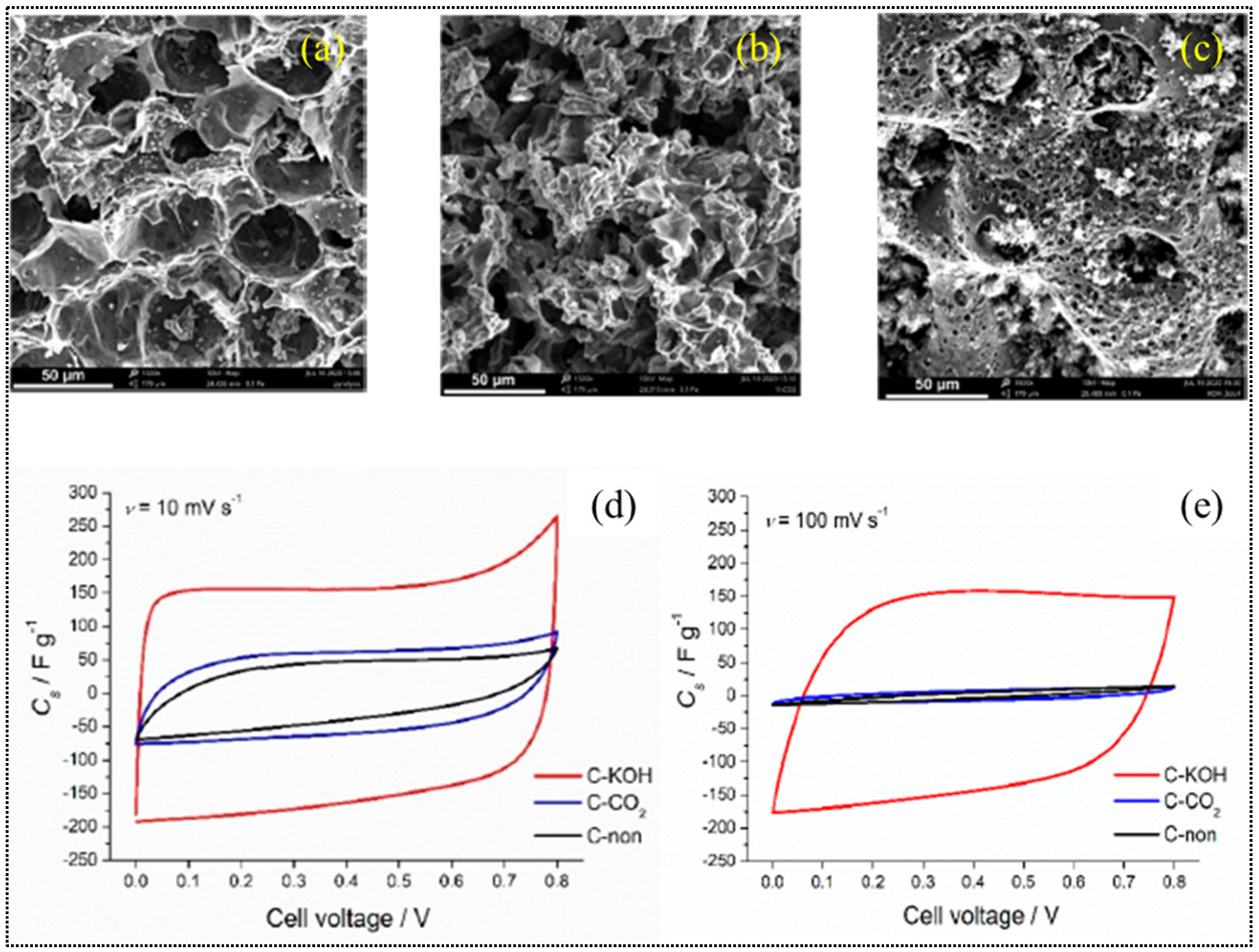
3.1.3. Physiochemical Activation of BC
| Activation Method | Advantages | Disadvantages |
|---|---|---|
| Physical activation | Commercial scale application, easy adoptability, biochar with enhanced porosity and physical strength | Lower yield of biochar, longer activation time, high activation temperature, high energy consumption |
| Chemical activation | Higher biochar yield, high surface area, developed and larger porosity and lower activation temperature | Expensive chemicals, post-washing of these chemicals, unwanted hazardous by-products |
| Physicochemical activation | It is utilized when it is difficult to remove the activating agent used in activation process through washing | Less studied method of activation, lower char yield, high temperature |
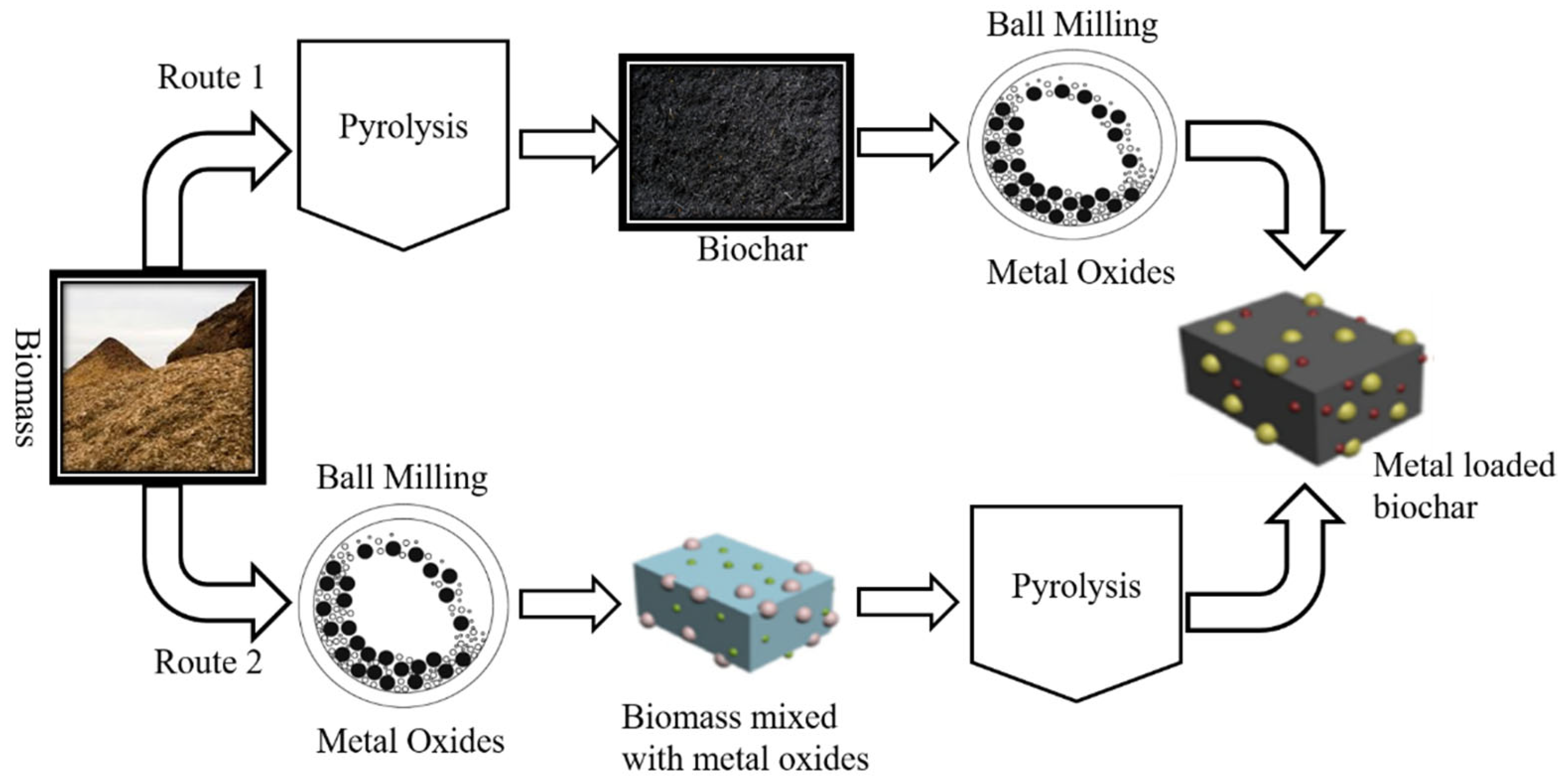
3.2. Metal Oxide Loaded Modified BC for Supercapacitors
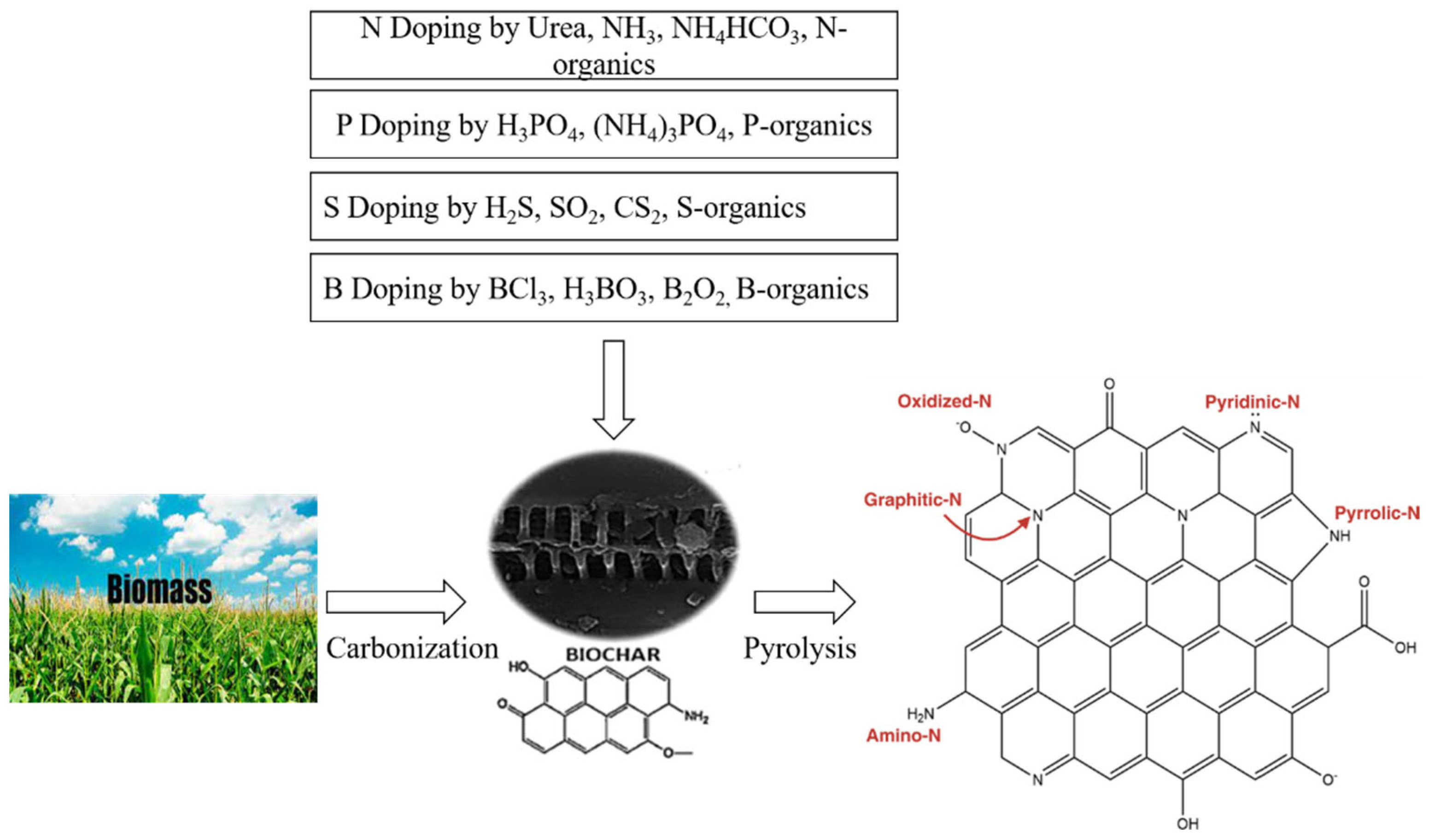
3.3. Heteroatoms Doped BC for Supercapacitors
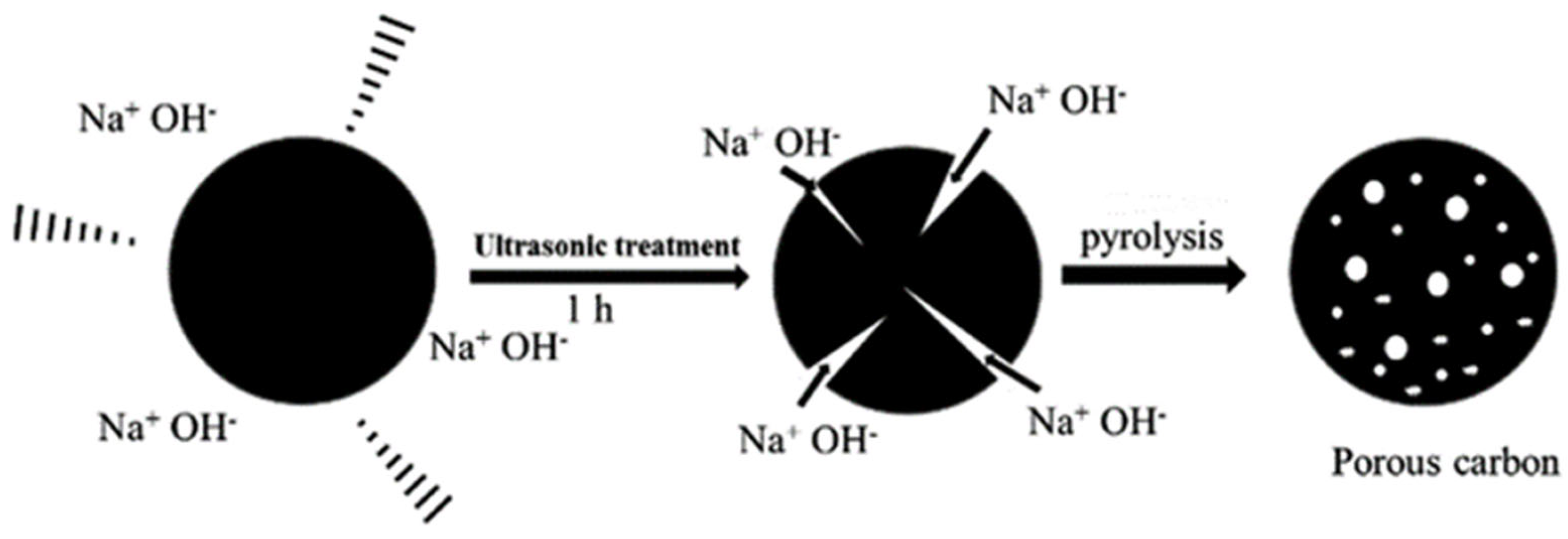
3.4. Modification of BC Using Sono-Chemical Method for Supercapacitors
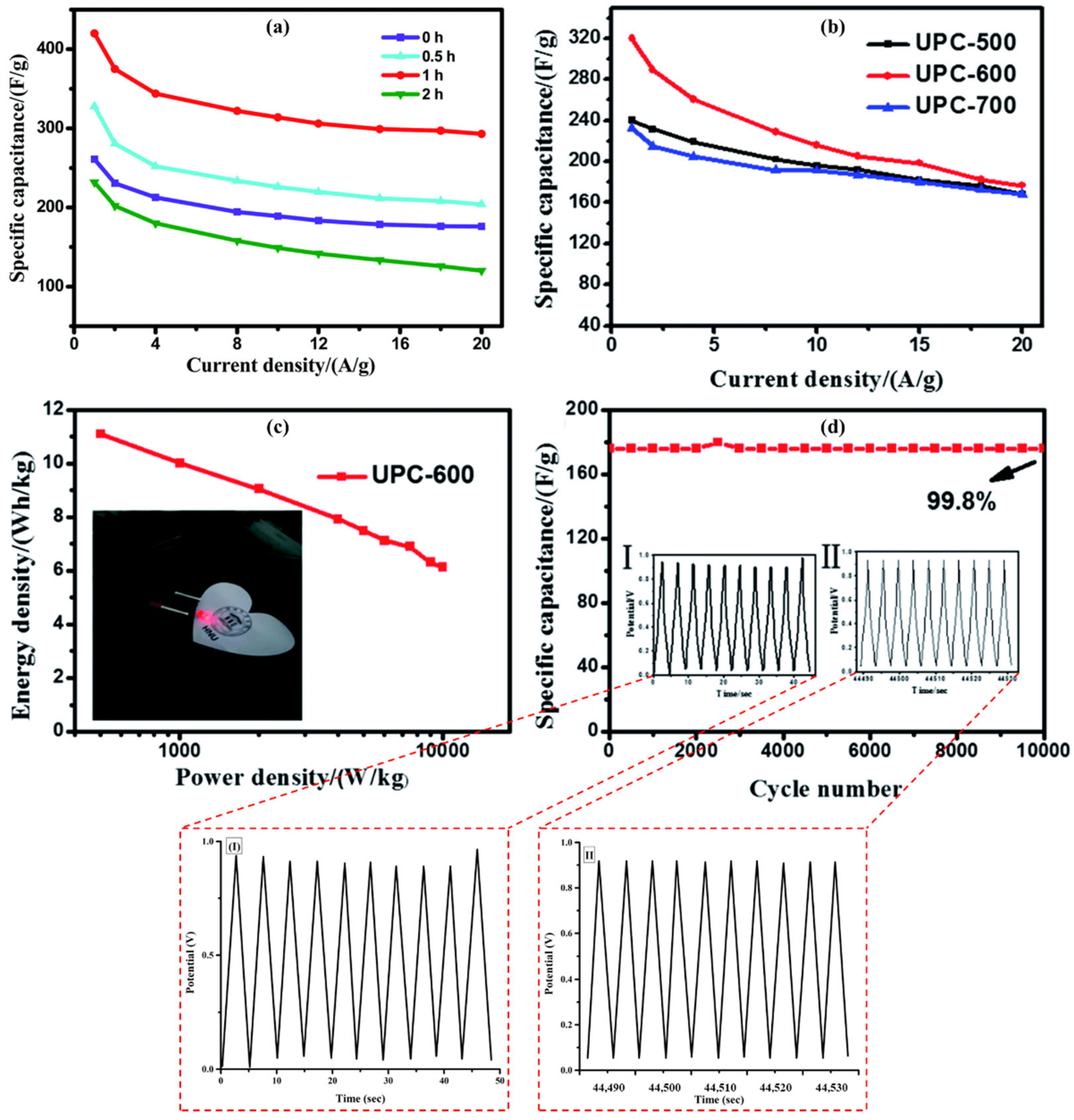
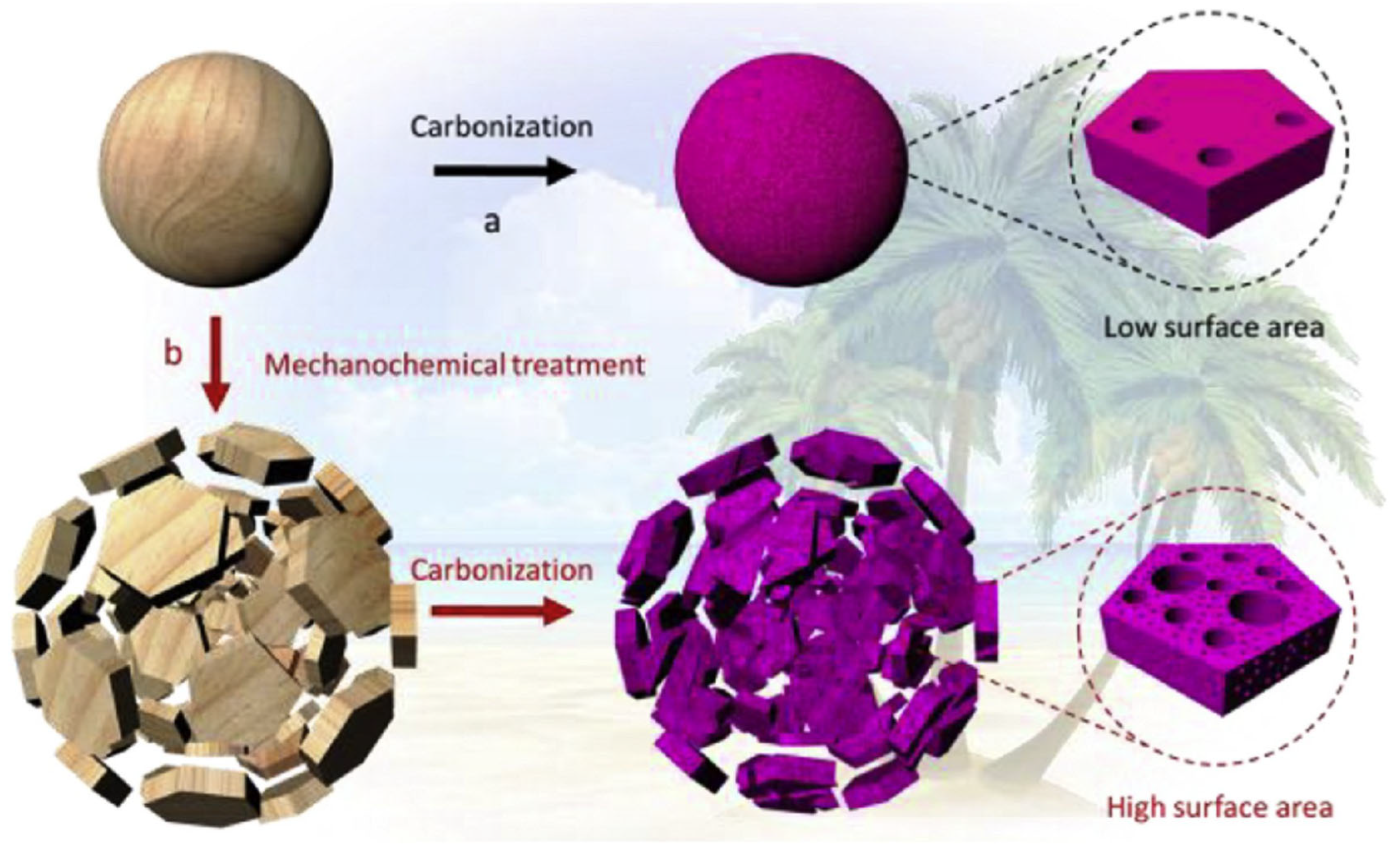
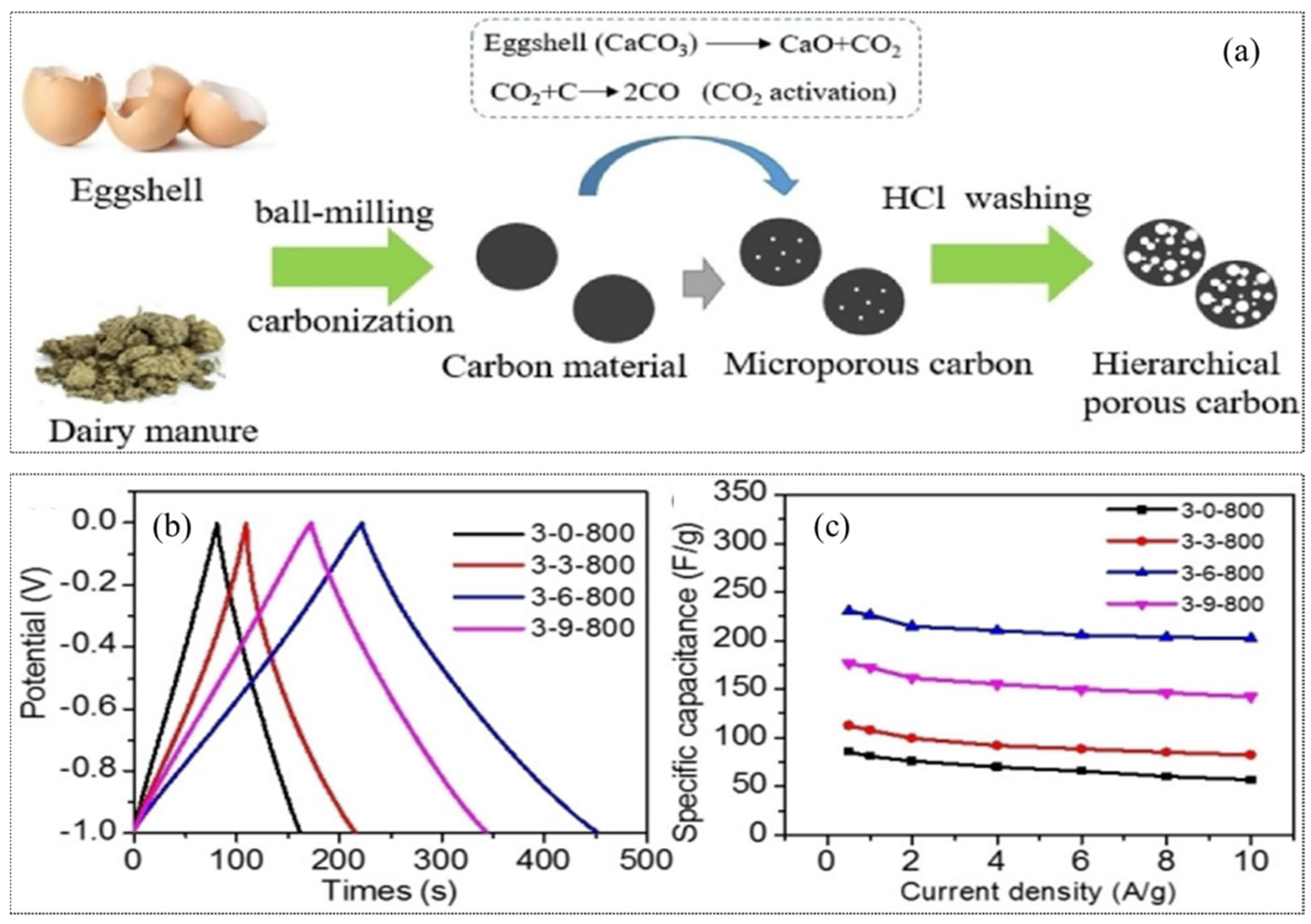
3.5. Modification of BC Using Mechano-Chemical Method for Supercapacitors
| Lignocellulosic Biomass | Modification Techniques | Specific Surface Area (m2/g) | Specific Capacitance (F/g) | Current Density (A/g) | Electrolyte | Energy Density (Wh/kg) | Power Density (W/kg) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Rice straw | Ultrasound assisted activation, which lowers activation temperature | 1820.2 | 420 | 1 | 11.1 | 500 | [219] | |
| Coconut shell | Ultrasound assisted with KOH activation | 2700 | 487 with sonication and 296 without sonication | [224] | ||||
| Garlic peel | Ultrasound assisted modification | 3887 | 426 | 1 | 6 M KOH | 59.57 | 190.06 | [248] |
| Lotus root | Carbonization at 700 °C for 4 h at 2 °C/min. ball milling coupled with K2CO3 as an activating agent in a mass ratio of 1:1 for 5 h at 500 rpm with ethanol as a reaction medium. Activation was carried out at 600 °C, 700 °C and 800 °C | Highest specific surface area of 1400 at 700 °C compared to 600 °C and 800 °C | 390 with mechano-chemical modification & 236 with chemical activation | 0.4 | 3 M KOH | 9 | 80.8 | [246] |
| Corn stover | Mechano-chemically BC activated at different temperatures | 2440.6 | 398 | 0.5 | 1 M H2SO4 | 5 | 100 | [247] |
4. Knowledge Gap and Future Perspective
- Further research is requisite to understand the reactions occurring during BC production and surface modification at a macroscopic and microscopic level to be lined with biochar performance in supercapacitor applications.
- Techno-economic analysis of BC-based material production via pyrolysis and surface modification techniques is also necessary for prospects in supercapacitor applications.
- An interconnected hierarchical pore-structured BC would be a good direction to achieve the required objective. BC with improved energy storage ability and electrochemical performance need future energy storage devices.
- Efficiency comparison of different modification methods is difficult; therefore, a comprehensive comparison needs to be made at individual optimum conditions on the same BC prepared by different methods for the same energy storage application.
- More emphasized work must be conducted on the solvent-free technique mechano-chemical modification method, and also for the ultrasound-assisted method to obtain the required surface structure of BC-based materials. A combination of the ultrasound and milling effect can be more efficient to produce modified BC-based materials for energy applications.
5. Conclusive Remarks
- Physical activation is advantageous due to commercial-scale applications and adaptability. The BC materials produced by this method show the promising properties of enhanced porosity and physical strength, which are critically required to improve the electrochemical performance of supercapacitors. On the other hand, low BC yield, longer time of activation, and higher activation temperature leading to high energy consumption are issues associated with this method.
- A higher carbon yield, larger porosity, and lower pyrolysis temperature are some of the advantages of chemical activation to achieve an improved performance of supercapacitors. However, expansive chemicals and post-washing of these chemicals and by-products are some of the challenges of this method.
- Metals oxide loading on the biochar surface increases the surface redox activity. It also improves the electrical conductivity and surface area of the BC, which enhances the performance of the supercapacitor. The uniform distribution of metals is one of the challenges to be considered in this method.
- Heteroatom doping plays its role in improving the surface area, electrical conductivity, and stability of the BC for enhanced supercapacitor applications.
- Sono-chemical surface modification can modify the surface in lesser time with a lower activation temperature and improved surface area and capacitance for supercapacitors.
- Mechano-chemical modification is a solvent-free technique for surface modification of BC to achieve a high surface area, enhanced specific capacitance, and high energy density for supercapacitors. However, high energy consumption is an issue associated with this method. This technique can be coupled with various other techniques to modify the surface features of the BC for supercapacitor applications.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hatfield-Dodds, S.; Schandl, H.; Newth, D.; Obersteiner, M.; Cai, Y.; Baynes, T.; West, J.; Havlik, P. Assessing global resource use and greenhouse emissions to 2050, with ambitious resource efficiency and climate mitigation policies. J. Clean. Prod. 2017, 144, 403–414. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, Y.M.; Kim, S.; Ryu, C.; Park, S.H.; Park, Y.K. Review of the use of activated biochar for energy and environmental applications. Carbon Lett. 2018, 26, 1–10. [Google Scholar]
- Merlet, C.; Rotenberg, B.; Madden, P.A.; Taberna, P.-L.; Simon, P.; Gogotsi, Y.; Salanne, M. On the molecular origin of supercapacitance in nanoporous carbon electrodes. Nat. Mater. 2012, 11, 306–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent advances in utilization of biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Melikoglu, M. Pumped hydroelectric energy storage: Analysing global development and assessing potential applications in Turkey based on Vision 2023 hydroelectricity wind and solar energy targets. Renew. Sustain. Energy Rev. 2017, 72, 146–153. [Google Scholar] [CrossRef]
- Chen, Z.; Mo, F.; Wang, T.; Yang, Q.; Huang, Z.; Wang, D.; Liang, G.; Chen, A.; Li, Q.; Guo, Y.; et al. Zinc/selenium conversion battery: A system highly compatible with both organic and aqueous electrolytes. Energy Environ. Sci. 2021, 14, 2441–2450. [Google Scholar] [CrossRef]
- Bi, Z.; Kong, Q.; Cao, Y.; Sun, G.; Su, F.; Wei, X.; Li, X.; Ahmad, A.; Xie, L.; Chen, C.-M. Biomass-derived porous carbon materials with different dimensions for supercapacitor electrodes: A review. J. Mater. Chem. A 2019, 7, 16028–16045. [Google Scholar] [CrossRef]
- Zhang, H.; He, X.; Wei, F.; Dong, S.; Xiao, N.; Qiu, J. Moss-covered rock-like hybrid porous carbons with enhanced electrochemical properties. ACS Sustain. Chem. Eng. 2020, 8, 3065–3071. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [Green Version]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 320–329. [Google Scholar]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Wu, K.C.W. Agricultural waste-derived biochar for environmental management. In Biochar in Agriculture for Achieving Sustainable Development Goals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 3–13. [Google Scholar]
- Sun, L.; Tian, C.; Li, M.; Meng, X.; Wang, L.; Wang, R.; Yin, J.; Fu, H. From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. [Google Scholar] [CrossRef]
- Saikia, B.K.; Benoy, S.M.; Bora, M.; Tamuly, J.; Pandey, M.; Bhattacharya, D. A brief review on supercapacitor energy storage devices and utilization of natural carbon resources as their electrode materials. Fuel 2020, 282, 118796. [Google Scholar] [CrossRef]
- Yakaboylu, G.A.; Jiang, C.; Yumak, T.; Zondlo, J.W.; Wang, J.; Sabolsky, E.M. Engineered hierarchical porous carbons for supercapacitor applications through chemical pretreatment and activation of biomass precursors. Renew. Energy 2020, 163, 276–287. [Google Scholar] [CrossRef]
- Ye, Y.-Y.; Qian, T.-T.; Jiang, H. Co loaded N-doped Biochar as a High Performance of Oxygen Reduction Reaction Electrocatalyst by Combined Pyrolysis of Biomass. Ind. Eng. Chem. Res. 2020, 59, 15614–15623. [Google Scholar] [CrossRef]
- Garg, R.; Elmas, S.; Nann, T.; Andersson, M.R. Deposition methods of graphene as electrode material for organic solar cells. Adv. Energy Mater. 2016, 7, 1601393. [Google Scholar] [CrossRef]
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Deng, J.; Xiong, T.; Wang, H.; Zheng, A.; Wang, Y. Effects of cellulose, hemicellulose, and lignin on the structure and morphology of porous carbons. ACS Sustain. Chem. Eng. 2016, 4, 3750–3756. [Google Scholar] [CrossRef]
- Chu, G.; Zhao, J.; Huang, Y.; Zhou, D.; Liu, Y.; Wu, M.; Peng, H.; Zhao, Q.; Pan, B.; Steinberg, C.E. Phosphoric acid pretreatment enhances the specific surface areas of biochars by generation of micropores. Environ. Pollut. 2018, 240, 1–9. [Google Scholar] [CrossRef]
- Sharma, R.; Wooten, J.; Baliga, V.; Hajaligol, M. Characterization of chars from biomass-derived materials: Pectin chars. Fuel 2001, 80, 1825–1836. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Yang, R.-X.; Dutta, S.; Ok, Y.S.; Wu, K.C.-W. Recent progress in the development of biomass-derived nitrogen-doped porous carbon. J. Mater. Chem. A 2020, 9, 3703–3728. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.-Y.; Egiebor, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2018, 35, 735–776. [Google Scholar] [CrossRef]
- Sajjadi, B.; Zubatiuk, T.; Leszczynska, D.; Leszczynski, J.; Chen, W.Y. Chemical activation of biochar for energy and environmental applications: A comprehensive review. Rev. Chem. Eng. 2018, 35, 777–815. [Google Scholar] [CrossRef]
- Huang, W.-H.; Lee, D.-J.; Huang, C. Modification on biochars for applications: A research update. Bioresour. Technol. 2020, 319, 124100. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Xiong, X.; Fu, J.; Yang, J.; Qiu, M.; Qi, X.; Tsang, D.C. Recent advances in mechanochemical production of chemicals and carbon materials from sustainable biomass resources. Renew. Sustain. Energy Rev. 2020, 130, 109944. [Google Scholar] [CrossRef]
- Gaudino, E.C.; Cravotto, G.; Manzoli, M.; Tabasso, S. Sono- and mechanochemical technologies in the catalytic conversion of biomass. Chem. Soc. Rev. 2020, 50, 1785–1812. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Xiu, S.; Shahbazi, A.; Li, R. Characterization, modification and application of biochar for energy storage and catalysis: A review. Trends Renew. Energy 2017, 3, 86–101. [Google Scholar] [CrossRef] [Green Version]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2020, 763, 144204. [Google Scholar] [CrossRef]
- Maggi, R.; Delmon, B. Comparison between ‘slow’and ‘flash’pyrolysis oils from biomass. Fuel 1994, 73, 671–677. [Google Scholar] [CrossRef]
- Román, S.; Valente Nabais, J.M.; Ledesma, B.; González, J.F.; Laginhas, C.; Titirici, M.M. Production of low-cost adsorbents with tunable surface chemistry by conjunction of hydrothermal carbonization and activation processes. Microporous Mesoporous Mater. 2013, 165, 127–133. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A. Influence of activation conditions on the physicochemical properties of activated biochar: A review. Biomass Convers. Biorefinery 2020, 12, 925–947. [Google Scholar] [CrossRef]
- Demirbaş, A. Mechanisms of liquefaction and pyrolysis reactions of biomass. Energy Convers. Manag. 2000, 41, 633–646. [Google Scholar] [CrossRef]
- Goldstein, I.S. Organic Chemicals from Biomass; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Khan, S.R.; Zeeshan, M.; Masood, A. Enhancement of hydrocarbons production through co-pyrolysis of acid-treated biomass and waste tire in a fixed bed reactor. Waste Manag. 2020, 106, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Zeeshan, M. Catalytic potential of low-cost natural zeolite and influence of various pretreatments of biomass on pyro-oil up-gradation during co-pyrolysis with scrap rubber tires. Energy 2021, 238, 121820. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, Y.; Wu, Y.; Xu, J.; Peng, H.; Liu, X.; Zhang, W.; Wang, S. In-depth comparison of the physicochemical characteristics of bio-char derived from biomass pseudo components: Hemicellulose, cellulose, and lignin. J. Anal. Appl. Pyrolysis 2019, 140, 195–204. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, I.K.; Tsang, D.C.; Fan, J.; Clark, J.H.; Luo, G.; Zhang, S.; Khan, E.; Graham, N.J. Tailored design of graphitic biochar for high-efficiency and chemical-free microwave-assisted removal of refractory organic contaminants. Chem. Eng. J. 2020, 398, 125505. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Kang, M.; Zhang, Z.; Jin, J.; Wang, Z.; Pan, Z.; Xu, D.; Wu, F.; Xing, B. Impact of deashing treatment on biochar structural properties and potential sorption mechanisms of phenanthrene. Environ. Sci. Technol. 2013, 47, 11473–11481. [Google Scholar] [CrossRef]
- Antal, M.J.; Grønli, M. The art, science, and technology of charcoal production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Kumar, J.V.; Pratt, B.C. Compositional analysis of some renewable biofuels. Am. Lab. 1996, 28, 15–20. [Google Scholar]
- Irfan, M.; Chen, Q.; Yue, Y.; Pang, R.; Lin, Q.; Zhao, X.; Chen, H. Co-production of biochar, bio-oil and syngas from halophyte grass (Achnatherum splendens L.) under three different pyrolysis temperatures. Bioresour. Technol. 2016, 211, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Pallarés, J.; González-Cencerrado, A.; Arauzo, I. Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenergy 2018, 115, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: Abingdon, UK, 2015. [Google Scholar]
- Leng, L.; Huang, H. An overview of the effect of pyrolysis process parameters on biochar stability. Bioresour. Technol. 2018, 270, 627–642. [Google Scholar] [CrossRef]
- Zhao, B.; O’Connor, D.; Zhang, J.; Peng, T.; Shen, Z.; Tsang, D.C.W.; Hou, D. Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J. Clean. Prod. 2018, 174, 977–987. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Cen, K.; Luo, M.; Li, H.; Lu, B. Pyrolysis polygeneration of poplar wood: Effect of heating rate and pyrolysis temperature. Bioresour. Technol. 2016, 218, 780–788. [Google Scholar] [CrossRef]
- Bouchelta, C.; Medjram, M.S.; Zoubida, M.; Chekkat, F.A.; Ramdane, N.; Bellat, J.-P. Effects of pyrolysis conditions on the porous structure development of date pits activated carbon. J. Anal. Appl. Pyrolysis 2012, 94, 215–222. [Google Scholar] [CrossRef]
- Liu, R.; Liu, G.; Yousaf, B.; Abbas, Q. Operating conditions-induced changes in product yield and characteristics during thermal-conversion of peanut shell to biochar in relation to economic analysis. J. Clean. Prod. 2018, 193, 479–490. [Google Scholar] [CrossRef]
- Sakhiya, A.K.; Anand, A.; Kaushal, P. Production, activation, and applications of biochar in recent times. Biochar 2020, 2, 253–285. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Vijay, V.K.; Chandra, R.; Kumar, H. Production and characterization of biochar produced from slow pyrolysis of pigeon pea stalk and bamboo. Clean. Eng. Technol. 2021, 3, 100101. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Malekshahian, M.; Hill, J.M. Effect of pyrolysis and CO2 gasification pressure on the surface area and pore size distribution of petroleum coke. Energy Fuels 2011, 25, 5250–5256. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Salema, A.A.; Afzal, M.T. Numerical simulation of heating behaviour in biomass bed and pellets under multimode microwave system. Int. J. Therm. Sci. 2015, 91, 12–24. [Google Scholar] [CrossRef]
- Namazi, A.B.; Allen, D.; Jia, C.Q. Probing microwave heating of lignocellulosic biomasses. J. Anal. Appl. Pyrolysis 2015, 112, 121–128. [Google Scholar] [CrossRef]
- Zhang, T.; Walawender, W.P.; Fan, L.; Fan, M.; Daugaard, D.; Brown, R. Preparation of activated carbon from forest and agricultural residues through CO2 activation. Chem. Eng. J. 2004, 105, 53–59. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, X.S. Biomass-derived carbon electrode materials for supercapacitors. Sustain. Energy Fuels 2017, 1, 1265–1281. [Google Scholar] [CrossRef]
- Cheng, B.-H.; Zeng, R.J.; Jiang, H. Recent developments of post-modification of biochar for electrochemical energy storage. Bioresour. Technol. 2017, 246, 224–233. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, Y.; Song, N.; Li, X. Biomass-derived renewable carbon materials for electrochemical energy storage. Mater. Res. Lett. 2016, 5, 69–88. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, S.A.; Ansari, M.O.; Cho, M.H. Manganese dioxide nanorods intercalated reduced graphene oxide nanocomposite toward high performance electrochemical supercapacitive electrode materials. J. Colloid Interface Sci. 2017, 506, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ma, F.; Wu, G.; Ma, D.; Geng, W.; Wan, J. Facile self-templating large scale preparation of biomass-derived 3D hierarchical porous carbon for advanced supercapacitors. J. Mater. Chem. A 2015, 3, 18154–18162. [Google Scholar] [CrossRef]
- Sun, Q. Porous carbon material based on biomass prepared by MgO template method and ZnCl2 activation method as Electrode for high performance supercapacitor. Int. J. Electrochem. Sci. 2019, 14, 1–14. [Google Scholar] [CrossRef]
- Qin, L. Porous carbon derived from pine nut shell prepared by steam activation for supercapacitor electrode material. Int. J. Electrochem. Sci. 2019, 8907–8918. [Google Scholar] [CrossRef]
- Zhang, Z.; He, J.; Tang, X.; Wang, Y.; Yang, B.; Wang, K.; Zhang, D. Supercapacitors based on a nitrogen doped hierarchical porous carbon fabricated by self-activation of biomass: Excellent rate capability and cycle stability. Carbon Lett. 2019, 29, 585–594. [Google Scholar] [CrossRef]
- Sundriyal, S.; Shrivastav, V.; Pham, H.D.; Mishra, S.; Deep, A.; Dubal, D.P. Advances in bio-waste derived activated carbon for supercapacitors: Trends, challenges and prospective. Resour. Conserv. Recycl. 2021, 169, 105548. [Google Scholar] [CrossRef]
- Osman, N.; Shamsuddin, N.; Uemura, Y. Activated carbon of oil palm empty fruit bunch (EFB); core and shaggy. Procedia Eng. 2016, 148, 758–764. [Google Scholar] [CrossRef] [Green Version]
- Taer, E.; Iwantono; Manik, S.T.; Taslim, R.; Dahlan, D.; Deraman, M. Preparation of activated carbon monolith electrodes from sugarcane bagasse by physical and physical-chemical activation process for supercapacitor application. Adv. Mater. Res. 2014, 896, 179–182. [Google Scholar] [CrossRef]
- Xiao, H.; Peng, H.; Deng, S.; Yang, X.; Zhang, Y.; Li, Y. Preparation of activated carbon from edible fungi residue by microwave assisted K2CO3 activation—application in reactive black 5 adsorption from aqueous solution. Bioresour. Technol. 2012, 111, 127–133. [Google Scholar] [CrossRef]
- Januszewicz, K.; Cymann-Sachajdak, A.; Kazimierski, P.; Klein, M.; Łuczak, J.; Wilamowska-Zawłocka, M. Chestnut-derived activated carbon as a prospective material for energy storage. Materials 2020, 13, 4658. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, D.; Liu, Y.; Wang, H.; Wang, L. Recent advances and challenges in biomass-derived porous carbon nanomaterials for supercapacitors. Chem. Eng. J. 2020, 397, 125418. [Google Scholar] [CrossRef]
- Nabais, J.M.V.; Nunes, P.; Carrott, P.J.; Carrott MM, L.R.; García, A.M.; Díaz-Díez, M.A. Production of activated carbons from coffee endocarp by CO2 and steam activation. Fuel Processing Technol. 2008, 89, 262–268. [Google Scholar] [CrossRef]
- Aworn, A.; Thiravetyan, P.; Nakbanpote, W. Preparation and characteristics of agricultural waste activated carbon by physical activation having micro- and mesopores. J. Anal. Appl. Pyrolysis 2008, 82, 279–285. [Google Scholar] [CrossRef]
- Bouchelta, C.; Medjram, M.S.; Bertrand, O.; Bellat, J.P. Preparation and characterization of activated carbon from date stones by physical activation with steam. J. Anal. Appl. Pyrolysis 2008, 82, 70–77. [Google Scholar] [CrossRef]
- Cabal, B.; Budinova, T.; Ania, C.O.; Tsyntsarski, B.; Parra, J.B.; Petrova, B. Adsorption of naphthalene from aqueous solution on activated carbons obtained from bean pods. J. Hazard. Mater. 2009, 161, 1150–1156. [Google Scholar] [CrossRef] [Green Version]
- Cagnon, B.; Py, X.; Guillot, A.; Stoeckli, F.; Chambat, G. Contributions of hemicellulose, cellulose and lignin to the mass and the porous properties of chars and steam activated carbons from various lignocellulosic precursors. Bioresour. Technol. 2009, 100, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Nabais, J.V.; Teixeira, J.G.; Almeida, I. Development of easy made low cost bindless monolithic electrodes from biomass with controlled properties to be used as electrochemical capacitors. Bioresour. Technol. 2011, 102, 2781–2787. [Google Scholar] [CrossRef] [Green Version]
- Nabais, J.V.; Carrott, P.; Carrott, M.R.; Luz, V.; Ortiz, A.L. Influence of preparation conditions in the textural and chemical properties of activated carbons from a novel biomass precursor: The coffee endocarp. Bioresour. Technol. 2008, 99, 7224–7231. [Google Scholar] [CrossRef]
- Haffner-Staton, E.; Balahmar, N.; Mokaya, R. High yield and high packing density porous carbon for unprecedented CO2 capture from the first attempt at activation of air-carbonized biomass. J. Mater. Chem. A 2016, 4, 13324–13335. [Google Scholar] [CrossRef]
- Niu, J.; Liu, M.; Xu, F.; Zhang, Z.; Dou, M.; Wang, F. Synchronously boosting gravimetric and volumetric performance: Biomass-derived ternary-doped microporous carbon nanosheet electrodes for supercapacitors. Carbon 2018, 140, 664–672. [Google Scholar] [CrossRef]
- Shang, T.-X.; Ren, R.-Q.; Zhu, Y.-M.; Jin, X.-J. Oxygen- and nitrogen-co-doped activated carbon from waste particleboard for potential application in high-performance capacitance. Electrochim. Acta 2015, 163, 32–40. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J.; Hou, D.; Yin, J.; Liu, D.; He, Y.; Lin, H. Hierarchical porous carbon prepared from biomass through a facile method for supercapacitor applications. J. Colloid Interface Sci. 2018, 530, 338–344. [Google Scholar] [CrossRef]
- Abechi, S.E.; Gimba, C.E.; Uzairu, A.; Dallatu, Y.A. Preparation and characterization of activated carbon from palm kernel shell by chemical activation. Res. J. Chem. Sci. 2013, 3, 54–61. [Google Scholar]
- González-García, P.; Centeno, T.A.; Urones-Garrote, E.; Ávila-Brande, D.; Otero-Díaz, L.C. Microstructure and surface properties of lignocellulosic-based activated carbons. Appl. Surf. Sci. 2013, 265, 731–737. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Zapata-Benabithe, Z.; Carrasco-Marín, F.; Moreno-Castilla, C. Activated carbons from KOH-activation of argan (Argania spinosa) seed shells as supercapacitor electrodes. Bioresour. Technol. 2012, 111, 185–190. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, Q.; Qu, N.; Yang, H.; Wang, M.; Liu, A.; Yang, J. Ultrathin 2D nitrogen-doped carbon nanosheets for high performance supercapacitors: Insight into the effects of graphene oxides. Nanoscale 2019, 11, 8588–8596. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, W.; Wu, R.; Sun, L.; Zhang, Y.; Cui, Y.; Liu, S.; Wang, H.; Shan, B. Marine-biomass-derived porous carbon sheets with a tunable n-doping content for superior sodium-ion storage. ACS Appl. Mater. Interfaces 2018, 10, 38376–38386. [Google Scholar] [CrossRef]
- Ramakrishnan, K.; Nithya, C.; Karvembu, R. High-performance sodium ion capacitor based on MoO2@rGO nanocomposite and goat hair derived carbon electrodes. ACS Appl. Energy Mater. 2018, 1, 841–850. [Google Scholar] [CrossRef]
- Qiao, Y.; Ma, M.; Liu, Y.; Li, S.; Lu, Z.; Yue, H.; Dong, H.; Cao, Z.; Yin, Y.; Yang, S. First-principles and experimental study of nitrogen/sulfur co-doped carbon nanosheets as anodes for rechargeable sodium ion batteries. J. Mater. Chem. A 2016, 4, 15565–15574. [Google Scholar] [CrossRef]
- Yang, K.; Peng, J.; Srinivasakannan, C.; Zhang, L.; Xia, H.; Duan, X. Preparation of high surface area activated carbon from coconut shells using microwave heating. Bioresour. Technol. 2010, 101, 6163–6169. [Google Scholar] [CrossRef] [PubMed]
- Tay, T.; Ucar, S.; Karagöz, S. Preparation and characterization of activated carbon from waste biomass. J. Hazard. Mater. 2009, 165, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, X.; Gu, Z.; Polin, J. Carbon materials from high ash biochar for supercapacitor and improvement of capacitance with HNO3 surface oxidation. J. Power Sources 2013, 236, 285–292. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, B.; Lin, X.; Xie, Z. Correction: Biomass-derived hierarchical porous carbons: Boosting the energy density of supercapacitors via an ionothermal approach. J. Mater. Chem. A 2017, 5, 25090. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Yu, D.; Zhao, G.; Du, B.; Tang, W.; Sun, L.; Sun, Y.; Besenbacher, F.; Yu, M. Three-dimensional scaffolding framework of porous carbon nanosheets derived from plant wastes for high-performance supercapacitors. Nano Energy 2016, 27, 377–389. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, W.; Li, T.; Zhi, L.; Dang, L.; Liu, Z.; Lei, Z. Capacitive performance of porous carbon nanosheets derived from biomass cornstalk. RSC Adv. 2017, 7, 1067–1074. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Zhang, G.; Li, M.-Y.; Yin, Y.-X.; Li, J.-Y.; Li, G.; Wang, W.-P.; Peng, W.; Cao, F.-F.; Guo, Y.-G. Porous carbon for high-energy density symmetrical supercapacitor and lithium-ion hybrid electrochemical capacitors. Chem. Eng. J. 2019, 375, 122020. [Google Scholar] [CrossRef]
- Raj, C.J.; Rajesh, M.; Manikandan, R.; Yu, K.H.; Anusha, J.; Ahn, J.H.; Kim, D.-W.; Park, S.Y.; Kim, B.C. High electrochemical capacitor performance of oxygen and nitrogen enriched activated carbon derived from the pyrolysis and activation of squid gladius chitin. J. Power Sources 2018, 386, 66–76. [Google Scholar] [CrossRef]
- Yu, D.; Ma, Y.; Chen, M.; Dong, X. KOH activation of wax gourd-derived carbon materials with high porosity and heteroatom content for aqueous or all-solid-state supercapacitors. J. Colloid Interface Sci. 2018, 537, 569–578. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Mei, C.; Xu, J.; Zhou, S.; Wong, C.-P. Evaluating biomass-derived hierarchically porous carbon as the positive electrode material for hybrid Na-ion capacitors. J. Power Sources 2017, 342, 48–55. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.-J. MnO2 and biomass-derived 3D porous carbon composites electrodes for high performance supercapacitor applications. J. Alloys Compd. 2018, 741, 360–367. [Google Scholar] [CrossRef]
- Takeuchi, K.; Fujishige, M.; Ishida, N.; Kunieda, Y.; Kato, Y.; Tanaka, Y.; Ochi, T.; Shirotori, H.; Uzuhashi, Y.; Ito, S.; et al. High porous bio-nanocarbons prepared by carbonization and NaOH activation of polysaccharides for electrode material of EDLC. J. Phys. Chem. Solids 2018, 118, 137–143. [Google Scholar] [CrossRef]
- Zhou, L.; Cao, H.; Zhu, S.; Hou, L.; Yuan, C. Hierarchical micro-/mesoporous N-and O-enriched carbon derived from disposable cashmere: A competitive cost-effective material for high-performance electrochemical capacitors. Green Chem. 2015, 17, 2373–2382. [Google Scholar] [CrossRef]
- Cruz, G.; Pirilä, M.; Huuhtanen, M.; Carrión, L.; Alvarenga, E.; Keiski, R.L. Production of Activated Carbon from Cocoa (Theobroma cacao) Pod Husk. J. Civ. Environ. Eng. 2012, 2, 1–6. [Google Scholar] [CrossRef]
- Selvaraj, A.R.; Chinnadurai, D.; Cho, I.; Bak, J.S.; Prabakar, K. Bio-waste wood-derived porous activated carbon with tuned microporosity for high performance supercapacitors. J. Energy Storage 2022, 52, 104928. [Google Scholar] [CrossRef]
- Kumagai, S.; Tashima, D. Electrochemical performance of activated carbons prepared from rice husk in different types of non-aqueous electrolytes. Biomass Bioenergy 2015, 83, 216–223. [Google Scholar] [CrossRef]
- Chen, L.; Ji, T.; Mu, L.; Zhu, J. Cotton fabric derived hierarchically porous carbon and nitrogen doping for sustainable capacitor electrode. Carbon 2017, 111, 839–848. [Google Scholar] [CrossRef]
- Libich, J.; Máca, J.; Vondrák, J.; Čech, O.; Sedlaříková, M. Supercapacitors: Properties and applications. J. Energy Storage 2018, 17, 224–227. [Google Scholar] [CrossRef]
- Du, J.; Liu, L.; Hu, Z.; Yu, Y.; Zhang, Y.; Hou, S.; Chen, A. Raw-cotton-derived n-doped carbon fiber aerogel as an efficient electrode for electrochemical capacitors. ACS Sustain. Chem. Eng. 2018, 6, 4008–4015. [Google Scholar] [CrossRef]
- Wei, X.; Wan, S.; Gao, S. Self-assembly-template engineering nitrogen-doped carbon aerogels for high-rate supercapacitors. Nano Energy 2016, 28, 206–215. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Li, Z.; Cui, K.; Karpuzov, D.; Tan, X.; Kohandehghan, A.; Mitlin, D. Peanut shell hybrid sodium ion capacitor with extreme energy–power rivals lithium ion capacitors. Energy Environ. Sci. 2014, 8, 941–955. [Google Scholar] [CrossRef]
- Im, U.-S.; Kim, J.; Lee, S.H.; Lee, S.M.; Lee, B.-R.; Peck, D.-H.; Jung, D.-H. Preparation of activated carbon from needle coke via two-stage steam activation process. Mater. Lett. 2018, 237, 22–25. [Google Scholar] [CrossRef]
- Ismanto, A.E.; Wang, S.; Soetaredjo, F.E.; Ismadji, S. Preparation of capacitor’s electrode from cassava peel waste. Bioresour. Technol. 2010, 101, 3534–3540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, P.; Reed, A. Development of activated carbon pore structure via physical and chemical activation of biomass fibre waste. Biomass Bioenergy 2006, 30, 144–152. [Google Scholar] [CrossRef]
- Abioye, A.M.; Ani, F.N. Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: A review. Renew. Sustain. Energy Rev. 2015, 52, 1282–1293. [Google Scholar] [CrossRef]
- Cuong, D.V.; Liu, N.-L.; Nguyen, V.A.; Hou, C.-H. Meso/micropore-controlled hierarchical porous carbon derived from activated biochar as a high-performance adsorbent for copper removal. Sci. Total Environ. 2019, 692, 844–853. [Google Scholar] [CrossRef]
- Jiang, C.; Yakaboylu, G.A.; Yumak, T.; Zondlo, J.W.; Sabolsky, E.M.; Wang, J. Activated carbons prepared by indirect and direct CO2 activation of lignocellulosic biomass for supercapacitor electrodes. Renew. Energy 2020, 155, 38–52. [Google Scholar] [CrossRef]
- Olorundare, O.F.; Msagati, T.; Krause, R.W.M.; Okonkwo, J.O.; Mamba, B.B. Activated Carbon from lignocellulosic waste residues: Effect of activating agent on porosity characteristics and use as adsorbents for organic species. Water Air Soil Pollut. 2014, 225, 1876. [Google Scholar] [CrossRef]
- Girgis, B.S.; Soliman, A.M.; Fathy, N.A. Development of micro-mesoporous carbons from several seed hulls under varying conditions of activation. Microporous Mesoporous Mater. 2011, 142, 518–525. [Google Scholar] [CrossRef]
- Qian, L.; Guo, F.; Jia, X.; Zhan, Y.; Zhou, H.; Jiang, X.; Tao, C. Recent development in the synthesis of agricultural and forestry biomass-derived porous carbons for supercapacitor applications: A review. Ionics 2020, 26, 3705–3723. [Google Scholar] [CrossRef]
- Fu, K.; Yue, Q.; Gao, B.; Sun, Y.; Zhu, L. Preparation, characterization and application of lignin-based activated carbon from black liquor lignin by steam activation. Chem. Eng. J. 2013, 228, 1074–1082. [Google Scholar] [CrossRef]
- Suhas; Carrott, P.; Carrott, M.R.; Singh, R.; Singh, L.; Chaudhary, M. An innovative approach to develop microporous activated carbons in oxidising atmosphere. J. Clean. Prod. 2017, 156, 549–555. [Google Scholar] [CrossRef]
- Vinayagam, M.; Babu, R.S.; Sivasamy, A.; de Barros, A.L.F. Biomass-derived porous activated carbon from Syzygium cumini fruit shells and Chrysopogon zizanioides roots for high-energy density symmetric supercapacitors. Biomass Bioenergy 2020, 143, 105838. [Google Scholar] [CrossRef]
- Lim, W.; Srinivasakannan, C.; Balasubramanian, N. Activation of palm shells by phosphoric acid impregnation for high yielding activated carbon. J. Anal. Appl. Pyrolysis 2010, 88, 181–186. [Google Scholar] [CrossRef]
- Gunasekaran, S.S.; Badhulika, S. High-performance solid-state supercapacitor based on sustainable synthesis of meso-macro porous carbon derived from hemp fibres via CO2 activation. J. Energy Storage 2021, 41, 102997. [Google Scholar] [CrossRef]
- Ahmida, K.; Darmoon, M.; Al-Tohami, F.; Erhayem, M.; Zidan, M. Effect of Physical and Chemical Preparation on Characteristics of Activated Carbon from Agriculture Solid Waste and Their Potential Application. In Proceedings of the International Conference on Chemical, Civil and Environmental Engineering, Istanbul, Turkey, 5–6 June 2015. [Google Scholar]
- Sarwar, A.; Ali, M.; Khoja, A.H.; Nawar, A.; Waqas, A.; Liaquat, R.; Naqvi, S.R.; Asjid, M. Synthesis and characterization of biomass-derived surface-modified activated carbon for enhanced CO2 adsorption. J. CO2 Util. 2021, 46, 101476. [Google Scholar] [CrossRef]
- Hesas, R.H.; Arami-Niya, A.; Daud, W.M.A.W.; Sahu, J.N. Preparation of granular activated carbon from oil palm shell by microwave-induced chemical activation: Optimisation using surface response methodology. Chem. Eng. Res. Des. 2013, 91, 2447–2456. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B.; Mokaya, R. High density hydrogen storage in superactivated carbons from hydrothermally carbonized renewable organic materials. Energy Environ. Sci. 2011, 4, 1400–1410. [Google Scholar] [CrossRef] [Green Version]
- Dehkhoda, A.M.; Gyenge, E.; Ellis, N. A novel method to tailor the porous structure of KOH-activated biochar and its application in capacitive deionization and energy storage. Biomass Bioenergy 2016, 87, 107–121. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-L.; Li, S.-Y.; Tang, Z.-S.; Song, Z.-X.; Sun, J. Xanthoceras sorbifolia seed coats derived porous carbon with unique architecture for high rate performance supercapacitors. Diam. Relat. Mater. 2018, 91, 119–126. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, Y.; Chen, Y.; Guo, H. Activated biomass carbon made from bamboo as electrode material for supercapacitors. Mater. Res. Bull. 2018, 102, 391–398. [Google Scholar] [CrossRef]
- Li, Y.; Yu, N.; Yan, P.; Li, Y.; Zhou, X.; Chen, S.; Wang, G.; Wei, T.; Fan, Z. Fabrication of manganese dioxide nanoplates anchoring on biomass-derived cross-linked carbon nanosheets for high-performance asymmetric supercapacitors. J. Power Sources 2015, 300, 309–317. [Google Scholar] [CrossRef]
- Sudhan, N.; Subramani, K.; Karnan, M.; Ilayaraja, N.; Sathish, M. Biomass-derived activated porous carbon from rice straw for a high-energy symmetric supercapacitor in aqueous and non-aqueous electrolytes. Energy Fuels 2016, 31, 977–985. [Google Scholar] [CrossRef]
- Deng, J.; Xiong, T.; Xu, F.; Li, M.; Han, C.; Gong, Y.; Wang, H.; Wang, Y. Inspired by bread leavening: One-pot synthesis of hierarchically porous carbon for supercapacitors. Green Chem. 2015, 17, 4053–4060. [Google Scholar] [CrossRef]
- Sevilla, M.; Mokaya, R. Energy storage applications of activated carbons: Supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250–1280. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Wang, C.-Y.; Chen, M.-M.; Wang, J.; Shi, Z.-Q. Potato starch-based activated carbon spheres as electrode material for electrochemical capacitor. J. Phys. Chem. Solids 2009, 70, 1256–1260. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Lee, B.-J.; Suezaki, H.; Chino, T.; Abe, Y.; Yanagiura, T.; Park, K.C.; Endo, M. Preparation and characterization of bamboo-based activated carbons as electrode materials for electric double layer capacitors. Carbon 2006, 44, 1592–1595. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Geng, Z.; Li, B.; Zhang, C. High performance electrode materials for electric double-layer capacitors based on biomass-derived activated carbons. Electrochim. Acta 2015, 173, 377–384. [Google Scholar] [CrossRef]
- Arami-Niya, A.; Daud, W.M.A.W.; Mjalli, F.S.; Abnisa, F.; Shafeeyan, M.S. Production of microporous palm shell based activated carbon for methane adsorption: Modeling and optimization using response surface methodology. Chem. Eng. Res. Des. 2012, 90, 776–784. [Google Scholar] [CrossRef]
- Ooi, C.-H.; Cheah, W.-K.; Sim, Y.-L.; Pung, S.-Y.; Yeoh, F.-Y. Conversion and characterization of activated carbon fiber derived from palm empty fruit bunch waste and its kinetic study on urea adsorption. J. Environ. Manag. 2017, 197, 199–205. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Hamid, S.B.A.; Das, R.; Hasan, R.; Zain, S.M.; Khalid, K.; Uddin, N. Preparation of carbonaceous adsorbents from lignocellulosic biomass and their use in removal of contaminants from aqueous solution. BioResources 2013, 8, 6523–6555. [Google Scholar] [CrossRef]
- Hu, Z.; Guo, H.; Srinivasan, M.; Yaming, N. A simple method for developing mesoporosity in activated carbon. Sep. Purif. Technol. 2003, 31, 47–52. [Google Scholar] [CrossRef]
- Yuliusman; Nasruddin; Afdhol, M.K.; Haris, F.; Amiliana, R.A.; Hanafi, A.; Ramadhan, I.T. Production of activated carbon from coffee grounds using chemical and physical activation method. Adv. Sci. Lett. 2017, 23, 5751–5755. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Liu, B.; Su, Z. A critical review on the application and recent developments of post-modified biochar in supercapacitors. J. Clean. Prod. 2021, 310, 127428. [Google Scholar] [CrossRef]
- Peng, L.; Liang, Y.; Huang, J.; Xing, L.; Hu, H.; Xiao, Y.; Dong, H.; Liu, Y.; Zheng, M. Mixed-biomass wastes derived hierarchically porous carbons for high-performance electrochemical energy storage. ACS Sustain. Chem. Eng. 2019, 7, 10393–10402. [Google Scholar] [CrossRef]
- Rani, M.U.; Nanaji, K.; Rao, T.N.; Deshpande, A.S. Corn husk derived activated carbon with enhanced electrochemical performance for high-voltage supercapacitors. J. Power Sources 2020, 471, 228387. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, W.; Huang, W. Effect of removing silica in rice husk for the preparation of activated carbon for supercapacitor applications. Chin. Chem. Lett. 2019, 30, 1315–1319. [Google Scholar] [CrossRef]
- Zhang, J.; Gong, L.; Sun, K.; Jiang, J.; Zhang, X. Preparation of activated carbon from waste Camellia oleifera shell for supercapacitor application. J. Solid State Electrochem. 2012, 16, 2179–2186. [Google Scholar] [CrossRef]
- Khan, A.; Senthil, R.A.; Pan, J.; Sun, Y.; Liu, X.; Pan, Y. Hierarchically porous biomass carbon derived from natural withered rose flowers as high-performance material for advanced supercapacitors. Batter. Supercaps 2020, 3, 731–737. [Google Scholar] [CrossRef]
- Li, X.; Xing, W.; Zhuo, S.; Zhou, J.; Li, F.; Qiao, S.Z.; Lu, G.Q. Preparation of capacitor’s electrode from sunflower seed shell. Bioresour. Technol. 2011, 102, 1118–1123. [Google Scholar] [CrossRef]
- Gao, Y.; Li, L.; Jin, Y.; Wang, Y.; Yuan, C.; Wei, Y.; Chen, G.; Ge, J.; Lu, H. Porous carbon made from rice husk as electrode material for electrochemical double layer capacitor. Appl. Energy 2015, 153, 41–47. [Google Scholar] [CrossRef]
- Teo, E.Y.L.; Muniandy, L.; Ng, E.-P.; Adam, F.; Mohamed, A.R.; Jose, R.; Chong, K.F. High surface area activated carbon from rice husk as a high performance supercapacitor electrode. Electrochim. Acta 2016, 192, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Shen, W.; Wang, J.; Fan, W. Hierarchical Porous Carbons Derived from Renewable Poplar Anthers for High-Performance Supercapacitors. ChemElectroChem 2018, 5, 1451–1458. [Google Scholar] [CrossRef]
- Xu, B.; Chen, Y.; Wei, G.; Cao, G.; Zhang, H.; Yang, Y. Activated carbon with high capacitance prepared by NaOH activation for supercapacitors. Mater. Chem. Phys. 2010, 124, 504–509. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, H.; Zhanga, Z.; Yua, J.; Yangab, Z. In-built template synthesis of hierarchical porous carbon microcubes from biomass toward electrochemical energy storage. Carbon 2019, 155, 1–8. [Google Scholar] [CrossRef]
- Okonkwo, C.A.; Lv, T.; Hong, W.; Li, G.; Huang, J.; Deng, J.; Jia, L.; Wu, M.; Liu, H.; Guo, M. The synthesis of micromesoporous carbon derived from nitrogen-rich spirulina extract impregnated castor shell based on biomass self-doping for highly efficient supercapacitor electrodes. J. Alloys Compd. 2020, 825, 154009. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Zhang, Y.; He, J.; Wang, Y.; Wang, K.; Xu, Y.; Li, H.; Wang, Y. Biomass-derived microporous carbon with large micropore size for high-performance supercapacitors. J. Power Sources 2020, 448, 227369. [Google Scholar] [CrossRef]
- Chen, H.; Wang, G.; Chen, L.; Dai, B.; Yu, F. Three-dimensional honeycomb-like porous carbon with both interconnected hierarchical porosity and nitrogen self-doping from cotton seed husk for supercapacitor electrode. Nanomaterials 2018, 8, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, X.; Ma, H.; Li, Z.; Yan, S.; Ma, L.; Yu, F.; Wang, G.; Guo, X.; Ma, Y.; Wong, C. Flute type micropores activated carbon from cotton stalk for high performance supercapacitors. J. Power Sources 2017, 359, 88–96. [Google Scholar] [CrossRef]
- Peng, C.; Yan, X.B.; Wang, R.T.; Lang, J.W.; Ou, Y.J.; Xue, Q.J. Promising activated carbons derived from waste tea-leaves and their application in high performance supercapacitors electrodes. Electrochim. Acta 2013, 87, 401–408. [Google Scholar] [CrossRef]
- Balathanigaimani, M.S.; Shim, W.G.; Lee, M.J.; Kim, C.; Lee, J.W.; Moon, H. Highly porous electrodes from novel corn grains-based activated carbons for electrical double layer capacitors. Electrochem. Commun. 2008, 10, 868–871. [Google Scholar] [CrossRef]
- Sun, Y.; Xue, J.; Dong, S.; Zhang, Y.; An, Y.; Ding, B.; Zhang, T.; Dou, H.; Zhang, X. Biomass-derived porous carbon electrodes for high-performance supercapacitors. J. Mater. Sci. 2020, 55, 5166–5176. [Google Scholar] [CrossRef]
- Ahmad, M.; Usman, A.R.; Al-Faraj, A.S.; Abduljabbar, A.; Ok, Y.S.; Al-Wabel, M.I. Date palm waste-derived biochar composites with silica and zeolite: Synthesis, characterization and implication for carbon stability and recalcitrant potential. Environ. Geochem. Health 2019, 41, 1687–1704. [Google Scholar] [CrossRef]
- Lim, Y.S.; Lai, C.W.; Abd Hamid, S.B. Porous 3D carbon decorated Fe3O4 nanocomposite electrode for highly symmetrical supercapacitor performance. RSC Adv. 2017, 7, 23030–23040. [Google Scholar] [CrossRef] [Green Version]
- Norouzi, O.; Pourhosseini, S.E.M.; Naderi, H.R.; Di Maria, F.; Dutta, A. Integrated hybrid architecture of metal and biochar for high performance asymmetric supercapacitors. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Pourhosseini, S.; Norouzi, O.; Naderi, H.R. Study of micro/macro ordered porous carbon with olive-shaped structure derived from Cladophora glomerata macroalgae as efficient working electrodes of supercapacitors. Biomass Bioenergy 2017, 107, 287–298. [Google Scholar] [CrossRef]
- Pourhosseini, S.E.M.; Norouzisafsari, O.; Salimi, P.; Naderi, H.R. Synthesis of a novel interconnected 3D pore network algal biochar constituting iron nanoparticles derived from a harmful marine biomass as high-performance asymmetric supercapacitor electrodes. ACS Sustain. Chem. Eng. 2018, 6, 4746–4758. [Google Scholar] [CrossRef]
- Zhi, M.; Xiang, C.; Li, J.; Li, M.; Wu, N. Nanostructured carbon–metal oxide composite electrodes for supercapacitors: A review. Nanoscale 2013, 5, 72–88. [Google Scholar] [CrossRef]
- Thomas, D.; Fernandez, N.B.; Mullassery, M.D.; Surya, R. Iron oxide loaded biochar/polyaniline nanocomposite: Synthesis, characterization and electrochemical analysis. Inorg. Chem. Commun. 2020, 119, 108097. [Google Scholar] [CrossRef]
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Carbon-based composite materials for supercapacitor electrodes: A review. J. Mater. Chem. A 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Li, X.; Zhou, J.; Li, X.; Xin, M.; Cai, T.; Xing, W.; Chai, Y.; Xue, Q.; Yan, Z. Bifuntional petaloid nickel manganese layered double hydroxides decorated on a freestanding carbon foam for flexible asymmetric supercapacitor and oxygen evolution. Electrochim. Acta 2017, 252, 275–285. [Google Scholar] [CrossRef]
- Anjali, J.; Jose, V.K.; Lee, J.-M. Carbon-based hydrogels: Synthesis and their recent energy applications. J. Mater. Chem. A 2019, 7, 15491–15518. [Google Scholar] [CrossRef]
- Tan, S.; Kraus, T.J.; Li-Oakey, K.D. Understanding the supercapacitor properties of electrospun carbon nanofibers from Powder River Basin coal. Fuel 2019, 245, 148–159. [Google Scholar] [CrossRef]
- Deng, Y.; Ji, Y.; Wu, H.; Chen, F. Enhanced electrochemical performance and high voltage window for supercapacitor based on multi-heteroatom modified porous carbon materials. Chem. Commun. 2018, 55, 1486–1489. [Google Scholar] [CrossRef]
- Karamanova, B.; Stoyanova, R.; Shipochka, M.; Girginov, C. On the cycling stability of biomass-derived carbons as electrodes in supercapacitors. J. Alloys Compd. 2019, 803, 882–890. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, J.; Lv, R.; Liang, Q.; Zhan, C.; Bai, Y.; Huang, Z.-H.; Shen, W.; Kang, F. Facile synthesis of nitrogen-doped carbon nanosheets with hierarchical porosity for high performance supercapacitors and lithium–sulfur batteries. J. Mater. Chem. A 2015, 3, 18400–18405. [Google Scholar] [CrossRef]
- Li, J.; Liu, K.; Gao, X.; Yao, B.; Huo, K.; Cheng, Y.; Cheng, X.; Chen, D.; Wang, B.; Sun, W.; et al. Oxygen-and nitrogen-enriched 3D porous carbon for supercapacitors of high volumetric capacity. ACS Appl. Mater. Interfaces 2015, 7, 24622–24628. [Google Scholar] [CrossRef]
- Lian, J.; Xiong, L.; Cheng, R.; Pang, D.; Tian, X.; Lei, J.; He, R.; Yu, X.; Duan, T.; Zhu, W. Ultra-high nitrogen content biomass carbon supercapacitors and nitrogen forms analysis. J. Alloys Compd. 2019, 809, 151664. [Google Scholar] [CrossRef]
- Lyu, L.; Seong, K.-D.; Ko, D.; Choi, J.; Lee, C.; Hwang, T.; Cho, Y.; Jin, X.; Zhang, W.; Pang, H.; et al. Recent development of biomass-derived carbons and composites as electrode materials for supercapacitors. Mater. Chem. Front. 2019, 3, 2543–2570. [Google Scholar] [CrossRef]
- Lai, F.; Miao, Y.-E.; Zuo, L.; Lu, H.; Huang, Y.; Liu, T. Biomass-Derived Nitrogen-Doped Carbon Nanofiber Network: A Facile Template for Decoration of Ultrathin Nickel-Cobalt Layered Double Hydroxide Nanosheets as High-Performance Asymmetric Supercapacitor Electrode. Small 2016, 12, 3235–3244. [Google Scholar] [CrossRef]
- Deng, S.; Ai, C.; Luo, M.; Liu, B.; Zhang, Y.; Li, Y.; Lin, S.; Pan, G.; Xiong, Q.; Liu, Q.; et al. Coupled biphase (1T-2H)-MoSe2 on mold spore carbon for advanced hydrogen evolution reaction. Small 2019, 15, 1901796. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Li, S.; Chen, W.; Wu, T.; Peng, C. Biomass-derived materials for electrochemical energy storage and conversion: Overview and perspectives. Energy Environ. Mater. 2019, 2, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Vijayakumar, M.; Sankar, A.B.; Rohita, D.S.; Rao, T.N.; Karthik, M. Conversion of biomass waste into high performance supercapacitor electrodes for real-time supercapacitor applications. ACS Sustain. Chem. Eng. 2019, 7, 17175–17185. [Google Scholar] [CrossRef]
- Chen, D.; Yang, L.; Li, J.; Wu, Q. Effect of Self-Doped Heteroatoms in Biomass-Derived Activated Carbon for Supercapacitor Applications. ChemistrySelect 2019, 4, 1586–1595. [Google Scholar] [CrossRef]
- Sankar, S.; Ahmed, A.T.A.; Inamdar, A.I.; Im, H.; Bin Im, Y.; Lee, Y.; Kim, D.Y.; Lee, S. Biomass-derived ultrathin mesoporous graphitic carbon nanoflakes as stable electrode material for high-performance supercapacitors. Mater. Des. 2019, 169, 107688. [Google Scholar] [CrossRef]
- Huang, S.; Ding, Y.; Li, Y.; Han, X.; Xing, B.; Wang, S. Nitrogen and Sulfur Co-doped Hierarchical Porous Biochar Derived from the Pyrolysis of Mantis Shrimp Shell for Supercapacitor Electrodes. Energy Fuels 2021, 35, 1557–1566. [Google Scholar] [CrossRef]
- Norouzi, O.; Di Maria, F.; Dutta, A. Biochar-based composites as electrode active materials in hybrid supercapacitors with particular focus on surface topography and morphology. J. Energy Storage 2020, 29, 101291. [Google Scholar] [CrossRef]
- Zhou, B.; Sui, Y.; Qi, J.; He, Y.; Meng, Q.; Wei, F.; Ren, Y.; Zhang, X. Synthesis of Ultrathin MnO2 Nanosheets/Bagasse Derived Porous Carbon Composite for Supercapacitor with High Performance. J. Electron. Mater. 2019, 48, 3026–3035. [Google Scholar] [CrossRef]
- Golmohammadi, F.; Amiri, M. Facile synthesis of nanofiber composite based on biomass-derived material conjugated with nanoparticles of Ni–Co oxides for high-performance supercapacitors. J. Mater. Sci. Mater. Electron. 2020, 31, 2269–2279. [Google Scholar] [CrossRef]
- Jiang, X.; Shi, G.; Wang, G.; Mishra, P.; Du, J.; Zhang, Y. Fe2O3/hemp straw-based porous carbon composite for supercapacitor electrode materials. Ionics 2020, 26, 4039–4051. [Google Scholar] [CrossRef]
- Shang, Z.; An, X.; Zhang, H.; Shen, M.; Baker, F.; Liu, Y.; Liu, L.; Yang, J.; Cao, H.; Xu, Q.; et al. Houttuynia-derived nitrogen-doped hierarchically porous carbon for high-performance supercapacitor. Carbon 2020, 161, 62–70. [Google Scholar] [CrossRef]
- Raj, F.R.M.S.; Boopathi, G.; Jaya, N.V.; Kalpana, D.; Pandurangan, A. N, S codoped activated mesoporous carbon derived from the Datura metel seed pod as active electrodes for supercapacitors. Diam. Relat. Mater. 2019, 102, 107687. [Google Scholar] [CrossRef]
- Nirosha, B.; Selvakumar, R.; Jeyanthi, J.; Vairam, S. Elaeocarpus tectorius derived phosphorus-doped carbon as an electrode material for an asymmetric supercapacitor. New J. Chem. 2019, 44, 181–193. [Google Scholar] [CrossRef]
- Meng, X.; Jia, S.; Mo, L.; Wei, J.; Wang, F.; Shao, Z. O/N-co-doped hierarchically porous carbon from carboxymethyl cellulose ammonium for high-performance supercapacitors. J. Mater. Sci. 2020, 55, 7417–7431. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Xiao, J.; Jiang, H.; Hu, T.; Meng, C. Copper oxide/cuprous oxide/hierarchical porous biomass-derived carbon hybrid composites for high-performance supercapacitor electrode. J. Alloys Compd. 2018, 782, 1103–1113. [Google Scholar] [CrossRef]
- Wan, L.; Hu, J.; Liu, J.; Xie, M.; Zhang, Y.; Chen, J.; Du, C.; Tian, Z. Heteroatom-doped porous carbons derived from lotus pollen for supercapacitors: Comparison of three activators. J. Alloys Compd. 2020, 859, 158390. [Google Scholar] [CrossRef]
- Xu, H.; Yan, X.-H.; Meng, Z.; Xue, T.; Li, D.; Fang, G.; Shen, H. Nitrogen-doped mesoporous carbon/poly-o-phenylenediamine composites for high-performance hybrid supercapacitor electrodes. Mater. Res. Express 2019, 6, 095601. [Google Scholar] [CrossRef]
- Wan, L.; Wei, W.; Xie, M.; Zhang, Y.; Li, X.; Xiao, R.; Chen, J.; Du, C. Nitrogen, sulfur co-doped hierarchically porous carbon from rape pollen as high-performance supercapacitor electrode. Electrochim. Acta 2019, 311, 72–82. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, C.; Qiu, S.; Zhu, Y.; Qin, M.; Meng, Y.; Wang, Y.; Chu, H.; Zou, Y.; Xiang, C. Nitrogen-doped porous carbon derived from ginkgo leaves with remarkable supercapacitance performance. Diam. Relat. Mater. 2019, 98, 107475. [Google Scholar] [CrossRef]
- Lei, W.; Yang, B.; Sun, Y.; Xiao, L.; Tang, D.; Chen, K.; Sun, J.; Ke, J.; Zhuang, Y. Self-sacrificial template synthesis of heteroatom doped porous biochar for enhanced electrochemical energy storage. J. Power Sources 2021, 488, 229455. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Z.; Fei, B.; Ma, J.; Liu, X. Graphene functionalized bio-carbon xerogel for achieving high-rate and high-stability supercapacitors. Electrochim. Acta 2018, 282, 813–821. [Google Scholar] [CrossRef]
- Yao, L.; Yang, J.; Zhang, P.; Deng, L. In situ surface decoration of Fe3C/Fe3O4/C nanosheets: Towards bi-functional activated carbons with supercapacitance and efficient dye adsorption. Bioresour. Technol. 2018, 256, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Zhang, J.; Liu, X.; Liu, Y.; Guo, S.; Yu, S.; Filatov, S.; Yi, X. N, S self-doped hollow-sphere porous carbon derived from puffball spores for high performance supercapacitors. Appl. Surf. Sci. 2020, 542, 148697. [Google Scholar] [CrossRef]
- Gao, X.; Li, X.; Kong, Z.; Xiao, G.; Zhu, Y. Bifunctional 3D n-doped porous carbon materials derived from paper towel for oxygen reduction reaction and supercapacitor. Sci. Bull. 2018, 63, 621–628. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Yang, Q.; Sun, K.; Peng, H.; Ran, F.; Zhao, X.; Lei, Z. Nitrogen-doped porous carbon derived from biomass waste for high-performance supercapacitor. Bioresour. Technol. 2015, 197, 137–142. [Google Scholar] [CrossRef]
- Guan, L.; Pan, L.; Peng, T.; Gao, C.; Zhao, W.; Yang, Z.; Hu, H.; Wu, M. Synthesis of biomass-derived nitrogen-doped porous carbon nanosheests for high-performance supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 8405–8412. [Google Scholar] [CrossRef]
- Chen, H.; Liu, D.; Shen, Z.; Bao, B.; Zhao, S.; Wu, L. Functional biomass carbons with hierarchical porous structure for supercapacitor electrode materials. Electrochim. Acta 2015, 180, 241–251. [Google Scholar] [CrossRef]
- Zhao, G.; Li, Y.; Zhu, G.; Shi, J.; Lu, T.; Pan, L. Biomass-based N, P, and S self-doped porous carbon for high-performance supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 12052–12060. [Google Scholar] [CrossRef]
- Loow, Y.-L.; Wu, T.Y.; Yang, G.H.; Jahim, J.M.; Teoh, W.H.; Mohammad, A.W. Role of energy irradiation in aiding pretreatment of lignocellulosic biomass for improving reducing sugar recovery. Cellulose 2016, 23, 2761–2789. [Google Scholar] [CrossRef]
- Bai, P.; Wei, S.; Lou, X.; Xu, L. An ultrasound-assisted approach to bio-derived nanoporous carbons: Disclosing a linear relationship between effective micropores and capacitance. RSC Adv. 2019, 9, 31447–31459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokai, F.; Sorin, R.; Chigusa, H.; Hanai, K.; Koshio, A.; Ishihara, M.; Koga, Y.; Hasegawa, M.; Imanishi, N.; Takeda, Y. Ultrasonication fabrication of high quality multilayer graphene flakes and their characterization as anodes for lithium ion batteries. Diam. Relat. Mater. 2012, 29, 63–68. [Google Scholar] [CrossRef]
- Zhou, G.; Yin, J.; Sun, Z.; Gao, X.; Zhu, F.; Zhao, P.; Li, R.; Xu, J. An ultrasonic-assisted synthesis of rice-straw-based porous carbon with high performance symmetric supercapacitors. RSC Adv. 2020, 10, 3246–3255. [Google Scholar] [CrossRef]
- Thiemann, A.; Holsteyns, F.; Cairós, C.; Mettin, R. Sonoluminescence and dynamics of cavitation bubble populations in sulfuric acid. Ultrason. Sonochem. 2017, 34, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Gireesan, S.; Pandit, A.B. Modeling the effect of carbon-dioxide gas on cavitation. Ultrason. Sonochem. 2017, 34, 721–728. [Google Scholar] [CrossRef]
- Merouani, S.; Hamdaoui, O.; Rezgui, Y.; Guemini, M. Theoretical estimation of the temperature and pressure within collapsing acoustical bubbles. Ultrason. Sonochem. 2014, 21, 53–59. [Google Scholar] [CrossRef]
- Feng, L.; Cao, Y.; Xu, D.; Wang, S.; Zhang, J. Molecular weight distribution, rheological property and structural changes of sodium alginate induced by ultrasound. Ultrason. Sonochem. 2017, 34, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Leng, C.; Sun, K.; Li, J.; Jiang, J. The reconstruction of char surface by oxidized quantum-size carbon dots under the ultrasonic energy to prepare modified activated carbon materials as electrodes for supercapacitors. J. Alloys Compd. 2017, 714, 443–452. [Google Scholar] [CrossRef]
- Chen, F.; Ji, Y.; Deng, Y.; Ren, F.; Tan, S.; Wang, Z. Ultrasonic-assisted fabrication of porous carbon materials derived from agricultural waste for solid-state supercapacitors. J. Mater. Sci. 2020, 55, 11512–11523. [Google Scholar] [CrossRef]
- Wang, T.; Li, G.; Yang, K.; Zhang, X.; Wang, K.; Cai, J.; Zheng, J. Enhanced ammonium removal on biochar from a new forestry waste by ultrasonic activation: Characteristics, mechanisms and evaluation. Sci. Total Environ. 2021, 778, 146295. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2011, 41, 413–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panchal, M.; Raghavendra, G.; Ojha, S.; Omprakash, M.; Acharya, S.K. A single step process to synthesize ordered porous carbon from coconut shells-eggshells biowaste. Mater. Res. Express 2019, 6, 115613. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Lv, H.; Chen, A. N/B-co-doped ordered mesoporous carbon spheres by ionothermal strategy for enhancing supercapacitor performance. J. Colloid Interface Sci. 2021, 587, 780–788. [Google Scholar] [CrossRef]
- Mehdi, R.; Raza, N.; Naqvi, S.R.; Khoja, A.H.; Mehran, M.T.; Farooq, M.; Tran, K.-Q. A comparative assessment of solid fuel pellets production from torrefied agro-residues and their blends. J. Anal. Appl. Pyrolysis 2021, 156, 105125. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, M.; Lu, C. Morphological and structural development of hardwood cellulose during mechanochemical pretreatment in solid state through pan-milling. Cellulose 2007, 14, 447–456. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Hou, T.; Chen, X.; Gao, C.; Han, L.; Xiao, W. Mechanical deconstruction of corn stover as an entry process to facilitate the microwave-assisted production of ethyl levulinate. Fuel Process. Technol. 2018, 174, 53–60. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Fujimoto, S.; Hirata, S.; Hassan, M.A. Ball milling pretreatment of oil palm biomass for enhancing enzymatic hydrolysis. Appl. Biochem. Biotechnol. 2014, 173, 1778–1789. [Google Scholar] [CrossRef]
- Mattonai, M.; Pawcenis, D.; del Seppia, S.; Łojewska, J.; Ribechini, E. Effect of ball-milling on crystallinity index, degree of polymerization and thermal stability of cellulose. Bioresour. Technol. 2018, 270, 270–277. [Google Scholar] [CrossRef]
- Shen, F.; Sun, S.; Yang, J.; Qiu, M.; Qi, X. Coupled pretreatment with liquid nitrogen and ball milling for enhanced cellulose hydrolysis in water. ACS Omega 2019, 4, 11756–11759. [Google Scholar] [CrossRef]
- Khan, A.S.; Man, Z.; Bustam, M.A.; Kait, C.F.; Khan, M.I.; Muhammad, N.; Nasrullah, A.; Ullah, Z.; Ahmad, P. Impact of ball-milling pretreatment on pyrolysis behavior and kinetics of crystalline cellulose. Waste Biomass Valorization 2015, 7, 571–581. [Google Scholar] [CrossRef]
- Lin, X.; Liang, Y.; Lu, Z.; Lou, H.; Zhang, X.; Liu, S.; Zheng, B.; Liu, R.; Fu, R.; Wu, D. Mechanochemistry: A green, activation-free and top-down strategy to high-surface-area carbon materials. ACS Sustain. Chem. Eng. 2017, 5, 8535–8540. [Google Scholar] [CrossRef]
- Shen, F.; Qiu, M.; Hua, Y.; Qi, X. Dual-Functional Templated Methodology for the Synthesis of Hierarchical Porous Carbon for Supercapacitor. ChemistrySelect 2018, 3, 586–591. [Google Scholar] [CrossRef]
- Bahuguna, A.; Kumar, A.; Krishnan, V. carbon-support-based heterogeneous nanocatalysts: Synthesis and applications in organic reactions. Asian J. Org. Chem. 2019, 8, 1263–1305. [Google Scholar] [CrossRef]
- Shan, D.; Deng, S.; Zhao, T.; Wang, B.; Wang, Y.; Huang, J.; Yu, G.; Winglee, J.; Wiesner, M.R. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. J. Hazard. Mater. 2015, 305, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filiciotto, L.; Balu, A.M.; Romero, A.A.; Rodríguez-Castellón, E.; van der Waal, J.C.; Luque, R. Benign-by-design preparation of humin-based iron oxide catalytic nanocomposites. Green Chem. 2017, 19, 4423–4434. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Liu, X.; Wang, M.; Huang, J.; Jiang, X.; Pang, J.; Xu, F.; Zhang, X. Synthesis of N-doped carbon quantum dots from bio-waste lignin for selective irons detection and cellular imaging. Int. J. Biol. Macromol. 2019, 128, 537–545. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, H.-L.; Hao, L.T.; Kong, H.; Park, J.M.; Jung, S.-H.; Gil Cha, H.; Lee, J.Y.; Kim, H.; Hwang, S.Y.; et al. A ball milling-based one-step transformation of chitin biomass to organo-dispersible strong nanofibers passing highly time and energy consuming processes. Int. J. Biol. Macromol. 2018, 125, 660–667. [Google Scholar] [CrossRef]
- Peterson, S.C.; Jackson, M.A.; Kim, S.; Palmquist, D.E. Increasing biochar surface area: Optimization of ball milling parameters. Powder Technol. 2012, 228, 115–120. [Google Scholar] [CrossRef]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Huang, H.; Tang, J. Effects of ball milling on the physicochemical and sorptive properties of biochar: Experimental observations and governing mechanisms. Environ. Pollut. 2018, 233, 54–63. [Google Scholar] [CrossRef]
- Rajendiran, R.; Nallal, M.; Park, K.H.; Li, O.L.; Kim, H.-J.; Prabakar, K. Mechanochemical assisted synthesis of heteroatoms inherited highly porous carbon from biomass for electrochemical capacitor and oxygen reduction reaction electrocatalysis. Electrochim. Acta 2019, 317, 1–9. [Google Scholar] [CrossRef]
- Huang, L.; Wu, Q.; Liu, S.; Yu, S.; Ragauskas, A.J. Solvent-free production of carbon materials with developed pore structure from biomass for high-performance supercapacitors. Ind. Crop. Prod. 2020, 150, 112384. [Google Scholar] [CrossRef]
- Teng, Z.; Han, K.; Li, J.; Gao, Y.; Li, M.; Ji, T. Ultrasonic-assisted preparation and characterization of hierarchical porous carbon derived from garlic peel for high-performance supercapacitors. Ultrason. Sonochem. 2020, 60, 104756. [Google Scholar] [CrossRef] [PubMed]

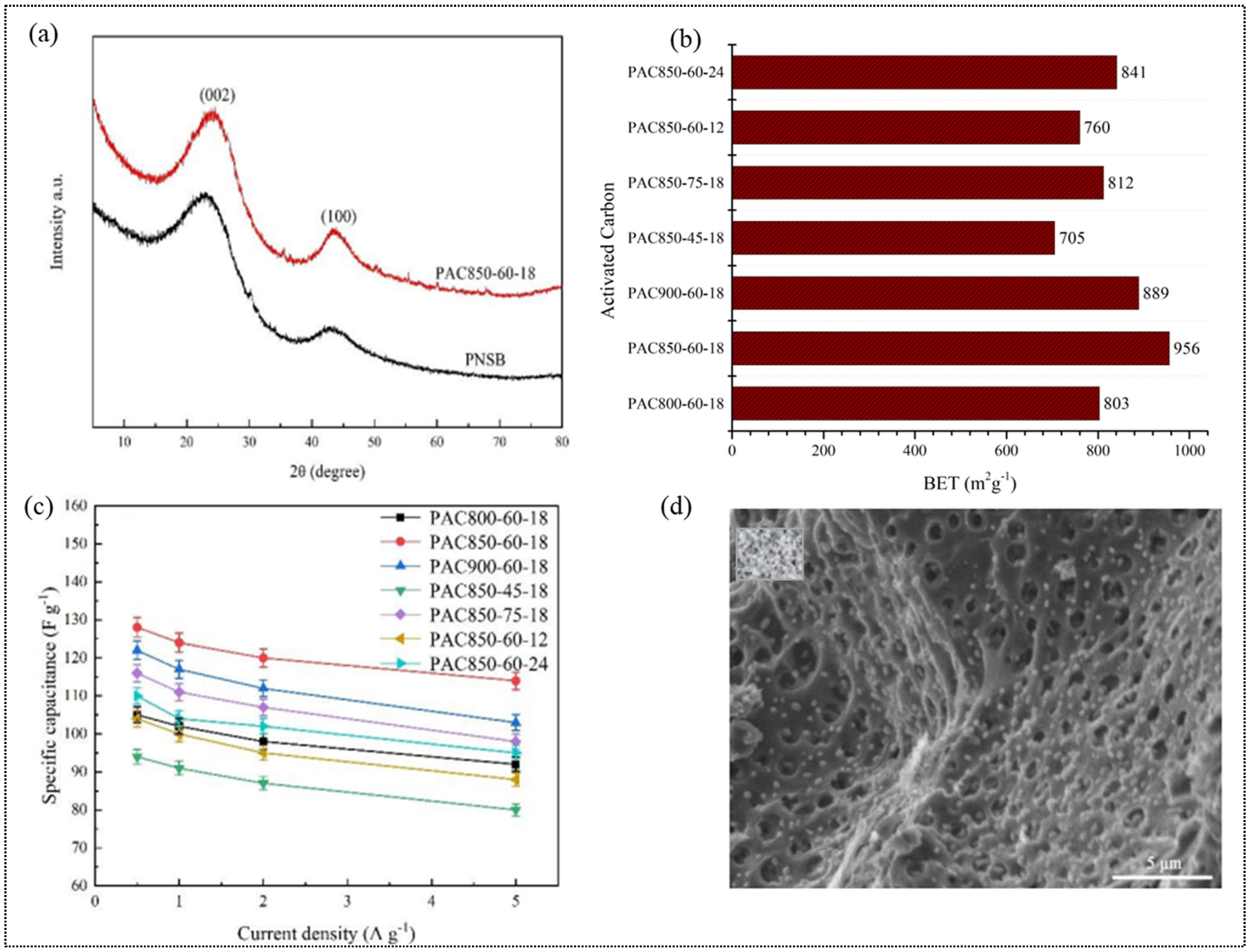
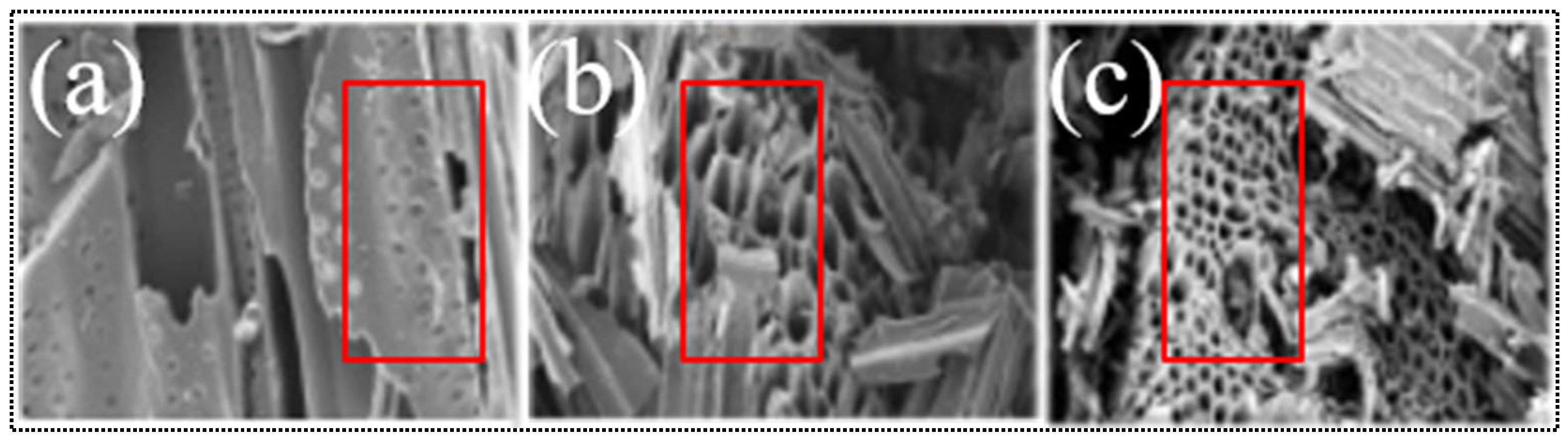
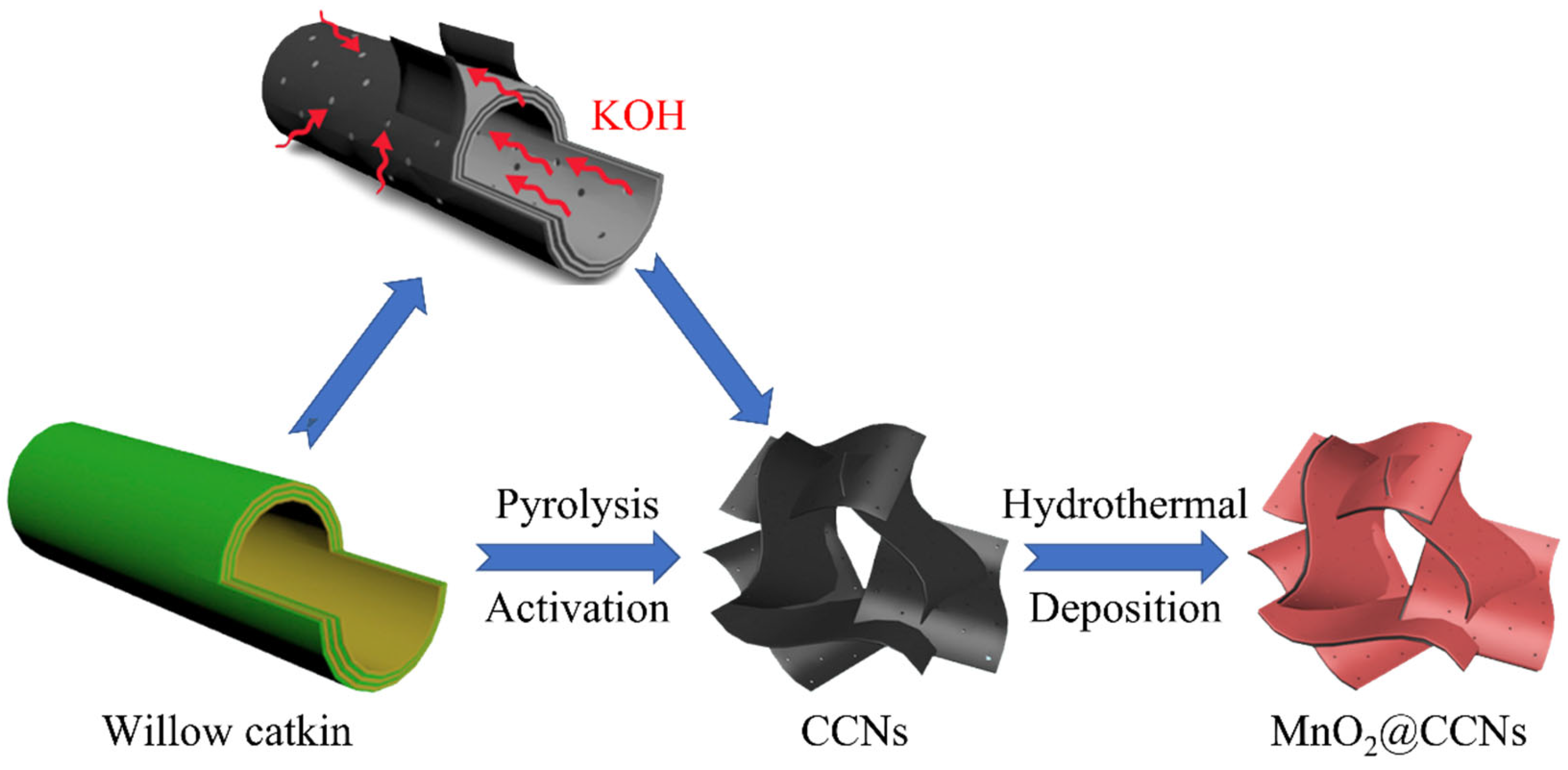
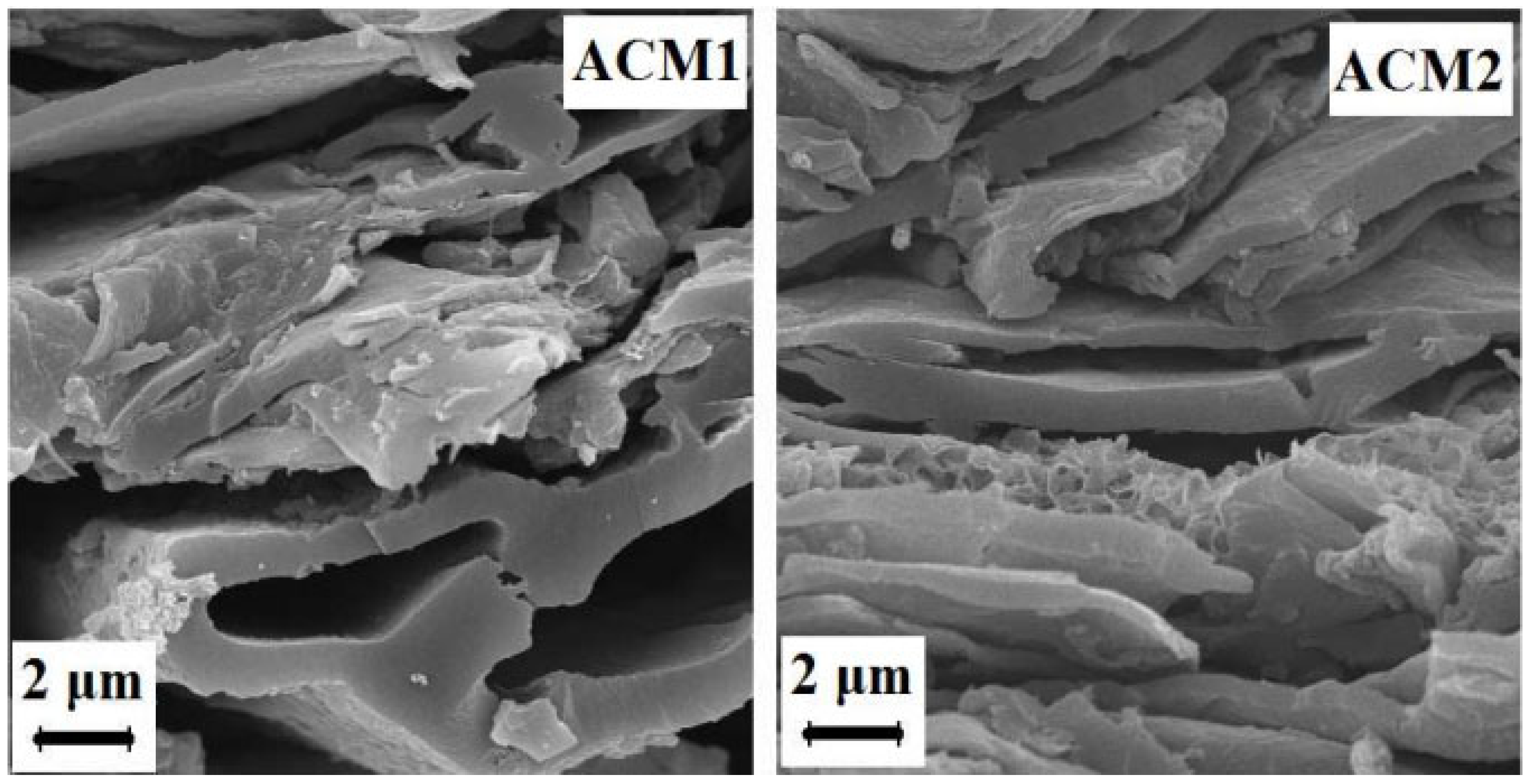
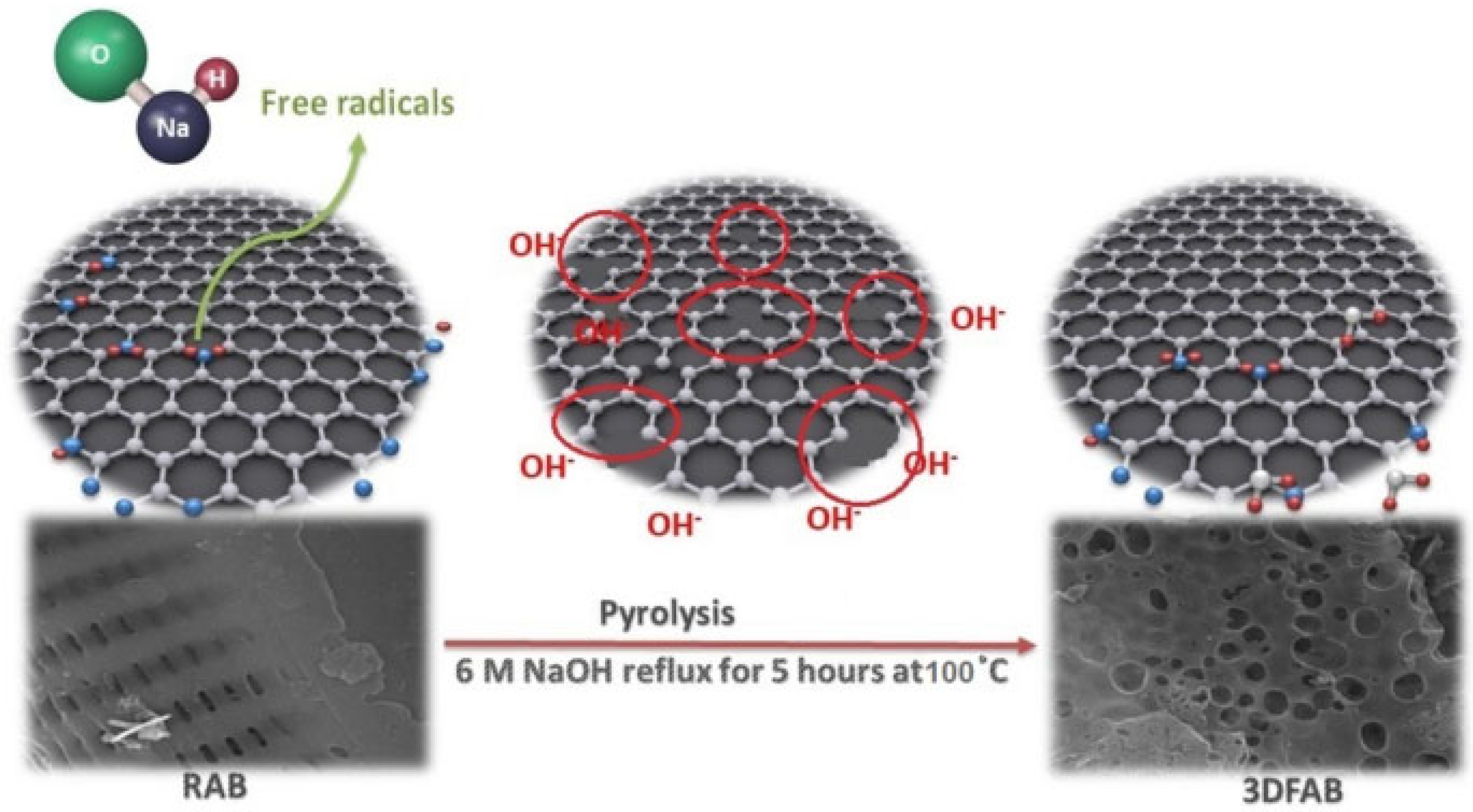
| Method of Activation | Lignocellulosic Biomass | Activation Agent | Ref. |
|---|---|---|---|
| Physical activation |
| CO2 and steam | [78,79,80] |
| Steam/CO2 | [81,82,83,84] | |
| CO2 and air | [48,85] | |
| Steam | [70] | |
| Chemical activation | Fish skin, onion, palm kernel shell, bamboo species, argan (Argania spinosa) seed shells, potato starch, goat hair, coconut shell, soybean oil cake, distillers dried grains, elm samara, cornstalk | KOH | [86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101] |
| Silkworm excretment, of squid gladius chitin, wax ground, cuttle bones, wheat straw, rice straw, cotton stalk, soybean stalk, peanut shell, banana peel, polysaccharides | NaOH, and NaOH/KOH | [102,103,104,105,106,107] | |
| palm kernel shell, cashmere, cocoa pod husk | KOH/K2CO3 | [89,108,109] | |
| Shrimp shell, waste particleboard, wood waste sticks | H3PO4/KOH | [82,84,110] | |
| teakwood sawdust, potato waste, S. bengalense, residue, Persian ironwood | ZnCl2 | [111,112,113] | |
| Raw cotton, bean curd | Zn(NO)3 and CH3COOK | [114,115] | |
| Physicochemical activation | Peanut shell | KOH and air | [116] |
| Needle coke | Steam | [117] | |
| Cassava peel waste | KOH and CO2 | [118] |
| Lignocellulosic Biomass | Activation Methods | Specific Surface Area (m2/g) | Current Density (Ag−1) | Specific Capacitance (Fg−1) | Electrolyte | Energy Density (Wh/kg) | Power Density (W/kg) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Rice husks and crab shells | Chemical activation by KOH | 3557 | 0.5 | 474 | 6 M KOH | --- | --- | [151] |
| Corn husk | Activation with KOH | 1370 | 1 | 127 and 80 | 6 M KOH and 1 M TEABF4/AN | 20 | 681 | [152] |
| Sisal | KOH activation | 2289 | 0.5 | 415 | 6 M KOH | ---- | ---- | [153] |
| Camellia oleifera shell | Chemical activation with ZnCl2 | 1935 | 0.2 | 374 and 266 | 1 M H2SO4 and 6 M KOH | ---- | ---- | [154] |
| Rose flower | Chemical activation with KOH/KNO3 | 1980 | 1 | 350 | 6 M KOH | ---- | ---- | [155] |
| Sunflower seed shell | KOH activation | 1162 | 0.25 | 244 | 30 wt% KOH | 4.8 | 2.4 | [156] |
| Rice husk | Carbonization at 450 °C and chemical activation by KOH at 400–900 °C | 3145 | 2.27 | 367 for aqueous electrolyte and 174 for organic electrolyte | 6 M KOH | ---- | ---- | [157] |
| Rice husk | Carbonization at 400 °C in muffle furnace of NaOH pretreated rice husk, activation by KOH at 750 °C, 850 °C, and 950 °C | 2696 at 850 °C | 0.1 | 147 at 850 °C | 6 M KOH | 5.11 | ---- | [158] |
| Poplar anthers | Chemical activation with KOH | 3639 | 0.5 | 361.5 | 6 M KOH | [159] | ||
| Apricot shell | NaOH activation | 2335 | 0.5 | 339 | 6 M KOH | ---- | ---- | [160] |
| Rice husk | Chemical activation with KOH | 3263 | 0.5 | 315 | 6 M KOH | [161] | ||
| Castor shell | Chemical activation with KOH | 1527 | 1 | 365 | 6 M KOH | ---- | ---- | [162] |
| Cornstalk | Chemical activation with K2C2O4·H2O | 2054 | 0.5 | 461 | 1M Na2SO4 | ---- | ---- | [163] |
| Husk of cotton Seed | Chemical activation with KOH | 1694.1 | 0.5 | 1694.1 | 6 M KOH | ---- | ---- | [164] |
| Cotton stalk | Chemical activation with KOH | 1964.46 | 0.2 | 254 | 1 M Na2SO4 | ---- | ---- | [165] |
| Waste tea leaves | Chemical activation with KOH | 2841 | 1 | 330 | 2 M KOH | ---- | ---- | [166] |
| Corn grains | Chemical activation with KOH | 3199 | 0.5 | 257 | 6 M KOH | ---- | ---- | [167] |
| Mantis shrimp shell | Self-activation at 700 °C, 750 °C, 800 °C, 850 °C, and 900 °C with N-S co-doping | 401 | 1 | 201 | 6 M KOH | ---- | ---- | [25] |
| Grape Marcs | Doping with N and CA with KOH at 700 °C | 2221.4 | 0.5 | 446 | 1M H2SO4 | 16.3 | 348.3 | [150] |
| Quinoa | N doping with CA with different KOH ratios. | 2597 | 0.5 | 330 | 6 M KOH | 9.5 in aqueous electrolyte and 22 in organic electrolyte | ---- | [168] |
| Lignocellulosic Biomass | Modification Method for Supercapacitor Materials | Capacitance (F/g) | Current Density (F/g) | Electrolyte | Cycle Stability (%) | Ref. |
|---|---|---|---|---|---|---|
| Bagasse | MnO2/Porous carbon | 492.5 | 1 | 6 M KOH | 92.1% after 5000 cycles | [195] |
| Typha domingensis | Ni–Co oxides/nanofibers composite | 142 | 1 | 6 M KOH | 78.4% after 5000 cycles | [196] |
| Hemp straw | Fe2O3/porous carbon nanocomposites | 256 | 1 | 6 M KOH | 77.71% after 5000 cycles | [197] |
| Houttuynia | nitrogen-doped hierarchically porous carbon | 473.5 | 1 | 6 M KOH | 95.74% after 10,000 cycles | [198] |
| Peach gum | Ni(OH)2/carbon nanosheet | 350 | 1 | 6 M KOH | 83.9% after 5000 cycles | [104] |
| Datura metel seed pod | N, S codoped activated mesoporous carbon | 340 | 1 | 1 M H2SO4 | 95.24% after 3000 cycles | [199] |
| Elaeocarpus tectorius | Phosphorus-doped porous carbon | 385 | 0.2 | 1 M H2SO4 | 96% after 1000 cycles | [200] |
| Carboxy methyl cellulose ammonium | co-doped hierarchically porous | 465 | 1 | 3 M KOH | 86.3% after 10,000 cycles | [201] |
| Bamboo leaves | Copper oxide/cuprous oxide/hierarchical porous carbon | 147 | 1 | 3 M KOH | 93% after 10,000 cycles | [202] |
| Lotus pollen | CuCl2-activated carbon | 496 | 1 | 1 M Na2SO4 | 90.8% after 10,000 cycles | [203,204] |
| Rape pollen | Co-doping of Nitrogen and sulfur to make hierarchically porous carbon | 361 | 1 | 6 M KOH | 94.5% after 20,000 cycles | [205] |
| Ginkgo leaves | porous carbon doped with nitrogen | 323.2 | 0.5 | 6 M KOH | 99% after 12,000 cycles | [206] |
| Peanut shells | doped BC with nitrogen | 447 | 0.2 | 1 M H2SO4 | 91.4% after 10,000 cycles | [207] |
| Bamboo | Graphene functionalized bio-carbon xerogel | 189 | 1 | 6M KOH | 10% after 10,000 cycles | [208] |
| Tofu | Fe3C/Fe3O4 nanosheets | 315 | 0.5 | 6 M KOH | – | [209] |
| Banana peel | MnO2 and biomass-derived 3D porous carbon composites | 170 | 10 | 1 M Na2SO4 | 98% after 3000 cycles | [106] |
| Cotton Seed Husk | 3D Porous Carbon like Honeycomb | 238 | 0.5 | 6 M KOH | 91% after 5000 cycles | [164] |
| Puffball spores | Self-doped hollow-sphere porous carbon doped with N & S | 285 | 0.5 | 2 M KOH | 80.3% after 5000 cycles | [210] |
| Paper towel | Bifunctional 3D n-doped carbon materials | 379.5 | 1 | 6 M KOH | 94.5% after 10,000 cycles | [211] |
| Potato waste | N-doped carbon activated ZnCl2 and melamine | 255 | 0.5 | 2 M KOH | 93.7 after 5000 cycles | [212] |
| Pine nut shells | N-doped BC with KOH and melamine activation | 324 | 0.5 | 6 M KOH | ---- | [213] |
| Bamboo shootShells | N, S-doped BC | 302.5 | 0.5 | 1 M H2SO4 | ---- | [82] |
| Bamboo | KOH activated, and HA-doped BC with N, B | 281 | 0.2 | 1 M KOH | ----- | [214] |
| Peanut meal | Carbonization, ZnCl2 and Mg(NO3)2·6H2O activation | 525 | 1 | 1 M H2SO4 | ---- | [215] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehdi, R.; Khoja, A.H.; Naqvi, S.R.; Gao, N.; Amin, N.A.S. A Review on Production and Surface Modifications of Biochar Materials via Biomass Pyrolysis Process for Supercapacitor Applications. Catalysts 2022, 12, 798. https://doi.org/10.3390/catal12070798
Mehdi R, Khoja AH, Naqvi SR, Gao N, Amin NAS. A Review on Production and Surface Modifications of Biochar Materials via Biomass Pyrolysis Process for Supercapacitor Applications. Catalysts. 2022; 12(7):798. https://doi.org/10.3390/catal12070798
Chicago/Turabian StyleMehdi, Rifat, Asif Hussain Khoja, Salman Raza Naqvi, Ningbo Gao, and Nor Aishah Saidina Amin. 2022. "A Review on Production and Surface Modifications of Biochar Materials via Biomass Pyrolysis Process for Supercapacitor Applications" Catalysts 12, no. 7: 798. https://doi.org/10.3390/catal12070798
APA StyleMehdi, R., Khoja, A. H., Naqvi, S. R., Gao, N., & Amin, N. A. S. (2022). A Review on Production and Surface Modifications of Biochar Materials via Biomass Pyrolysis Process for Supercapacitor Applications. Catalysts, 12(7), 798. https://doi.org/10.3390/catal12070798