Performance Optimization and Toxicity Effects of the Electrochemical Oxidation of Octogen
Abstract
:1. Introduction
2. Results and Discussion
2.1. Performance Optimization of HMX Degradation by Electrochemical Oxidation
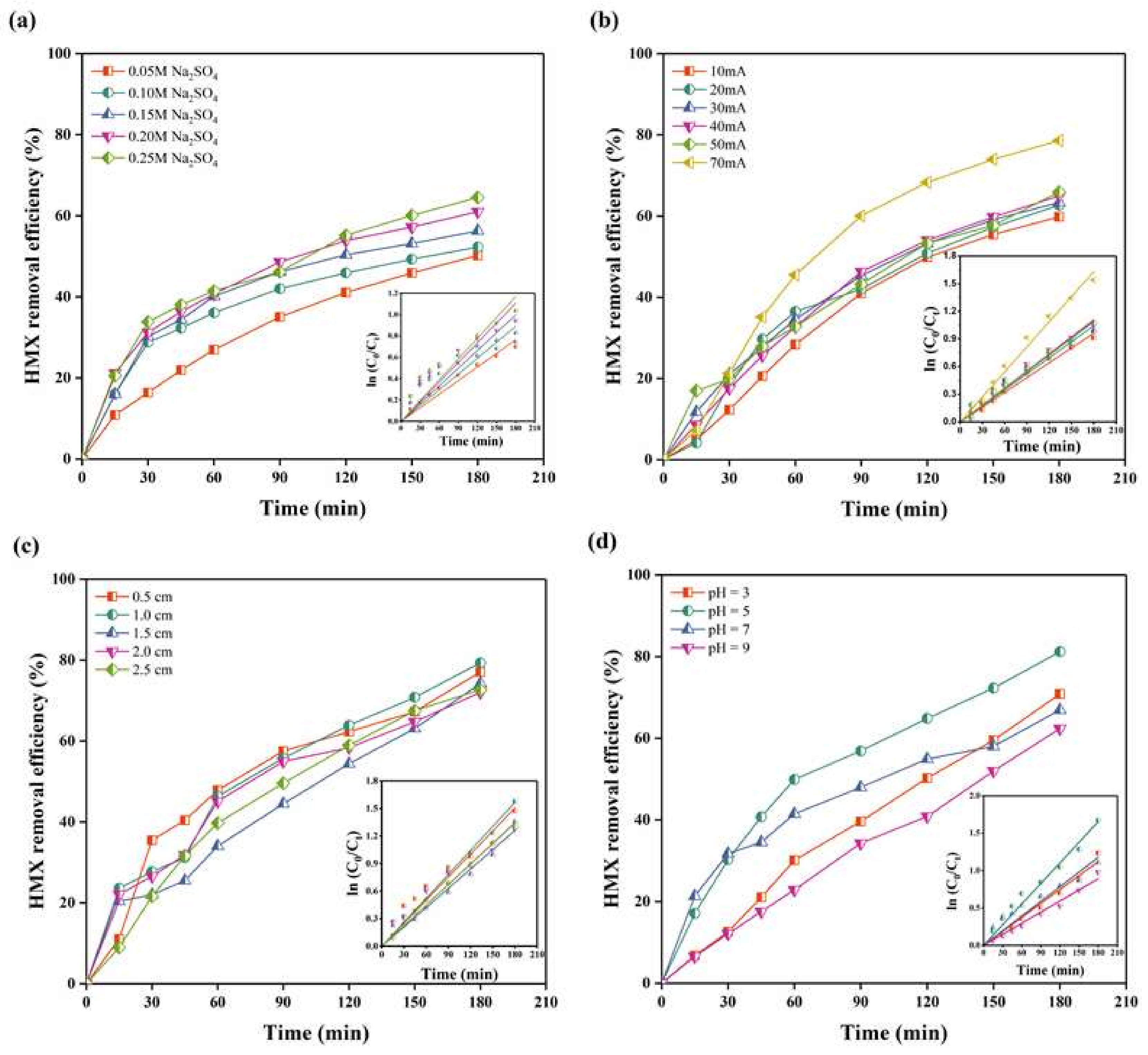
2.1.1. Electrolyte Concentration
2.1.2. Current Density
2.1.3. Interelectrode Distance
2.1.4. pH
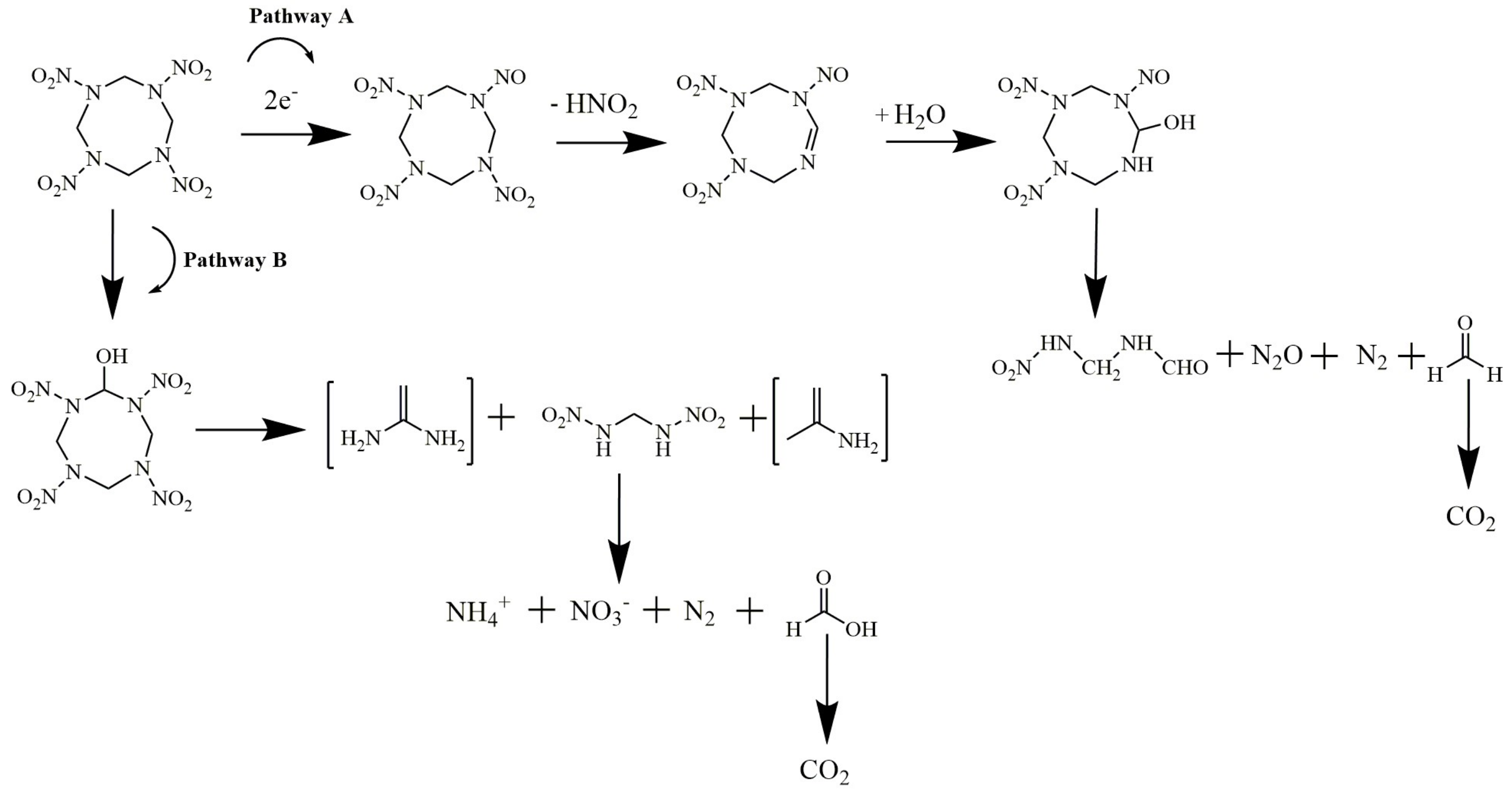
2.2. Possible Electrochemical Degradation Mechanism of HMX
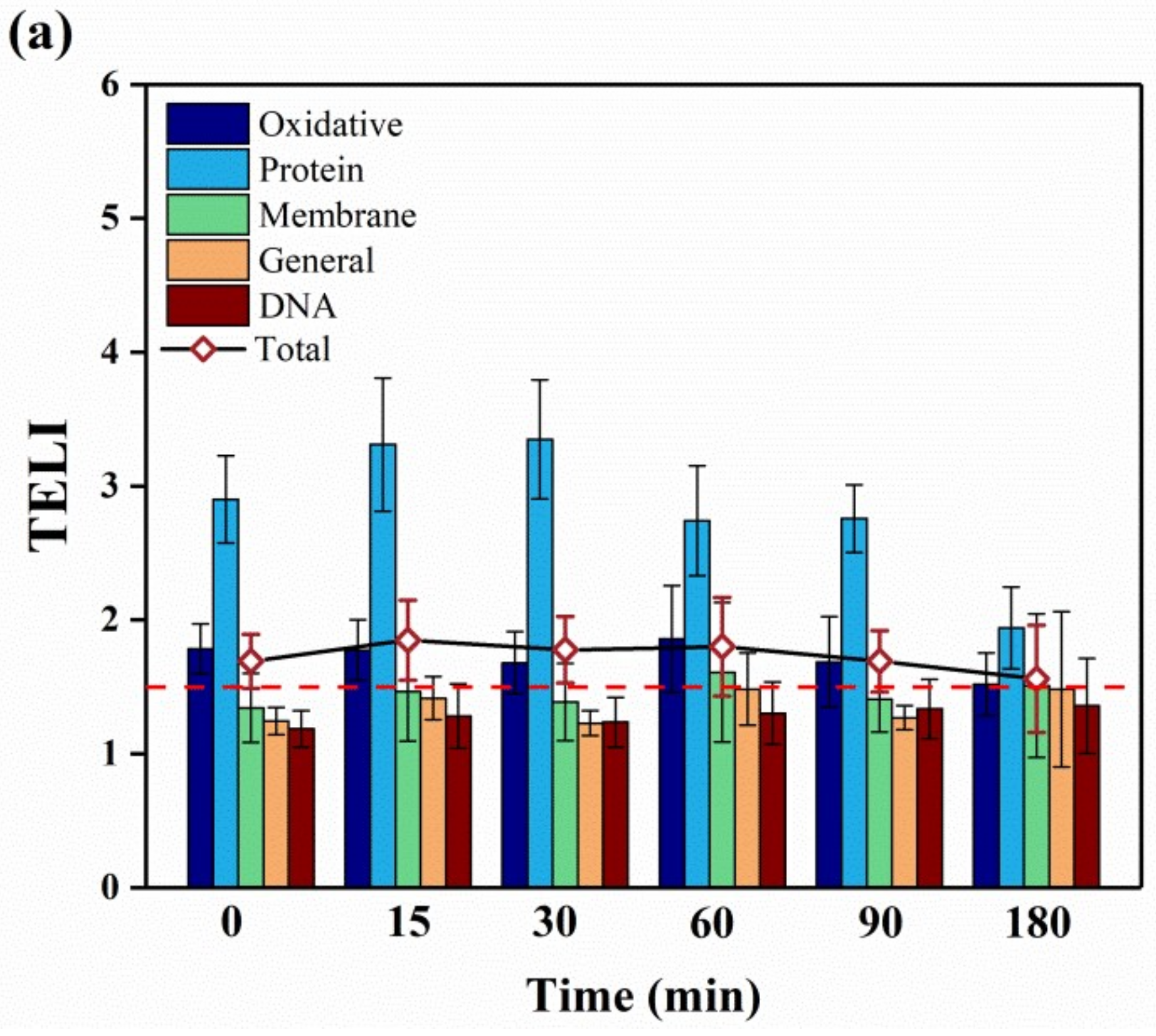
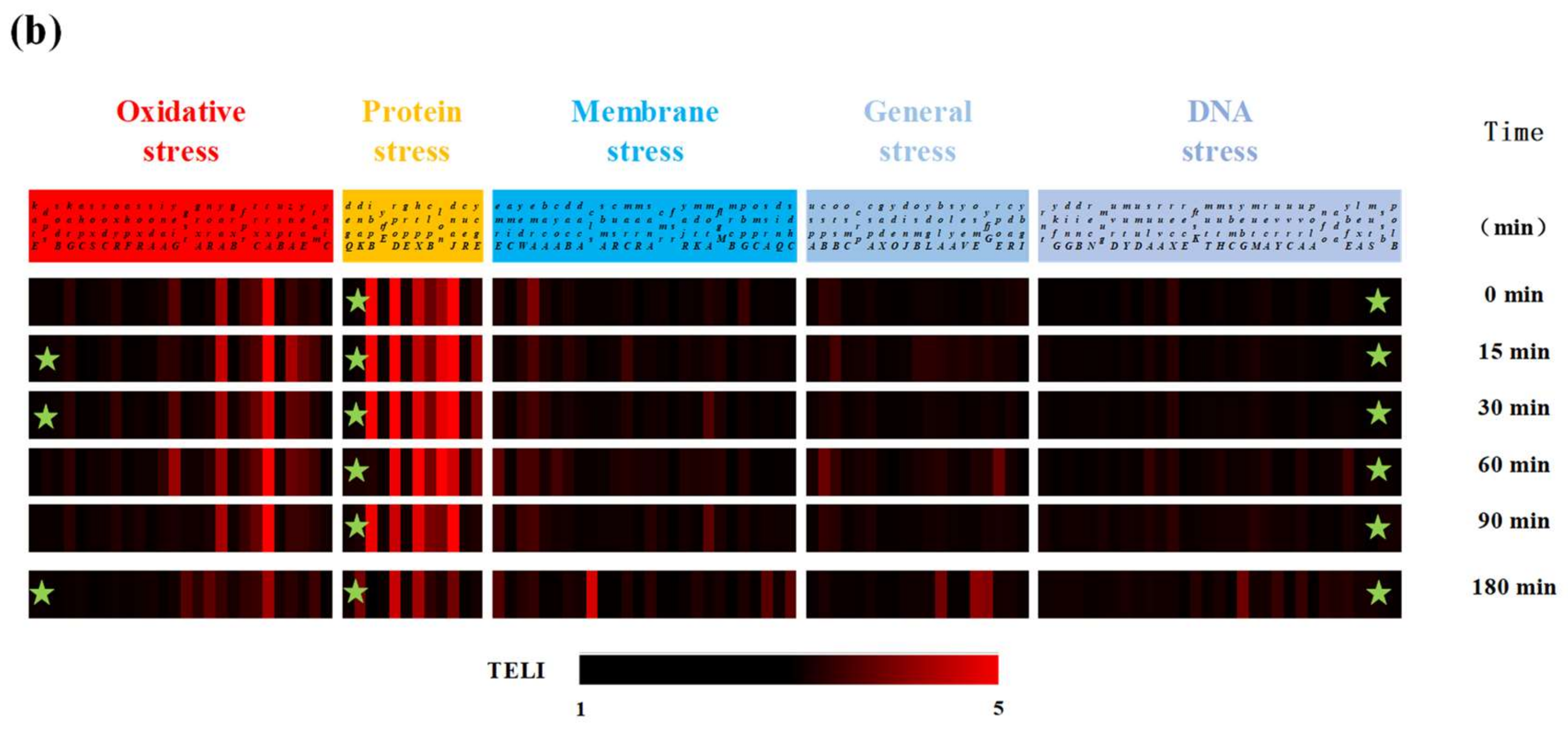
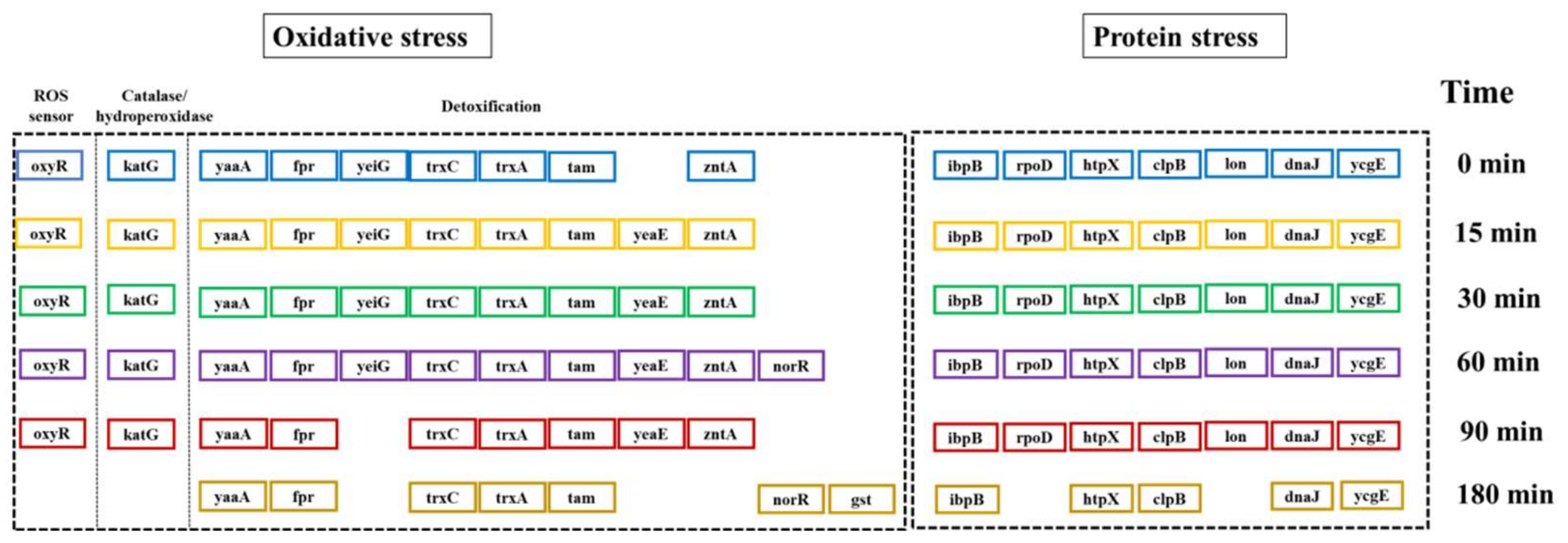
2.3. Molecular-Level Toxicity Evolution during HMX Degradation
2.3.1. Molecular Toxicity Potency
2.3.2. Molecular-Level Toxicity Effects
3. Materials and Methods
3.1. Chemicals
3.2. Experimental Design
3.3. Chemical Analysis
3.4. Radical Scavenger Experiment
3.5. Toxicity Analysis
3.6. Toxicogenomics Data Analysis
3.7. Cost Estimation
3.8. Data Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lyman, J.L.; Liau, Y.-C.; Brand, H.V. Thermochemical functions for gas-phase, 1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetraazacyclooctane (HMX), its condensed phases, and its larger reaction products. Combust. Flame 2002, 130, 185–203. [Google Scholar] [CrossRef]
- Yang, X.; Lai, J.-l.; Li, J.; Zhang, Y.; Luo, X.-G.; Han, M.-W.; Zhu, Y.-B.; Zhao, S.-P. Biodegradation and physiological response mechanism of Bacillus aryabhattai to cyclotetramethylenete-tranitramine (HMX) contamination. J. Environ. Manag. 2021, 288, 112247. [Google Scholar] [CrossRef] [PubMed]
- McMurry, S.T.; Jones, L.E.; Smith, P.; Cobb, G.; Anderson, T.; Lovern, M.B.; Cox, S.; Pan, X. Accumulation and effects of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) exposure in the green anole (Anolis carolinensis). Ecotoxicology 2012, 21, 304–314. [Google Scholar] [CrossRef]
- USEPA. Integrated Risk Information System (IRIS); Chemical Assessment Summary; USEPA: Washington, DC, USA, 2016.
- Savard, K.; Berthelot, Y.; Auroy, A.; Spear, P.A.; Trottier, B.; Robidoux, P.Y. Effects of HMX-lead mixtures on reproduction of the earthworm Eisenia Andrei. Arch. Environ. Contam. Toxicol. 2007, 53, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Hawari, J.; Thiboutot, S.; Ampleman, G.; Sunahara, G. Toxicity of octahydro-1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetrazocine (HMX) to soil microbes. Bull. Environ. Contam. Toxicol. 2002, 69, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Payne, Z.M.; Lamichhane, K.M.; Babcock, R.W.; Turnbull, S.J. Pilot-scale in situ bioremediation of HMX and RDX in soil pore water in Hawaii. Environ. Sci. Processes Impacts 2013, 15, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Panja, S.; Sarkar, D.; Datta, R. Vetiver grass (Chrysopogon zizanioides) is capable of removing insensitive high explosives from munition industry wastewater. Chemosphere 2018, 209, 920–927. [Google Scholar] [CrossRef]
- Adrian, N.R.; Arnett, C.M. Anaerobic biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by Acetobacterium malicum strain HAAP-1 isolated from a methanogenic mixed culture. Curr. Microbiol. 2004, 48, 332–340. [Google Scholar] [CrossRef]
- Bhatt, M.; Zhao, J.-S.; Monteil-Rivera, F.; Hawari, J. Biodegradation of cyclic nitramines by tropical marine sediment bacteria. J. Ind. Microbiol. Biotechnol. 2005, 32, 261–267. [Google Scholar] [CrossRef]
- Singh, R.; Singh, A. Biodegradation of military explosives RDX and HMX. In Microbial Degradation of Xenobiotics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 235–261. [Google Scholar]
- Zhao, J.-S.; Greer, C.W.; Thiboutot, S.; Ampleman, G.; Hawari, J. Biodegradation of the nitramine explosives hexahydro-1, 3, 5-trinitro-1,3,5-triazine and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine in cold marine sediment under anaerobic and oligotrophic conditions. Can. J. Microbiol. 2004, 50, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Kanekar, S.; Kanekar, P.; Sarnaik, S.; Gujrathi, N.; Shede, P.; Kedargol, M.; Reardon, K. Bioremediation of nitroexplosive wastewater by an yeast isolate Pichia sydowiorum MCM Y-3 in fixed film bioreactor. J. Ind. Microbiol. Biotechnol. 2009, 36, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Shang, K.; Wang, X.; Li, J.; Wang, H.; Lu, N.; Jiang, N.; Wu, Y. Synergetic degradation of Acid Orange 7 (AO7) dye by DBD plasma and persulfate. Chem. Eng. J. 2017, 311, 378–384. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Z.; Hong, Y.; Chelme-Ayala, P.; Meng, L.; Wu, Z.; El-Din, M.G. The treatment of electroplating wastewater using an integrated approach of interior microelectrolysis and Fenton combined with recycle ferrite. Chemosphere 2022, 286, 131543. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, L.; Han, W.; Wang, L.; Sun, X.; Li, J. Treatment of high explosive production wastewater containing RDX by combined electrocatalytic reaction and anoxic–oxic biodegradation. Chem. Eng. J. 2011, 168, 1256–1262. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.; Brillas, E.; Vilar, V.J. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Martinez-Huitle, C.A.; Ferro, S. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Direct and mediated anodic oxidation of organic pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Moreira, F.C.; Soler, J.; Fonseca, A.; Saraiva, I.; Boaventura, R.A.; Brillas, E.; Vilar, V.J. Incorporation of electrochemical advanced oxidation processes in a multistage treatment system for sanitary landfill leachate. Water Res. 2015, 81, 375–387. [Google Scholar] [CrossRef]
- Dai Lam, T.; Van Chat, N.; Bach, V.Q.; Loi, V.D.; Van Anh, N. Simultaneous degradation of 2,4,6-trinitrophenyl-N-methylnitramine (Tetryl) and hexahydro-1,3,5-trinitro-1,3,5 triazine (RDX) in polluted wastewater using some advanced oxidation processes. J. Ind. Eng. Chem. 2014, 20, 1468–1475. [Google Scholar]
- Bonin, P.M.; Bejan, D.; Radovic-Hrapovic, Z.; Halasz, A.; Hawari, J.; Bunce, N.J. Indirect oxidation of RDX, HMX, and CL-20 cyclic nitramines in aqueous solution at boron-doped diamond electrodes. Environ. Chem. 2005, 2, 125–129. [Google Scholar] [CrossRef]
- Kacem, S.B.; Elaoud, S.C.; Asensio, A.M.; Panizza, M.; Clematis, D. Electrochemical and sonoelectrochemical degradation of Allura Red and Erythrosine B dyes with Ti-PbO2 anode. J. Electroanal. Chem. 2021, 889, 115212. [Google Scholar] [CrossRef]
- Song, S.; Zhan, L.; He, Z.; Lin, L.; Tu, J.; Zhang, Z.; Chen, J.; Xu, L. Mechanism of the anodic oxidation of 4-chloro-3-methyl phenol in aqueous solution using Ti/SnO2–Sb/PbO2 electrodes. J. Hazard. Mater. 2010, 175, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayanan, V.; Nakazawa, I.; Yoshihara, S.; Shirakashi, T. The influence of electrolyte media on the deposition/dissolution of lead dioxide on boron-doped diamond electrode–A surface morphologic study. J. Electroanal. Chem. 2006, 592, 175–182. [Google Scholar] [CrossRef]
- García-Gómez, C.; Drogui, P.; Seyhi, B.; Gortáres-Moroyoqui, P.; Buelna, G.; Estrada-Alvgarado, M.; Alvarez, L.H. Combined membrane bioreactor and electrochemical oxidation using Ti/PbO2 anode for the removal of carbamazepine. J. Taiwan Inst. Chem. Eng. 2016, 64, 211–219. [Google Scholar] [CrossRef]
- Wang, J.; Wang, N.; Nan, W.; Wang, C.; Chen, X.; Qi, X.; Yan, S.; Dai, S. Enhancement of electrochemical performance of LiCoO2 cathode material at high cut-off voltage (4.5 V) by partial surface coating with graphene nanosheets. Int. J. Electrochem. Sci. 2020, 15, 9282–9293. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.-S.; Spain, J.; Thiboutot, S.; Ampleman, G.; Greer, C.; Hawari, J. Phylogeny of cyclic nitramine-degrading psychrophilic bacteria in marine sediment and their potential role in the natural attenuation of explosives. FEMS Microbiol. Ecol. 2004, 49, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Hawari, J.; Halasz, A.; Beaudet, S.; Paquet, L.; Ampleman, G.; Thiboutot, S. Biotransformation routes of octahydro-1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetrazocine by municipal anaerobic sludge. Environ. Sci. Technol. 2001, 35, 70–75. [Google Scholar] [CrossRef]
- Mukhi, S.; Patiño, R. Effects of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) in zebrafish: General and reproductive toxicity. Chemosphere 2008, 72, 726–732. [Google Scholar] [CrossRef]
- Nipper, M.; Carr, R.; Biedenbach, J.; Hooten, R.; Miller, K.; Saepoff, S. Development of marine toxicity data for ordnance compounds. Arch. Environ. Contam. Toxicol. 2001, 41, 308–318. [Google Scholar] [CrossRef]
- Gou, N.; Onnis-Hayden, A.; Gu, A.Z. Mechanistic toxicity assessment of nanomaterials by whole-cell-array stress genes expression analysis. Environ. Sci. Technol. 2010, 44, 5964–5970. [Google Scholar] [CrossRef]
- Lin, Y.; Sevillano-Rivera, M.; Jiang, T.; Li, G.; Cotto, I.; Vosloo, S.; Carpenter, C.M.; Larese-Casanova, P.; Giese, R.W.; Helbling, D.E. Impact of Hurricane Maria on drinking water quality in Puerto Rico. Environ. Sci. Technol. 2020, 54, 9495–9509. [Google Scholar] [CrossRef] [PubMed]
- El-Desoky, H.S.; Ghoneim, M.M.; Zidan, N.M. Decolorization and degradation of Ponceau S azo-dye in aqueous solutions by the electrochemical advanced Fenton oxidation. Desalination 2010, 264, 143–150. [Google Scholar] [CrossRef]
- Dai, Q.; Zhou, J.; Weng, M.; Luo, X.; Feng, D.; Chen, J. Electrochemical oxidation metronidazole with Co modified PbO2 electrode: Degradation and mechanism. Sep. Purif. Technol. 2016, 166, 109–116. [Google Scholar] [CrossRef]
- Samarghandi, M.R.; Ansari, A.; Dargahi, A.; Shabanloo, A.; Nematollahi, D.; Khazaei, M.; Nasab, H.Z.; Vaziri, Y. Enhanced electrocatalytic degradation of bisphenol A by graphite/β-PbO2 anode in a three-dimensional electrochemical reactor. J. Environ. Chem. Eng. 2021, 9, 106072. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Z.; Chen, X. Effects of experimental parameters on 2,4-dichlorphenol degradation over Er-chitosan-PbO2 electrode. J. Hazard. Mater. 2010, 178, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Klidi, N.; Clematis, D.; Delucchi, M.; Gadri, A.; Ammar, S.; Panizza, M. Applicability of electrochemical methods to paper mill wastewater for reuse. Anodic oxidation with BDD and TiRuSnO2 anodes. J. Electroanal. Chem. 2018, 815, 16–23. [Google Scholar] [CrossRef]
- Zhu, X.; Hu, W.; Feng, C.; Chen, N.; Chen, H.; Kuang, P.; Deng, Y.; Ma, L. Electrochemical oxidation of aniline using Ti/RuO2-SnO2 and Ti/RuO2-IrO2 as anode. Chemosphere 2021, 269, 128734. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Q.; Ni, J.; Xu, Y.; Song, N.; Gao, M. Highly efficient removal of amoxicillin from water by three-dimensional electrode system within granular activated carbon as particle electrode. J. Water Process Eng. 2020, 38, 101656. [Google Scholar] [CrossRef]
- Wu, H.; Xie, H.; He, G.; Guan, Y.; Zhang, Y. Effects of the pH and anions on the adsorption of tetracycline on iron-montmorillonite. Appl. Clay Sci. 2016, 119, 161–169. [Google Scholar] [CrossRef]
- Zaky, A.M.; Chaplin, B.P. Mechanism of p-substituted phenol oxidation at a Ti4O7 reactive electrochemical membrane. Environ. Sci. Technol. 2014, 48, 5857–5867. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.; Yao, B.; Yang, J.; Zhi, D. Current progress in electrochemical anodic-oxidation of pharmaceuticals: Mechanisms, influencing factors, and new technique. J. Hazard. Mater. 2021, 418, 126313. [Google Scholar] [CrossRef] [PubMed]
- Liou, M.-J.; Lu, M.-C.; Chen, J.-N. Oxidation of explosives by Fenton and photo-Fenton processes. Water Res. 2003, 37, 3172–3179. [Google Scholar] [CrossRef]
- Xiang, H.; Xiao, S.; Zhang, G.; Song, Y.; Cui, P.; Shao, H.; Li, H. Treatment of simulated berberine pharmaceutical wastewater by electrochemical oxidation process. J. Environ. Eng. 2011, 5, 5. [Google Scholar]
- Lin, H.; Niu, J.; Xu, J.; Li, Y.; Pan, Y. Electrochemical mineralization of sulfamethoxazole by Ti/SnO2-Sb/Ce-PbO2 anode: Kinetics, reaction pathways, and energy cost evolution. Electrochim. Acta 2013, 97, 167–174. [Google Scholar] [CrossRef]
- Lei, L.; Dai, Q.; Zhou, M.; Zhang, X. Decolorization of cationic red X-GRL by wet air oxidation: Performance optimization and degradation mechanism. Chemosphere 2007, 68, 1135–1142. [Google Scholar] [CrossRef]
- Radha, K.; Sridevi, V.; Kalaivani, K. Electrochemical oxidation for the treatment of textile industry wastewater. Bioresour. Technol. 2009, 100, 987–990. [Google Scholar] [CrossRef]
- Dargahi, A.; Barzoki, H.R.; Vosoughi, M.; Mokhtari, S.A. Enhanced electrocatalytic degradation of 2, 4-Dinitrophenol (2, 4-DNP) in three-dimensional sono-electrochemical (3D/SEC) process equipped with Fe/SBA-15 nanocomposite particle electrodes: Degradation pathway and application for real wastewater. Arab. J. Chem. 2022, 15, 103801. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Yang, W.; Chen, X.; Li, W.; Luo, B.; Wang, K. Preparation of 3D PbO2 nanospheres@ SnO2 nanowires/Ti electrode and its application in methyl orange degradation. Electrochim. Acta 2014, 146, 15–22. [Google Scholar] [CrossRef]
- Neto, S.A.; De Andrade, A. Electrooxidation of glyphosate herbicide at different DSA® compositions: pH, concentration and supporting electrolyte effect. Electrochim. Acta 2009, 54, 2039–2045. [Google Scholar] [CrossRef]
- Dargahi, A.; Vosoughi, M.; Mokhtari, S.A.; Vaziri, Y.; Alighadri, M. Electrochemical degradation of 2, 4-Dinitrotoluene (DNT) from aqueous solutions using three-dimensional electrocatalytic reactor (3DER): Degradation pathway, evaluation of toxicity and optimization using RSM-CCD. Arab. J. Chem. 2022, 15, 103648. [Google Scholar] [CrossRef]
- Rahmani, A.; Leili, M.; Seid-Mohammadi, A.; Shabanloo, A.; Ansari, A.; Nematollahi, D.; Alizadeh, S. Improved degradation of diuron herbicide and pesticide wastewater treatment in a three-dimensional electrochemical reactor equipped with PbO2 anodes and granular activated carbon particle electrodes. J. Clean. Prod. 2021, 322, 129094. [Google Scholar] [CrossRef]
- Li, C.; Xiong, K.; Li, D.; Liang, J.; Guo, B. Experimental study on treatment of antibiotic pharmaceutical wastewater by electro catalytic oxidation. Environ. Prot. Circ. Econ. 2017, 37, 5. [Google Scholar]
- Shao, D.; Wang, Z.; Zhang, C.; Li, W.; Xu, H.; Tan, G.; Yan, W. Embedding wasted hairs in Ti/PbO2 anode for efficient and sustainable electrochemical oxidation of organic wastewater. Chin. Chem. Lett. 2022, 33, 1288–1292. [Google Scholar] [CrossRef]
- Crocker, F.H.; Indest, K.J.; Fredrickson, H.L. Biodegradation of the cyclic nitramine explosives RDX, HMX, and CL-20. Appl. Microbiol. Biotechnol. 2006, 73, 274–290. [Google Scholar] [CrossRef] [PubMed]
- Fournier, D.; Halasz, A.; Thiboutot, S.; Ampleman, G.; Manno, D.; Hawari, J. Biodegradation of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) by Phanerochaete chrysosporium: New insight into the degradation pathway. Environ. Sci. Technol. 2004, 38, 4130–4133. [Google Scholar] [CrossRef] [PubMed]
- Anotai, J.; Tanvanit, P.; Garcia-Segura, S.; Lu, M.-C. Electro-assisted Fenton treatment of ammunition wastewater containing nitramine explosives. Process Saf. Environ. Prot. 2017, 109, 429–436. [Google Scholar] [CrossRef]
- Dugandžić, A.M.; Tomašević, A.V.; Radišić, M.M.; Šekuljica, N.Ž.; Mijin, D.Ž.; Petrović, S.D. Effect of inorganic ions, photosensitisers and scavengers on the photocatalytic degradation of nicosulfuron. J. Photochem. Photobiol. A Chem. 2017, 336, 146–155. [Google Scholar] [CrossRef]
- Sun, W.; Sun, Y.; Shah, K.J.; Chiang, P.-C.; Zheng, H. Electrocatalytic oxidation of tetracycline by Bi-Sn-Sb/γ-Al2O3 three-dimensional particle electrode. J. Hazard. Mater. 2019, 370, 24–32. [Google Scholar] [CrossRef]
- Yang, J.-S.; Lai, W.W.-P.; Panchangam, S.C.; Lin, A.Y.-C. Photoelectrochemical degradation of perfluorooctanoic acid (PFOA) with GOP25/FTO anodes: Intermediates and reaction pathways. J. Hazard. Mater. 2020, 391, 122247. [Google Scholar] [CrossRef]
- Keseler, I.M.; Bonavides-Martínez, C.; Collado-Vides, J.; Gama-Castro, S.; Gunsalus, R.P.; Johnson, D.A.; Krummenacker, M.; Nolan, L.M.; Paley, S.; Paulsen, I.T. EcoCyc: A comprehensive view of Escherichia coli biology. Nucleic Acids Res. 2009, 37, D464–D470. [Google Scholar] [CrossRef]
- Seaver, L.C.; Imlay, J.A. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 2001, 183, 7173–7181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seaver, L.C.; Imlay, J.A. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J. Bacteriol. 2001, 183, 7182–7189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeager, E. Electrocatalysts for O2 reduction. Electrochim. Acta 1984, 29, 1527–1537. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, X.; Templeton, L.J.; Smulski, D.R.; LaRossa, R.A.; Storz, G. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J. Bacteriol. 2001, 183, 4562–4570. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Bauer, S.C.; Imlay, J.A. The YaaA protein of the Escherichia coli OxyR regulon lessens hydrogen peroxide toxicity by diminishing the amount of intracellular unincorporated iron. J. Bacteriol. 2011, 193, 2186–2196. [Google Scholar] [CrossRef] [Green Version]
- Manchado, M.; Michán, C.; Pueyo, C. Hydrogen peroxide activates the SoxRS regulon in vivo. J. Bacteriol. 2000, 182, 6842–6844. [Google Scholar] [CrossRef] [Green Version]
- Burgess, R.R.; Travers, A.A.; Dunn, J.J.; Bautz, E.K. Factor stimulating transcription by RNA polymerase. Nature 1969, 221, 43–46. [Google Scholar] [CrossRef]
- Rosen, R.; Biran, D.; Gur, E.; Becher, D.; Hecker, M.; Ron, E.Z. Protein aggregation in Escherichia coli: Role of proteases. FEMS Microbiol. Lett. 2002, 207, 9–12. [Google Scholar] [CrossRef]
- Shineberg, B.; Zipser, D. The lon gene and degradation of β-galactosidase nonsense fragments. J. Bacteriol. 1973, 116, 1469–1471. [Google Scholar] [CrossRef] [Green Version]
- Gou, N.; Gu, A.Z. A new transcriptional effect level index (TELI) for toxicogenomics-based toxicity assessment. Environ. Sci. Technol. 2011, 45, 5410–5417. [Google Scholar] [CrossRef]
- Zeng, Q.; Zan, F.; Hao, T.; Biswal, B.K.; Lin, S.; van Loosdrecht, M.C.; Chen, G. Electrochemical pretreatment for stabilization of waste activated sludge: Simultaneously enhancing dewaterability, inactivating pathogens and mitigating hydrogen sulfide. Water Res. 2019, 166, 115035. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, Y.; Chen, K.; Chai, G.; Xi, P.; Yang, H.; Xie, L.; Qin, L.; Lin, Y.; Li, X.; Yan, W.; et al. Performance Optimization and Toxicity Effects of the Electrochemical Oxidation of Octogen. Catalysts 2022, 12, 815. https://doi.org/10.3390/catal12080815
Qian Y, Chen K, Chai G, Xi P, Yang H, Xie L, Qin L, Lin Y, Li X, Yan W, et al. Performance Optimization and Toxicity Effects of the Electrochemical Oxidation of Octogen. Catalysts. 2022; 12(8):815. https://doi.org/10.3390/catal12080815
Chicago/Turabian StyleQian, Yishi, Kai Chen, Guodong Chai, Peng Xi, Heyun Yang, Lin Xie, Lu Qin, Yishan Lin, Xiaoliang Li, Wei Yan, and et al. 2022. "Performance Optimization and Toxicity Effects of the Electrochemical Oxidation of Octogen" Catalysts 12, no. 8: 815. https://doi.org/10.3390/catal12080815
APA StyleQian, Y., Chen, K., Chai, G., Xi, P., Yang, H., Xie, L., Qin, L., Lin, Y., Li, X., Yan, W., & Wang, D. (2022). Performance Optimization and Toxicity Effects of the Electrochemical Oxidation of Octogen. Catalysts, 12(8), 815. https://doi.org/10.3390/catal12080815






