Abstract
Bimetallic catalysts have significantly contributed to the chemical community, especially in environmental science. In this work, a CoAl2O4 spinel bimetal oxide was synthesized by a facile co-precipitation method and used for the degradation of organic pollutants through peroxymonosulfate (PMS) activation. Compared with Co3O4, the as-prepared CoAl2O4 possesses a higher specific surface area and a larger pore volume, which contributes to its becoming increasingly conducive to the degradation of organic pollutants. Under optimal conditions (calcination temperature: 500 °C, catalyst: 0.1 g/L, and PMS: 0.1 g/L), the as-prepared CoAl2O4 catalyst could degrade over 99% of rhodamine B (RhB) at a degradation rate of 0.048 min−1, which is 2.18 times faster than Co3O4 (0.022 min−1). The presence of Cl− could enhance RhB degradation in the CoAl2O4/PMS system, while HCO3− and CO32− inhibit RhB degradation. Furthermore, the considerable reusability and universality of CoAl2O4 were testified. Through quenching tests, 1O2 and SO4•− were identified as the primary reactive species in RhB degradation. The toxicity evaluation verified that the degraded solution exhibited lower biological toxicity than the initial RhB solution. This study provides new prospects in the design of cost-effective and stable cobalt-based catalysts and promotes the application of PMS-based advanced oxidation processes for refractory wastewater treatment.
1. Introduction
A wide range of organic wastewater has been discharged into the natural water environment with the dramatic development of the printing and dyeing, textile, and medicine industries. These kinds of wastewater are characterized by stable composition, high pollutant concentration, massive variation of water quality and quantity, non-biodegradation [1], as well as high teratogenicity, carcinogenicity, and biotoxicity, seriously threatening human health and the aquatic ecosystem [2,3]. Conventional water treatment technologies, including adsorption [4], photocatalysis [5], and biological process [6], are generally plagued with drawbacks such as high operating cost, low degradation efficiency, and incomplete degradation [7]. Therefore, it is necessary to explore novel, low-cost, and efficient wastewater treatment techniques.
Recently, sulfate radical (SO4•−)-based advanced oxidation processes (SR-AOPs) have gained enormous attention in environmental remediation owing to their low cost and excellent oxidizing capability at a wide pH range [8,9]. SR-AOPs can generate SO4•− that exhibits a higher redox potential (E0 = 2.5–3.1 V) and a longer half-life (30–40 μs) than hydroxyl radical (•OH) and is independent of solution pH [10,11,12]. Among persulfate salts, peroxymonosulfate (PMS) with an asymmetric structure (HO−O−SO3−) and a longer superoxide bond (lo−o = 1.326 Å) can be more easily dissociated to generate more reactive oxygen species than peroxydisulfate (PDS) (lo−o = 1.322 Å) [13,14,15,16]. PMS can be activated in the presence of ultraviolet, heating, bases, ultrasound, electrochemical processes, and transition metal ions [17,18,19,20,21], among which, transition metal ions, especially Co2+ and cobalt oxides, demonstrate the best performance in PMS activation [22,23,24]. However, homogeneous PMS activation systems suffer from formidable separation and secondary pollution, while Co single−atom catalysts exhibit shortcomings of poor stability and low degradation efficiency, and these deficiencies remarkably restrict their practical application [25,26]. Thus, further exploration of efficient, stable, and reusable cobalt-based catalysts is still indispensable.
Bimetallic oxides have attracted extensive interest due to their dense active sites, synergistic effects between metal atoms, stable structure, and high catalytic activity [27,28,29]. For instance, Chen et al. [30] demonstrated that CuCo2O4 could effectively activate PMS for the degradation of sulfadiazine, which was superior to CuO and Co3O4, and the degradation efficiency was 98% within 30 min. Additionally, previous studies have shown that CoFe2O4 and NiCo2O4 exhibited enhanced mineralization of organic pollutants in the presence of PMS [31,32]. Since cobalt aluminate (CoAl2O4) possesses acid and alkali resistance, excellent chemical reactivity, prosperous optical properties, and thermal stability [33,34], it is extensively employed in the fields of high-temperature resistant coatings, plastics, ceramics, enamels, glass and coloring, paint, photocatalyst, and magnetic resonance imaging, etc. [35,36,37]. In our previous study [38], we synthesized CoAl2O4 by a combustion method, which exhibited remarkable performance in activating PMS to degrade organic pollutants. However, the synthesis of CoAl2O4 suffers from a complex process, high cost, and the degradation mechanism of PMS activation using CoAl2O4 needs further exploration.
Herein, CoAl2O4 was prepared via a cost-effective co-precipitation procedure and used as an efficient PMS activator to degrade multiple organic pollutants (rhodamine B (RhB), tetracycline hydrochloride (TCH), methylene blue (MB), acid orange (ARG), and acid orange (MO)) in an aqueous solution. The nanostructure, morphology, catalytic performance, university, and stability of CoAl2O4 were evaluated. A comprehensive study was conducted to determine the effect of various factors (including calcination temperature, catalyst dosage, PMS concentration, initial pH, and co-existing ions) on the CoAl2O4/PMS system against RhB. The degradation mechanism of CoAl2O4 by PMS activation was further clarified by the combination of quenching experiments and XPS analysis. Our study details insights into cobalt-based catalysts for efficient and sustainable remediation of organic pollutants.
2. Results and Discussion
2.1. Characterization of Catalysts
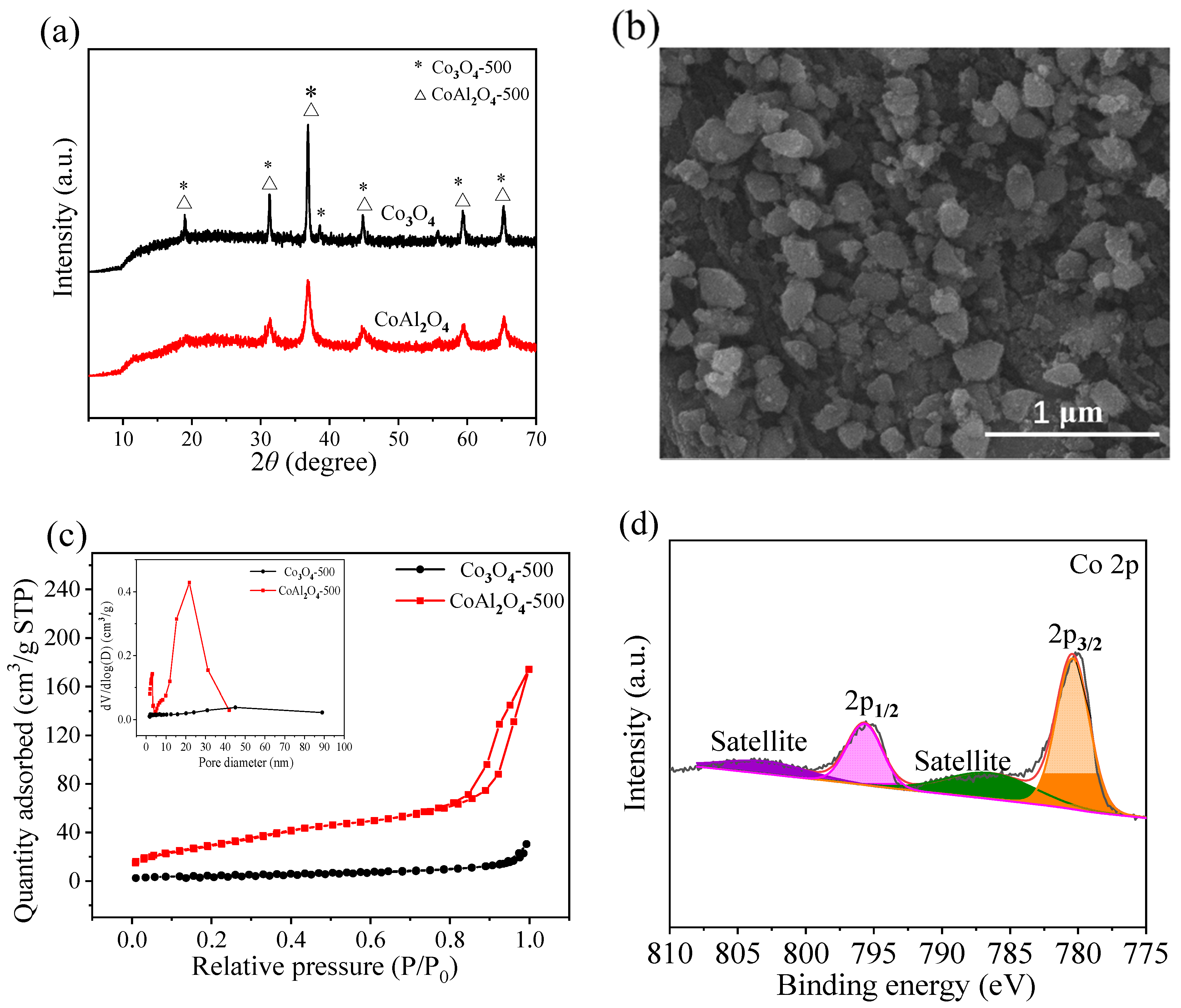
The XRD patterns of CoAl2O4-500 and Co3O4-500 prepared via the co-precipitation method are shown in Figure 1a. The characteristic peaks of CoAl2O4-500 appear at 2θ = 19.1°, 31.3°, 36.8°, 44.7°, 59.6°, and 65.3°, which correspond to the (111), (220), (311), (400), (511), and (440) lattice planes of CoAl2O4 (JCPDS No. 44-0160) [39]. The crystal size of CoAl2O4-500 was calculated to be 16.0 nm according to the Scherrer’s equation. A characteristic peak of Co3O4-500 is located at 2θ = 38.5°, which can be attributed to the (222) lattice planes of Co3O4 (JCPDS No. 43-1003) [40]. These results indicate that CoAl2O4 and Co3O4 were successfully synthesized.
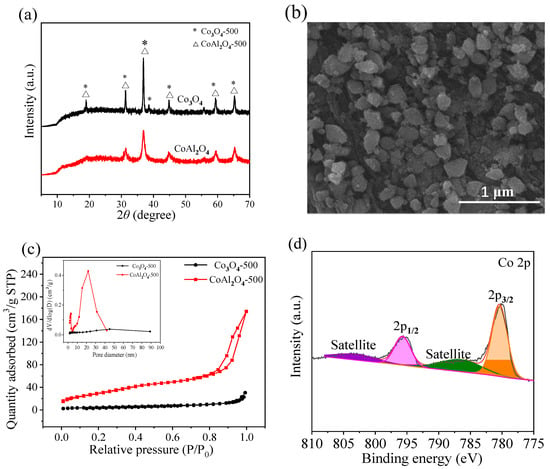
Figure 1.
(a) XRD patterns of CoAl2O4-500 and Co3O4-500, (b) SEM image of CoAl2O4-500, (c) N2 absorption-desorption isotherms and pore size distributions (inset) of CoAl2O4-500 and Co3O4-500, and (d) Co 2p XPS spectrum of CoAl2O4-500.
As illustrated in Figure 1b, the SEM micrograph shows the morphology of CoAl2O4-500. The CoAl2O4-500 particles presented irregular blocky morphology with a relatively smooth surface and a non-uniform particle size distribution ranging from 0.1 to 0.4 μm. It was granular with a coarse surface and dispersed distribution.
N2 adsorption–desorption isotherms were employed to determine the specific surface area and total pore volume of CoAl2O4-500 and Co3O4-500. As explicated in Figure 1c, the typical type IV profiles with H3 type hysteresis loop for CoAl2O4-500, according to the IUPC classification, indicate the presence of well-defined mesoporous structure in CoAl2O4-500 [41]. It could be seen that the pore size of Co3O4-500 was radically distributed in the range of 40–50 nm (inset in Figure 1c), while the pore structure of CoAl2O4-500 was primarily at the mesoporous region in the range of 2–4 nm and 20–25 nm. Compared with Co3O4-500, CoAl2O4-500 possesses a higher specific surface area (112.90 vs. 16.88 m2/g) and a larger total pore volume (0.2694 vs. 0.0470 cm3/g). Such a high surface area and porous nature of CoAl2O4-500 could potentially provide a higher quantity of active sites, lower mass transport resistance, and enhance PMS activation whereas vice in the case of Co3O4-500 [42].
The chemical state of Co was investigated via XPS analysis. The Co 2p spectrum possesses two major peaks at around 780.4 and 795.7 eV (Figure 1d), which are assigned to Co 2p3/2 and Co 2p1/2 peaks, respectively [43,44]. The peaks at 786.4 and 803.0 eV are indexed to the shake-up satellite of Co 2p3/2 and Co 2p1/2 peaks, respectively [45]. The Co 2p3/2 and Co 2p1/2 peaks for CoAl2O4-500 present a splitting value of 15.3 eV assigned to Co2+ [46,47]. There is no peak around 780.0 eV, implying the absence of Co2O3 or Co3O4 phases [48].
2.2. Catalytic Performance of Catalysts
2.2.1. Effect of Different Reaction Systems
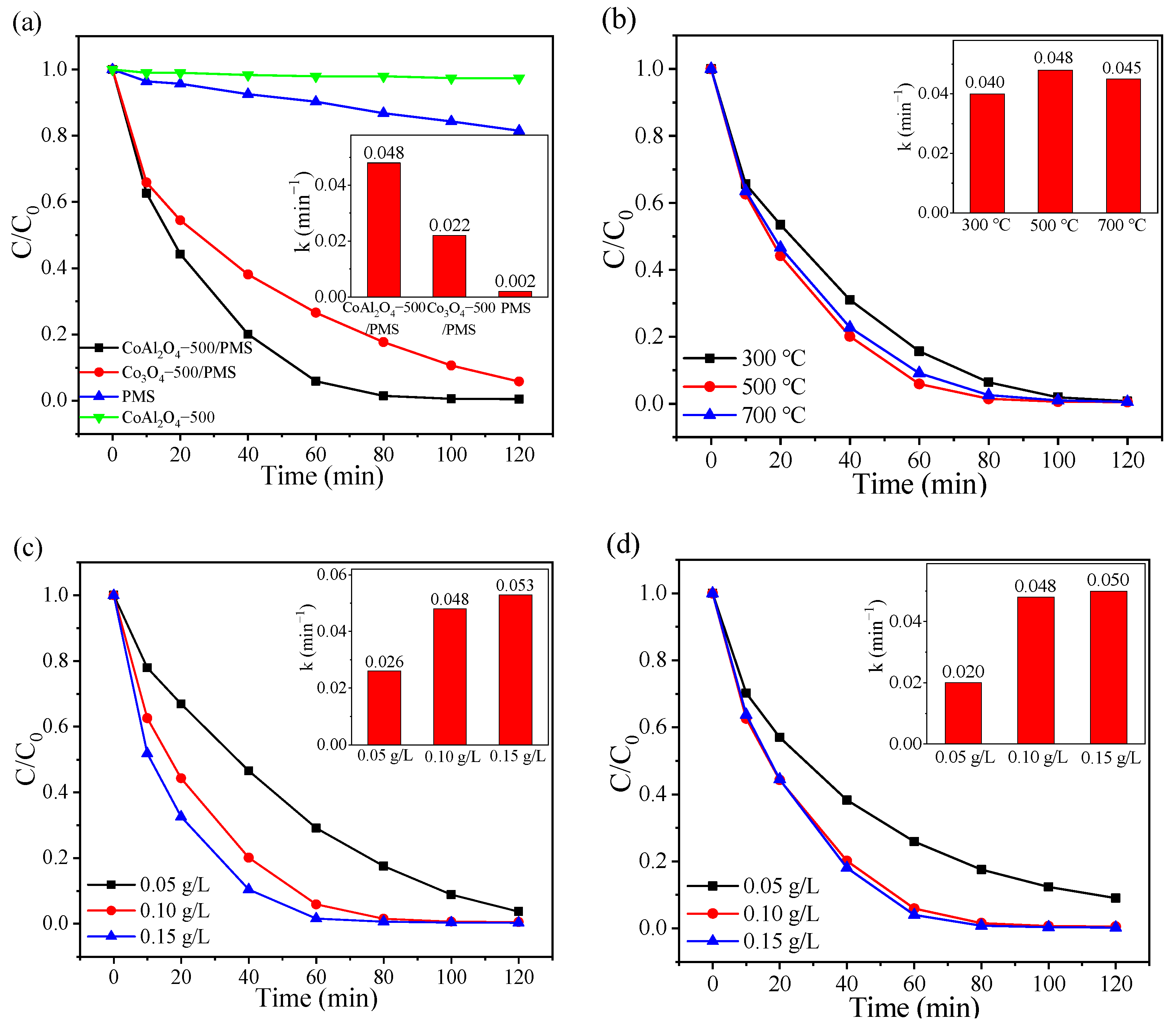
RhB, an artificial synthetic dye and a common component in dye wastewater, was employed as the simulated pollutant to investigate the catalytic performance of CoAl2O4 for PMS activation. As illustrated in Figure 2a, RhB can be barely degraded without the presence of PMS and PMS could oxidize only 18.46% of RhB within 120 min without adding a catalyst. In contrast, PMS was effectively activated by CoAl2O4-500 with 99% RhB elimination efficiency after 100 min, which was much higher than that of Co3O4-500. Meanwhile, the degradation rate constant of the CoAl2O4-500/PMS system was about 2.18 times higher than that of the Co3O4-500/PMS system (inset in Figure 2a). Moreover, the degradation of RhB by catalytic activation of PMS with bimetallic materials in recent years was summarized. As shown in Table 1, CoAl2O4 exhibited better catalytic performance for PMS activation at a wide pH range than the other bimetallic oxides.
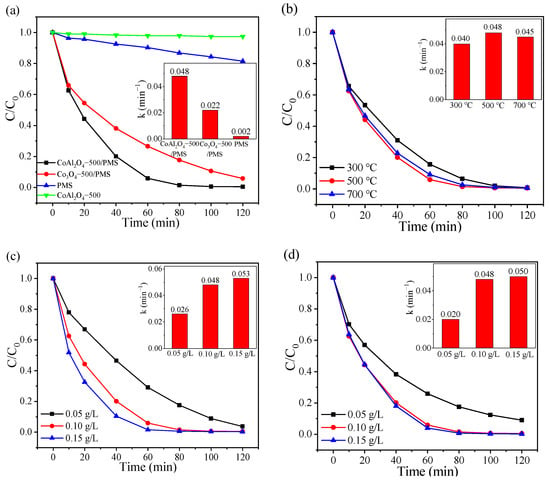
Figure 2.
(a) RhB degradation in various systems; effects of (b) calcination temperature; (c) catalyst dosage, and (d) PMS dosage on the RhB degradation and their corresponding pseudo-first-order model kinetic results (inset) in the CoAl2O4-500/PMS system.

Table 1.
Comparison of RhB degradation by bimetallic oxides through PMS activation.
2.2.2. Effect of Calcination Temperature
Figure 2b displays the degradation rate of RhB over the CoAl2O4 samples calcined at different temperatures. Notably, CoAl2O4-300, CoAl2O4-500, and CoAl2O4-700 attained 84.32%, 94.00%, and 90.82% RhB degradation in 60 min with the rate constants of 0.040, 0.048, and 0.045 min−1 (inset in Figure 2b), respectively. The results indicate that the degradation of RhB was slightly influenced by various calcination temperatures, and CoAl2O4-500 exhibited the best catalytic performance in RhB degradation. This might be because, with the increase in calcination temperature, the crystallinity and stability of the catalyst were enhanced [52]. However, when the calcination temperature was further elevated from 500 °C to 700 °C, the active components were over-sintered, and the agglomerated particle diameter size increased, inducing the reduction in active sites for PMS activation to degrade RhB.
2.2.3. Effect of Catalyst Dosage
Reasonable control of catalyst dosage can avoid side reactions and metal dissolution caused by excessive addition, thereby avoiding the degradation efficiency decline and secondary pollution. As observed in Figure 2c, when the dosage of CoAl2O4-500 increased from 0.05 to 0.10 g/L, the degradation efficiency of RhB increased from 96.27% after 120 min to 98.51% within 80 min, while the corresponding reaction rate constant of RhB noticeably increased from 0.026 to 0.048 min−1 (inset in Figure 2c). Increasing the catalyst dosage provides a higher total surface area and availability of abundant active sites on the surface of the catalyst for PMS activation to yield more reactive oxygen species [53]. When the catalyst dosage further increased to 0.15 g/L, RhB was almost completely degraded within 80 min, but the degradation rate increased insignificantly. As the fixed PMS dosage, the amount of generated active species is assumed constant. Hence, 0.10 g/L was selected as the optimized amount of CoAl2O4-500.
2.2.4. Effect of PMS Dosage
The effect of oxidant amount is also crucial, as this is the producer of active species and directly affects the degradation efficiency. As displayed in Figure 2d, when the PMS concentration increased from 0.05 g/L to 0.10 g/L, the degradation efficiency of RhB enhanced from 91.05% at 120 min to 94.13% within 60 min, and the rate constant gradually increased from 0.020 to 0.048 min−1 (inset in Figure 2d). The positive correlation between PMS dosage and RhB removal could be assigned to the more active species activated from increased PMS that facilitate RhB degradation [54]. However, no remarkable enhancement was observed when PMS concentration was further increased to 0.15 g/L. Although increased PMS could generate more oxidant species, the excess PMS would compete with target compounds for the reactive oxygen species [55]. Hence, the follow-up experiments were conducted with 0.10 g/L PMS as an optimum dosage for achieving the highest RhB degradation rate in the CoAl2O4/PMS system.
2.2.5. Effect of Initial pH
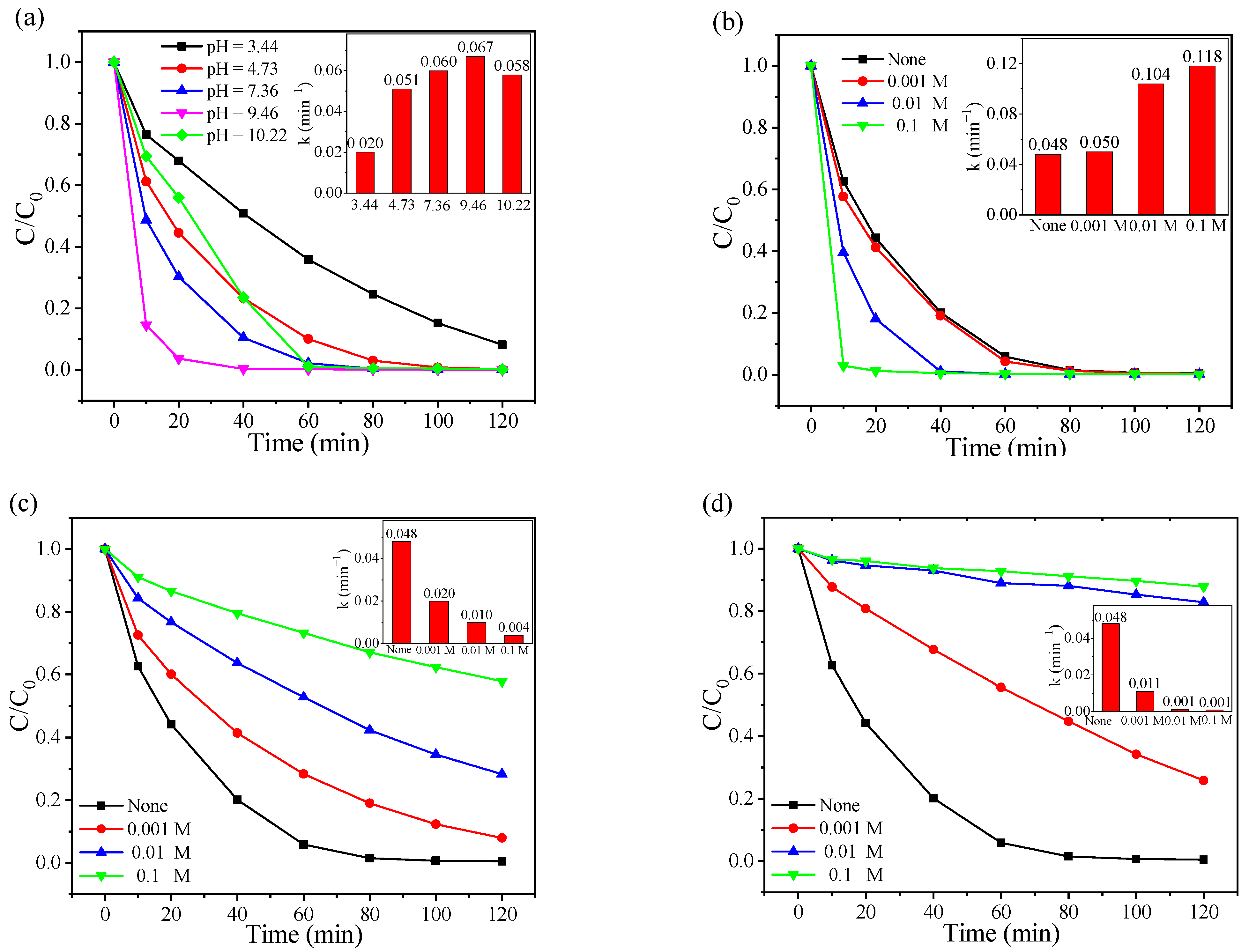
The pH in wastewater occasionally varies rapidly, and pH plays a key factor affecting physicochemical properties of reactants, possess of radical generation as well as the speciation of PMS [30]. Therefore, the effect of the solution pH on the CoAl2O4-500/PMS system was investigated over a wide range of pH from 3.44 to 10.22. As indicated in Figure 3a, the CoAl2O4-500/PMS system presented appreciable dramatical RhB degradation efficiency in a wide pH range, and the optimal pH range of the system was neutral or weak alkaline. Complete RhB degradation was achieved only in 20 min at pH = 9.46, and the reaction rate constants were 3.35, 1.31, 1.12 and 1.16 times of those at pH = 3.44, 4.73, 7.36 and 10.22, respectively (inset in Figure 3a). When the solution pH was 10.22, the degradation efficiency dramatically decreased, which might be because both OH− and H2O consumed SO4•− to produce •OH with lower activity and a shorter lifetime [56]. Meanwhile, Co(OH)2 might also be formed on the catalyst surface under strongly alkaline conditions [57], thus inhibiting the catalytic performance. When the solution pH was 3.44, the degradation efficiency of RhB was 91.87% at 120 min. In acidic pH conditions, strong H−bound could be formed between H+ and the O−O group in PMS (stabilization effect) [58,59], thus hindering the interaction between CoAl2O4-500 and PMS. Additionally, PMS is mainly present in the form of H2SO5 under strongly acidic conditions, rather than HSO5− [60], thus the activation of PMS could be inhibited and the degradation efficiency under acidic conditions was lower than that under alkaline conditions.
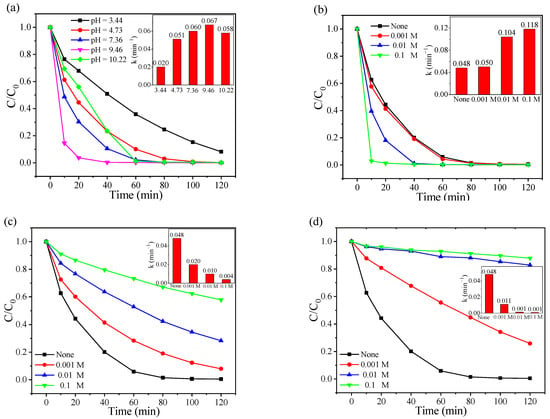
Figure 3.
Effects of (a) initial pH and different anions (b) Cl−, (c) HCO3−, and (d) CO32− on RhB degradation and their corresponding pseudo-first-order model kinetic results (inset) in the CoAl2O4-500/PMS system.
2.2.6. Effect of Different Anions
Inorganic anions are widely presented at various concentrations in actual wastewater, which also react with the reactive oxygen species and affect the catalytic reactions in the PMS oxidant system. Figure 3b–d reveal the effects of Cl−, HCO3−, and CO32− on the degradation efficiency of RhB in the CoAl2O4/PMS system, respectively. Compared with the oxidation system in the absence of inorganic anions, Cl− markedly facilitated the catalytic process, while HCO3− and CO32− inhibited the RhB degradation. As exhibited in Figure 3b, 97.13% of RhB removal occurred in the presence of 0.1 M of Cl− after 10 min, and the reaction rate constant was 2.46 times that without Cl− (inset in Figure 3b). Generally, in the presence of Cl−, the sulfate radical-based system is prone to form halogen radicals (e.g., Cl2•−, Cl•) with a higher oxidative potential. Cl2•−and Cl• can react to generate Cl2, further producing strong oxidizing ClO− [61]. With the addition of 0.1 M of HCO3− and CO32−, the RhB degradation declined from 99.51% to 42.13% and 12.15% within 120 min, respectively. The reaction rate constants without anions were 12 and 53 times of those with HCO3− and CO32−, respectively (inset in Figure 3c,d). Additionally, this phenomenon can be attributed to the reactive radicals scavenging ability of HCO3− [62], while CO32− can not only directly quench SO4•− but also quench •OH and SO4•− through hydrolysis to generate HCO3− [63]. Therefore, both HCO3− and CO32− can inhibit the degradation of the system, and the inhibitory effect of CO32− is more intense than that of HCO3−.
2.3. Versatility and Reusability of CoAl2O4
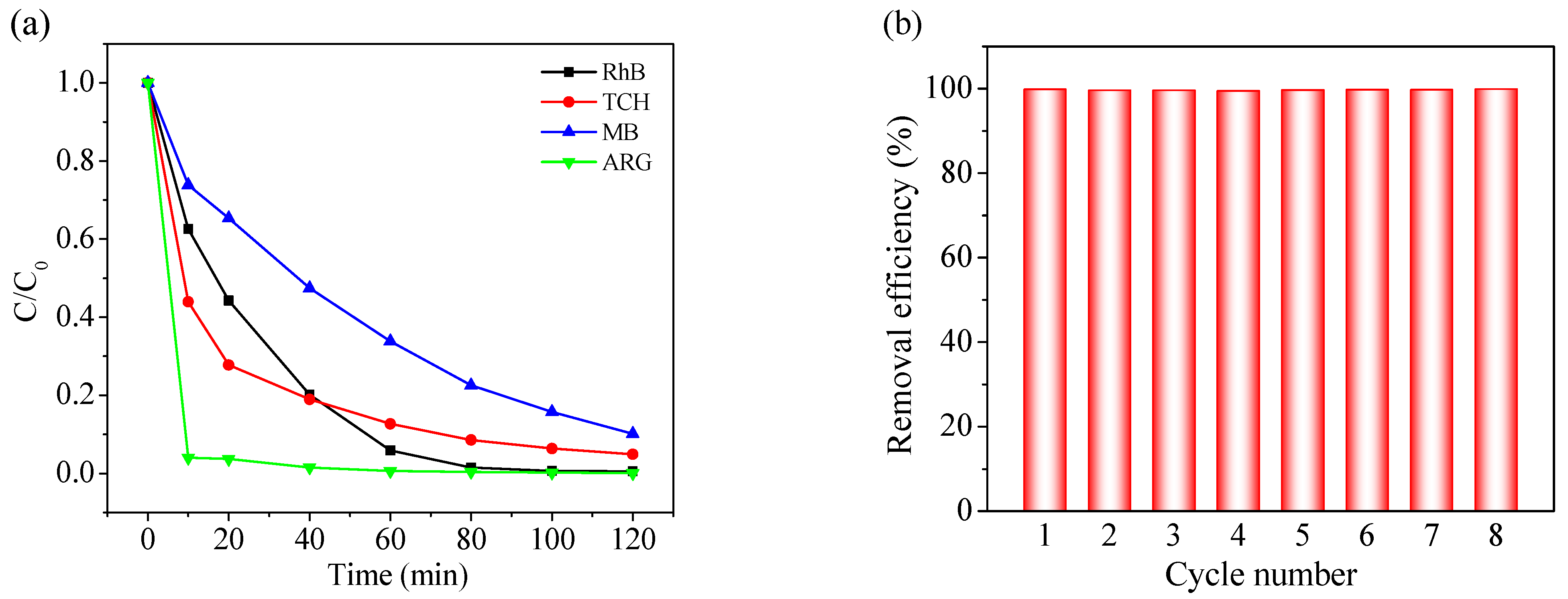
Versatility is one of the most crucial parameters for a catalyst with regard to its practical application. Therefore, to further explore the adaptability of CoAl2O4-500, the degradation efficiencies of TCH (an antibiotic), MB (a cationic dye), and ARG (an azo dye) by the CoAl2O4-500/PMS system were also evaluated. It can be seen from Figure 4a that the degradation efficiency of TCH and MB achieved 95.0% and 89.9% within 120 min, respectively, while ARG was basically completely degraded after 60 min. These results show that CoAl2O4-500 exhibits excellent degradation performance on TCH, MB, and ARG, indicating that the catalyst possesses remarkable universality. The results also reveal that CoAl2O4-500 as a heterogeneous catalyst has enormous application potential in the practical treatment of activated PMS degradation of most refractory organic wastewater.
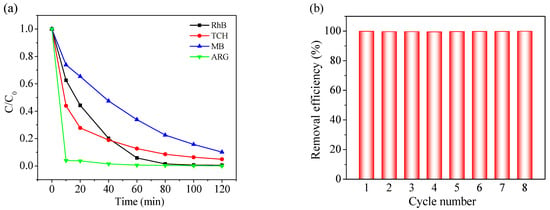
Figure 4.
(a) Degradation of TCH, MB, and ARG in the CoAl2O4-500/PMS system, (b) cycling experiments of CoAl2O4-500 toward RhB degradation in the presence of PMS.
For sustainable application in industrial fields, the stability and reusability of a catalyst are vital indicators. Therefore, the reusability of CoAl2O4-500 was evaluated by performing RhB degradation with the recovered catalyst. As demonstrated in Figure 4b, the regenerated CoAl2O4-500 provided complete RhB oxidation in eight consecutive cycles without significant loss of catalytic activity. In the CoAl2O4-500/PMS system, the concentration of the leached cobalt ions was determined to be 0.29 mg/L, which is lower than that in the Co3O4-500/PMS system (0.35 mg/L) and below the permissible limit of cobalt in livestock watering (1 mg/L) [64]. These results confirm that CoAl2O4-500 possesses excellent recyclability and practical application potential for wastewater treatment.
2.4. Activation Mechanism
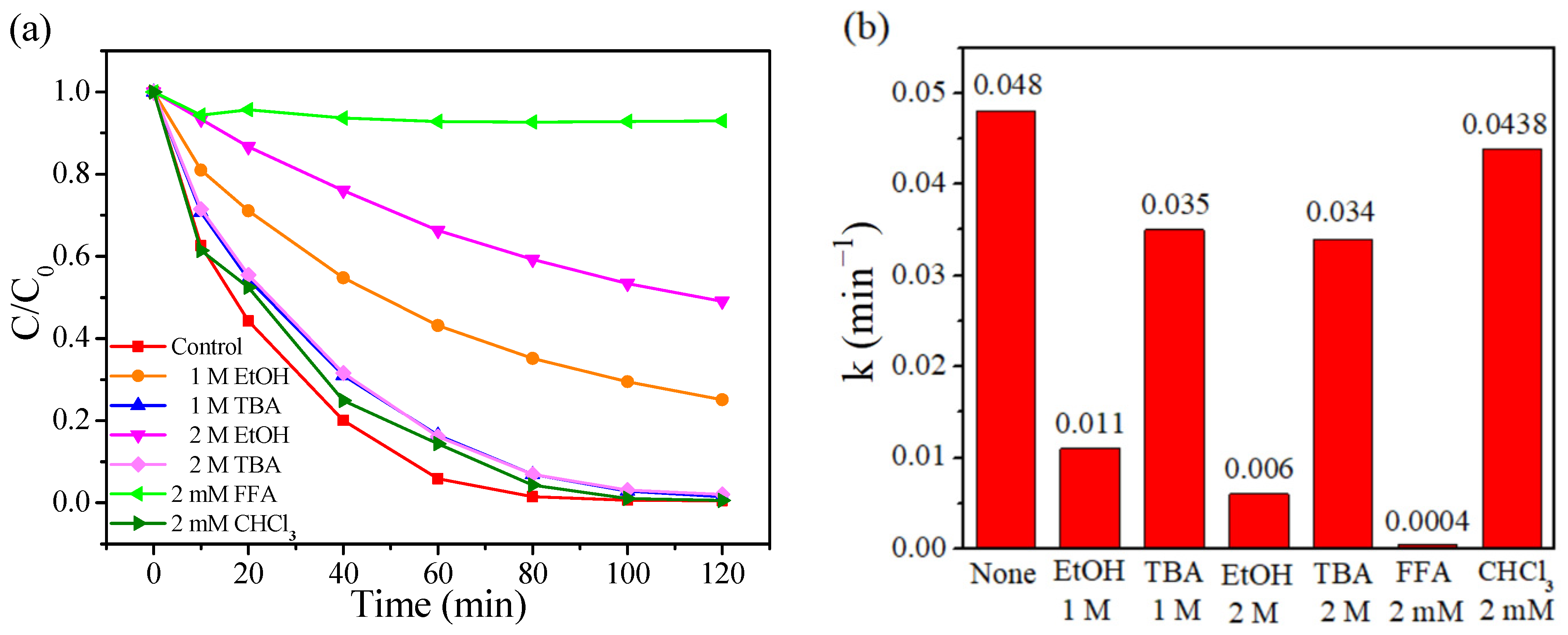
Quenching tests employing anhydrous ethanol (C2H5OH, EtOH), tertiary butanol (C4H9OH, TBA), trichloromethane (CHCl3), and furfuryl alcohol (FFA) as scavengers have been conducted to determine the dominated reactive oxygen species in the CoAl2O4-500/PMS system. EtOH can be used as a scavenger for both SO4•− and •OH, while TBA is only efficient for quenching •OH, CHCl3 is employed to verify the role of O2•−, and FFA is used to verify the contribution of 1O2 [65,66]. As depicted in Figure 5a, in the presence of 1 M of EtOH, the removal rate of RhB dropped from 99.76% to 74.92% after 120 min. When further increasing EtOH concentration from 1 to 2 M, the removal efficiency was suppressed dramatically, only 50.96% of RhB was degraded after 120 min. When 1 M of TBA was added, the reaction system degraded 98.49% of RhB after 120 min and the RhB removal efficiency was still higher than that of 1 M of EtOH. Nevertheless, with the concentration of TBA further increased, no enhanced inhibition of TBA toward RhB removal was observed, verifying that •OH exhibited limited suppression. However, stronger suppression to RhB removal was observed for 2 mM FFA compared to the case of 2 M of EtOH or 2 mM of CHCl3, whose reaction rate constant was the lowest (0.0004 min−1) (Figure 5b). This implies that 1O2 played a more significant role in RhB removal than SO4•− or O2•−. These results implied that O2•−, 1O2, SO4•− and •OH simultaneously contributed to the RhB degradation in the CoAl2O4-500/PMS system, while SO4•− and 1O2 were evidenced to be the decisive reactive oxygen species in RhB degradation.
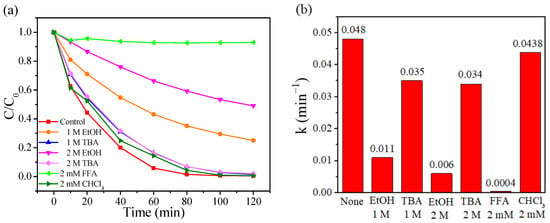
Figure 5.
(a) Effects of different capture agents for RhB degradation in the CoAl2O4-500/PMS system, (b) comparison of the reaction rate constant under different capture agents.
Based on the above analysis and discussion, the electron transfer between Co atoms in CoAl2O4-500 was the primary process for PMS activation by CoAl2O4-500 in the decomposition of RhB. Specifically, ≡Co(III)/≡Co(II) serve as primary catalytic sites in PMS activation, which are involved in the activation of PMS to generate SO4•−, 1O2, and SO5•− to degrade organic pollutants [67]. The specific reaction mechanism can be summarized as follows: firstly, RhB molecules are adsorbed to the surface by entering the mesopores of CoAl2O4-500. Subsequently, ≡Co(II) reacts with PMS on the surface of CoAl2O4-500 to generate 1O2 and SO4•− (Equations (1)–(3)) [68]. H2O2 and 1O2 are formed via the reaction of H2O and PMS (Equations (4) and (5)). Then, the reactions of H2O2 and ≡Co(III) result in the formation of 1O2 (Equations (6) and (7)) [69]. Then, SO4•− can react with H2O or OH− to form •OH (Equations (8) and (9)). Lastly, RhB molecules adsorbed on the catalyst surface are oxidized to CO2, H2O, NO3−, and NH4+ under the combined action of SO4•− and •OH.
≡Co(II) + HSO5− → ≡Co(III) + SO4•− + OH−
≡Co(II) + SO5•− → ≡Co(III) + SO42− + 1/21O2
≡Co(III) + HSO5− → ≡Co(II) + SO5•− + H+
H2O + 2SO5•− → 2HSO4− + 3/21O2
H2O + HSO5− → H2O2 + HSO4−
≡Co(III) + H2O2 → ≡Co(II) + O2•− + H+ + H2O
2O2•− + 2H+ → H2O2 + 1O2
SO4•− + H2O → H+ + SO42− + •OH
SO4•− + OH− → SO42− + •OH
2.5. Toxicity Evaluation
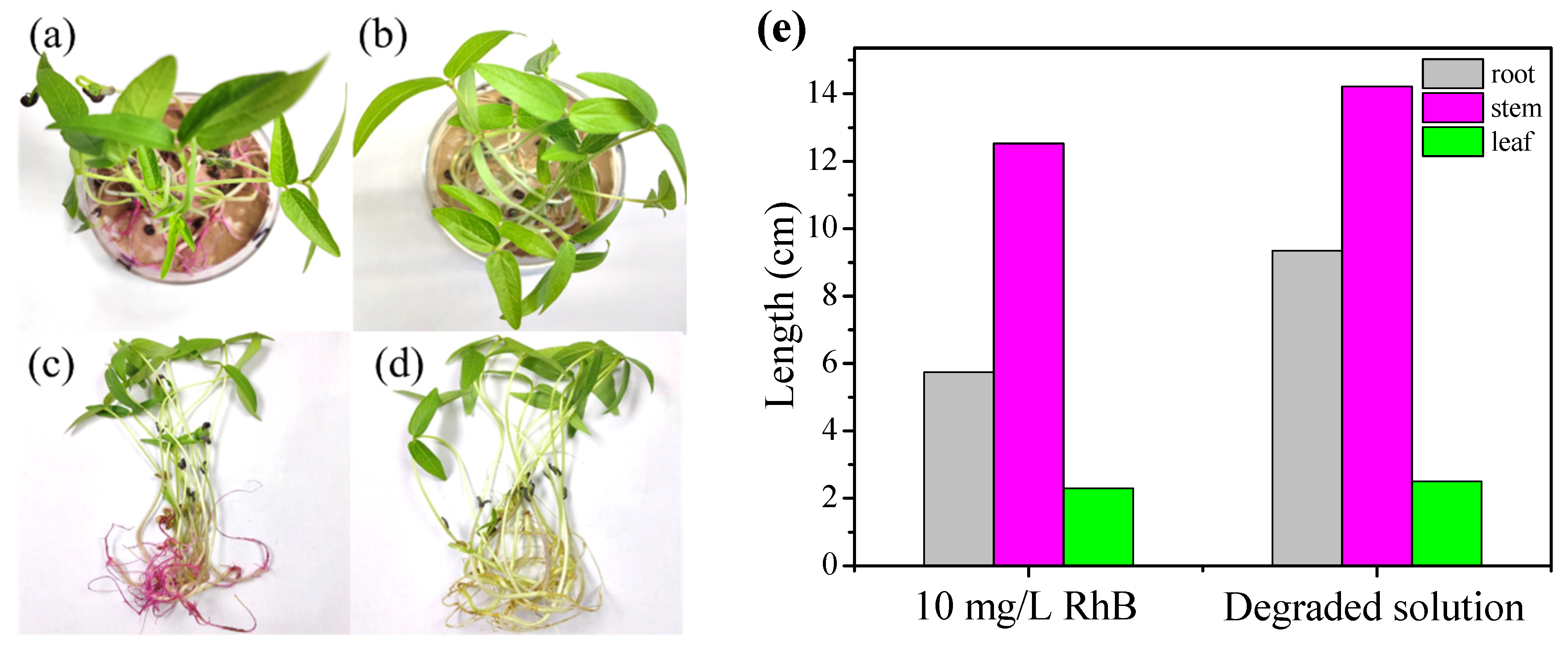
Mung bean seeds were cultivated for 9 days and their roots, stems, and leaves were measured to explore the ecotoxicity of the RhB degradation intermediates. Their growth states are exhibited in Figure 6a–d, the mung bean seeds cultivated in degraded solution are flourishing more than those grown in the initial solution. Additionally, the average lengths of the roots, stems, and leaves of the mung bean seeds cultivated in degraded solution were computed to be 9.35, 13.36, and 2.50 cm, which are longer than those developed in degraded solution (5.75, 12.53, and 2.30 cm) (Figure 6e). These results verify that the degraded solution exhibits lower biological toxicity than the initial RhB solution [70,71].
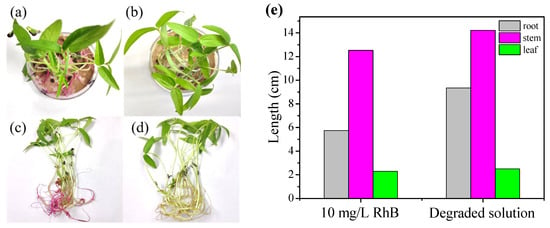
Figure 6.
Ecotoxicity tests with 10 mg/L of RhB solution (a,c) and the degraded solution (b,d). (e) Lengths of the roots, stems, and leaves of mature mung beans in the initial and degraded solution after 9 days.
3. Materials and Methods
3.1. Materials and Reagents
Cobalt nitrate hexahydrate (Co(NO3)2·6H2O), aluminum nitrate hexahydrate (Al(NO3)3·9H2O), RhB, CHCl3, FFA, and EtOH were bought from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). PMS and TBA were obtained from Aladdin Chemistry Co., Ltd. (Shanghai, China). ARG, TCH, and MB were purchased from Shanghai Macklin in Biochemical Reagent Co., Ltd (Shanghai, China). All reagents were analytical grade, and solutions were prepared with deionized water.
3.2. Preparation of CoAl2O4
According to the molar ratio of Co2+:Al3+ = 1:2, 20 mM of Al(NO3)3·9H2O and 10 mM of Co(NO3)2·6H2O were dissolved in deionized water (30 mL), respectively, at room temperature. Under continuously magnetic agitation, the Al(NO3)3 solution was added dropwise to the Co(NO3)2 solution. Subsequently, NaOH solution was added dropwise to the obtained solution until the pH value reached 12. The mixed solution was stirred for 1 h to generate precipitation and then separated by centrifugation. The resulting precipitate was washed with deionized water and dried at 100 °C. Finally, the sample was further grounded and calcinated in a muffle furnace at a certain temperature for 3 h to obtain the CoAl2O4 samples. To evaluate the impact of calcination temperature, the calcination temperatures were set to 300, 500, and 700 °C, respectively, to obtain CoAl2O4-X, where X represents the calcination temperature. For comparison, Co3O4 was prepared under the same procedure without adding Al(NO3)3·9H2O.
3.3. Characterization
The morphology and structure of the catalysts were examined by X-ray diffraction (XRD, D/MAX-RB, Rigaku, Tokyo, Japan). The software JADE (Shuyun Instruments, Shanghai, China) is employed for X-ray diffraction pattern analysis. The surface morphology of the material was analyzed by Scanning Electron Microscopy (SEM, FEI-Quanta 200, FEI Company, Eindhoven, The Netherlands). The specific surface areas and pore size distribution of the samples were measured by Brunauer–Emmett–Teller (BET, ASAP2020 HD88, Micromeritics, Shanghai, China) produced by the American Mike Instrument Company. Meanwhile, X-ray photoelectron spectroscopy (XPS, ESCALAB Xi, Thermo Fisher Scientific Co., Ltd., Waltham, MA, USA) equipped with Al Kα source was used to determine the surface elemental compositions and valence states of fresh and used CoAl2O4-500.
3.4. Experimental Procedure
All the degradation experiments were conducted in a 250 mL glass beaker containing 200 mL of RhB (10 mg/L) solution at room temperature under magnetic agitation. The degradation process was initiated by adding a certain amount of catalyst and PMS. At predetermined time intervals, 3 mL of solution was withdrawn, centrifuged, and analyzed immediately by measuring the absorbance of the supernatant at = 554 nm using a UV-vis spectrophotometer (UV-1990PC, AoE Instrument Co., Ltd., Shanghai, China). NaOH and HNO3 solutions (0.01 M) were utilized to adjust the initial pH value of the RhB solution. A certain amount of NaCl, NaHCO3, and Na2CO3 was added into the CoAl2O4/PMS system to investigate the effects of different anions. The degradation conditions of TCH, MB, and ARG were similar to those of RhB, and the concentrations of TCH, MB, and ARG were measured by absorbance at 356, 664, and 505 nm, respectively. Unless otherwise specified, the catalyst dosage and PMS concentration were both 0.10 g/L. The concentration of the leached cobalt ions was determined through an atomic absorption spectrometer (SP-3520AA, Spectrum Instruments, Shanghai, China). To investigate the primary active species, different scavengers such as EtOH, TBA, CHCl3, and FFA were employed before the addition of PMS.
4. Conclusions
In summary, a mesoporous CoAl2O4 spinel catalyst was successfully synthesized via a facile co-precipitation approach and applied to activate PMS to degrade RhB, TCH, MB, and ARG. CoAl2O4 exhibited much higher catalytic activity and stability than Co3O4 in activating PMS to degrade RhB due to its mesoporous and high specific surface area. Under optimal conditions (calcination temperature: 500 °C, catalyst: 0.1 g/L, and PMS: 0.1 g/L), the CoAl2O4 catalyst could degrade over 99% of RhB at a degradation rate of 0.048 min−1, which is 2.18 times faster than Co3O4 (0.022 min−1). RhB was efficiently degraded by the CoAl2O4-500/PMS over a wide pH range (3.44–10.22) and the catalyst possessed high stability for eight consecutive degradation cycles with insignificant cobalt leaching. The degraded solution exhibits lower biological toxicity than the initial RhB solution. Quenching tests confirmed that 1O2 and SO4•– were the primary reactive oxygen species in the CoAl2O4/PMS system. This work provides new insights into expanding the application of cobalt-based catalysts for environmental remediation.
Author Contributions
Conceptualization, S.G.; funding acquisition, S.G. and H.Z.; formal analysis, M.C.; investigation, L.Z.; project administration, S.G.; supervision, S.G., H.F. and H.Z.; software, L.Z. and M.C.; validation, F.A.; visualization, H.F. and F.A.; writing—original draft preparation, L.Z.; writing—review and editing, S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work received financial support from the National Natural Science Foundation of China (51604194), Natural Science Foundation of Hubei Province of China (No. 2021CFB296), and the Graduate Innovative Fund of Wuhan Institute of Technology (No. CX2021343).
Data Availability Statement
The data presented in this study are openly available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, K.D.; Chen, X.L.; Yan, D.K.; Xu, Z.C.; Hu, P.J.; Li, H.S. Petrochemical and municipal wastewater treatment plants activated sludge each own distinct core bacteria driven by their specific incoming wastewater. Sci. Total Environ. 2022, 826, 153962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.H.; Chen, M.L.; Xiong, Z.K.; Guo, Y.; Lai, B. Highly efficient degradation of emerging contaminants by magnetic CuO@FexOy derived from natural mackinawite (FeS) in the presence of peroxymonosulfate. Chin. Chem. Lett. 2021, 33, 948–952. [Google Scholar] [CrossRef]
- Manos, D.; Miserli, K.; Konstantinou, I. Perovskite and spinel catalysts for sulfate radical-based advanced oxidation of organic pollutants in water and wastewater systems. Catalysts 2020, 10, 1299. [Google Scholar] [CrossRef]
- Azhar, M.R.; Vijay, P.; Tadé, M.O.; Sun, H.Q.; Wang, S.B. Submicron sized water-stable metal organic framework (bio-MOF-11) for catalytic degradation of pharmaceuticals and personal care products. Chemosphere 2018, 196, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Nie, G.; Ding, Y.B. Metal-free enhanced photocatalytic activation of dioxygen by g-C3N4 doped with abundant oxygen-containing functional groups for selective N-deethylation of Rhodamine B. Catalysts 2019, 10, 6. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.N.; Zhang, F.; Li, J.; Li, D.B.; Liu, D.F.; Li, W.W.; Yu, H.Q. Exclusive extracellular bioreduction of methyl orange by azo reductase-free geobacter sulfurreducens. Environ. Sci. Technol. 2017, 51, 8616–8623. [Google Scholar] [CrossRef]
- Minella, M.; Bertinetti, S.; Hanna, K.; Minero, C.; Vione, D. Degradation of ibuprofen and phenol with a Fenton-like process triggered by zero-valent iron (ZVI-Fenton). Environ. Res. 2019, 179, 108750. [Google Scholar] [CrossRef]
- Li, C.Q.; Huang, Y.; Dong, X.B.; Sun, Z.M.; Duan, X.D.; Ren, B.X.; Zheng, S.L.; Dionysiou, D.D. Highly efficient activation of peroxymonosulfate by natural negatively-charged kaolinite with abundant hydroxyl groups for the degradation of atrazine. Appl. Catal. B-Environ. 2019, 247, 10–23. [Google Scholar] [CrossRef]
- Li, Y.Y.; Gan, P.F.; Zhao, Z.W.; Ye, J.Y.; Liu, W.; Tong, M.P.; Liang, J.L. Insight into the synergetic effect of photocatalysis and transition metal on sulfite activation: Different mechanisms for carbamazepine and diclofenac degradation. Sci. Total Environ. 2021, 787, 147626. [Google Scholar] [CrossRef]
- Meng, S.; Zhou, P.; Sun, Y.M.; Zhang, P.; Zhou, C.Y.; Xiong, Z.K.; Zhang, H.; Liang, J.; Lai, B. Reducing agents enhanced Fenton-like oxidation (Fe(III)/peroxydisulfate): Substrate specific reactivity of reactive oxygen species. Water Res. 2022, 218, 118412. [Google Scholar] [CrossRef]
- Dong, X.B.; Ren, B.X.; Sun, Z.M.; Li, C.Q.; Zhang, X.W.; Kong, M.H.; Zheng, S.L.; Dionysiou, D.D. Monodispersed CuFe2O4 nanoparticles anchored on natural kaolinite as highly efficient peroxymonosulfate catalyst for bisphenol A degradation. Appl. Catal. B-Environ. 2019, 253, 206–217. [Google Scholar] [CrossRef]
- Wang, Y.X.; Xie, Y.B.; Chen, C.M.; Duan, X.G.; Sun, H.Q.; Wang, S.B. Synthesis of magnetic carbon supported manganese catalysts for phenol oxidation by activation of peroxymonosulfate. Catalysts 2017, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Wang, H.; Yang, W.; Fida, H.; You, L.M.; Zhou, K. Scalable synthesis of Ca-doped α-Fe2O3 with abundant oxygen vacancies for enhanced degradation of organic pollutants through peroxymonosulfate activation. Appl. Catal. B-Environ. 2020, 262, 118250. [Google Scholar] [CrossRef]
- Khan, A.; Wang, H.B.; Liu, Y.; Jawad, A.; Ifthikar, J.; Liao, Z.W.; Wang, T.; Chen, Z.Q. Highly efficient α-Mn2O3@α-MnO2-500 nanocomposite for peroxymonosulfate activation: Comprehensive investigation of manganese oxides. J. Mater. Chem. A 2018, 6, 1590–1600. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhao, W.; Li, Z.; Lu, G.; Zhu, M. Visible-light-assisted peroxymonosulfate activation over Fe(II)/V(IV) self-doped FeVO4 nanobelts with enhanced sulfamethoxazole degradation: Performance and mechanism. Chem. Eng. J. 2021, 403, 126384. [Google Scholar] [CrossRef]
- Gao, Y.W.; Zhu, Y.; Chen, Z.H.; Zeng, Q.Y.; Hu, C. Insights into the difference in metal-free activation of peroxymonosulfate and peroxydisulfate. Chem. Eng. J. 2020, 394, 123936. [Google Scholar] [CrossRef]
- Lan, S.Y.; Chen, Y.X.; Zeng, L.X.; Ji, H.D.; Liu, W.; Zhu, M.S. Piezo-activation of peroxymonosulfate for benzothiazole removal in water. J. Hazard. Mater. 2020, 393, 122448. [Google Scholar] [CrossRef]
- Bu, Y.G.; Li, H.C.; Yu, W.J.; Pan, Y.F.; Li, L.J.; Wang, Y.F.; Pu, L.T.; Ding, J.; Gao, G.D.; Pan, B.C. Peroxydisulfate activation and singlet oxygen generation by oxygen vacancy for degradation of contaminants. Environ. Sci. Technol. 2021, 55, 2110–2120. [Google Scholar] [CrossRef]
- Zhao, X.F.; Niu, C.G.; Zhang, L.; Guo, H.; Wen, X.J.; Liang, C.; Zeng, G.M. Co-Mn layered double hydroxide as an effective heterogeneous catalyst for degradation of organic dyes by activation of peroxymonosulfate. Chemosphere 2018, 204, 11–21. [Google Scholar] [CrossRef]
- Tian, N.; Tian, X.K.; Nie, Y.L.; Yang, C.; Zhou, Z.X.; Li, Y. Biogenic manganese oxide: An efficient peroxymonosulfate activation catalyst for tetracycline and phenol degradation in water. Chem. Eng. J. 2018, 352, 469–476. [Google Scholar] [CrossRef]
- Ji, J.H.; Aleisa, R.M.; Duan, H.; Zhang, J.L.; Yin, Y.D.; Xing, M.Y. Metallic active sites on MoO2(110) surface to catalyze advanced oxidation processes for efficient pollutant removal. iScience 2020, 23, 100861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, X.G.; Su, C.; Miao, J.; Zhong, Y.J.; Shao, Z.P.; Wang, S.B.; Sun, H.Q. Insights into perovskite-catalyzed peroxymonosulfate activation: Maneuverable cobalt sites for promoted evolution of sulfate radicals. Appl. Catal. B-Environ 2018, 220, 626–634. [Google Scholar] [CrossRef]
- Xu, H.D.; Jiang, N.; Wang, D.; Wang, L.H.; Song, Y.F.; Chen, Z.Q.; Ma, J.; Zhang, T. Improving PMS oxidation of organic pollutants by single cobalt atom catalyst through hybrid radical and non-radical pathways. Appl. Catal. B-Environ. 2019, 263, 118350. [Google Scholar] [CrossRef]
- Zhou, G.L.; Sun, H.Q.; Wang, S.B.; Ang, H.M.; Tadé, M.O. Titanate supported cobalt catalysts for photochemical oxidation of phenol under visible light irradiations. Sep. Purif. Technol. 2011, 80, 626–634. [Google Scholar] [CrossRef]
- Du, W.Y.; Zhang, Q.Z.; Shang, Q.Z.; Shang, Y.N.; Wang, W.; Li, Q.; Yue, Q.Y.; Gao, B.Y.; Xu, X. Sulfate saturated biosorbent-derived Co-S@NC nanoarchitecture as an efficient catalyst for peroxymonosulfate activation. Appl. Catal. B-Environ. 2019, 262, 118302. [Google Scholar] [CrossRef]
- Xu, H.D.; Wang, D.; Ma, J.; Zhang, T.; Lu, X.H.; Chen, Z.Q. A superior active and stable spinel sulfide for catalytic peroxymonosulfate oxidation of bisphenol S. Appl. Catal. B-Environ. 2018, 238, 557–567. [Google Scholar] [CrossRef]
- Lu, Y.K.; Li, Z.X.; Xu, Y.L.; Tang, L.Q.; Xu, S.J.; Li, D.; Zhu, J.J.; Jiang, D.L. Bimetallic Co-Mo nitride nanosheet arrays as high-performance bifunctional electrocatalysts for overall water splitting. Chem. Eng. J. 2021, 411, 128433. [Google Scholar] [CrossRef]
- Guo, Z.G.; Wang, X.F.; Yang, F.S.; Liu, Z.F. Synergistic effect of Co and Fe bimetallic oxides/hydroxides composite structure as a bifunctional electrocatalyst for enhancing overall water splitting performance. J. Alloys Compd. 2022, 895, 162614. [Google Scholar] [CrossRef]
- Nie, C.Y.; Dai, Z.H.; Liu, W.J.; Duan, X.G.; Wang, C.Y.; Lai, B.; Ao, Z.M.; Wang, S.B.; An, T.C. Criteria of active sites in nonradical persulfate activation process from integrated experimental and theoretical investigations: Boron–nitrogen-co-doped nanocarbon-mediated peroxydisulfate activation as an example. Environ. Sci. Nano 2020, 7, 1899–1911. [Google Scholar] [CrossRef]
- Chen, C.; Liu, L.; Li, Y.X.; Li, W.; Zhou, L.X.; Lan, Y.Q.; Li, Y. Insight into heterogeneous catalytic degradation of sulfamethazine by peroxymonosulfate activated with CuCo2O4 derived from bimetallic oxalate. Chem. Eng. J. 2020, 384, 123257. [Google Scholar] [CrossRef]
- Niu, P.; Li, C.H.; Jia, C.X.; Wang, D.Q.; Liu, S.W. Facile synthesis of CoFe2O4 magnetic nanomaterial by natural cellulose template and catalytic performance in heterogeneous activation of peroxymonosulfate. J. Sol-Gel Sci. Technol. 2020, 93, 419–427. [Google Scholar] [CrossRef]
- Zhang, W.; Su, Y.; Zhang, X.M.; Yang, Y.; Guo, X.H. Facile synthesis of porous NiCo2O4 nanoflakes as magnetic recoverable catalysts towards the efficient degradation of RhB. RSC Adv. 2016, 6, 64626–64633. [Google Scholar] [CrossRef]
- Zhang, A.J.; Mu, B.; Luo, Z.H.; Wang, A.Q. Bright blue halloysite/CoAl2O4 hybrid pigments: Preparation, characterization and application in water-based painting. Dyes Pigment. 2017, 139, 473–481. [Google Scholar] [CrossRef]
- Liu, W.J.; Du, T.; Ru, Q.X.; Zuo, S.X.; Yang, X.Y.; Yao, C.; Kong, Y. Facile synthesis and characterization of 2D kaolin/CoAl2O4: A novel inorganic pigment with high near-infrared reflectance for thermal insulation. Appl. Clay Sci. 2018, 153, 239–245. [Google Scholar] [CrossRef]
- Mu, B.; Wang, Q.; Wang, A. Effect of different clay minerals and calcination temperature on the morphology and color of clay/CoAl2O4 hybrid pigments. RSC Adv. 2015, 5, 102674–102681. [Google Scholar] [CrossRef]
- Yang, H.; Mu, B.; Li, S.; Wang, X.W.; Wang, A.Q. Preparation and coloring mechanism of MAl2O4/CoAl2O4/quartz sand (M = Ca or Ba) composite pigments. Mate. Chem. Phys. 2022, 276, 125413. [Google Scholar] [CrossRef]
- Kim, J.H.; Son, B.R.; Yoon, D.H.; Hwang, K.T.; Noh, H.G.; Cho, W.S.; Kim, U.S. Characterization of blue CoAl2O4 nano-pigment synthesized by ultrasonic hydrothermal method. Ceram. Int. 2012, 38, 5707–5712. [Google Scholar] [CrossRef]
- Guo, S.; Tang, H.L.; You, L.M.; Zhang, H.L.; Li, J.; Zhou, K. Combustion synthesis of mesoporous CoAl2O4 for peroxymonosulfate activation to degrade organic pollutants. Chin. Chem. Lett. 2021, 32, 2828–2832. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, J.F.; Hu, Z.B.; Zhang, J.; Yan, J.; Sheng, J.W. Novel fabrication of rod-like CoAl2O4/halloysite hybrid pigment derived from Co-MOF/nano-clay and mechanism exploration. Dyes Pigment. 2022, 201, 110216. [Google Scholar] [CrossRef]
- Wei, P.P.; Liang, J.; Liu, Q.; Xie, L.S.; Tong, X.; Ren, Y.C.; Li, T.S.; Luo, Y.S.; Li, N.; Tang, B.; et al. Iron-doped cobalt oxide nanoarray for efficient electrocatalytic nitrate-to-ammonia conversion. J. Colloid Interface Sci. 2022, 615, 636–642. [Google Scholar] [CrossRef]
- Wang, D.L.; Hu, J.P.; Liu, B.C.; Hou, H.J.; Yang, J.K.; Li, Y.X.; Zhu, Y.; Sha, L.; Xiao, K.K. Degradation of refractory organics in dual-cathode electro-Fenton using air-cathode for H2O2 electrogeneration and microbial fuel cell cathode for Fe2+ regeneration. J. Hazard. Mater. 2021, 412, 125269. [Google Scholar] [CrossRef]
- Xue, W.D.; Zhou, Q.X.; Li, F.X.; Ondon, B.S. Zeolitic imidazolate framework-8 (ZIF-8) as robust catalyst for oxygen reduction reaction in microbial fuel cells. J. Power Sources 2019, 423, 9–17. [Google Scholar] [CrossRef]
- Wang, S.F.; Gao, H.J.; Chen, C.L.; Wei, Y.; Zhao, X.X. Irradiation assisted polyacrylamide gel route for the synthesize of the Mg1–xCoxAl2O4 nano-photocatalysts and its optical and photocatalytic performances. J. Sol-Gel Sci. Technol. 2019, 92, 186–199. [Google Scholar] [CrossRef]
- Basaleh, A.; Mahmoud, M.H.H. CoAl2O4-g-C3N4 nanocomposite photocatalysts for powerful visible-light-driven hydrogen production. ACS Omega 2021, 6, 10428–10436. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Zaki, Z.I. CoAl2O4–TiO2 nanocomposite photocatalyst for effective destruction of herbicide imazapyr under visible light. Appl. Nanosci. 2021, 11, 1009–1019. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Y.J.; Ji, Q.Y.; Li, T.Z.; Xu, C.M.; Qi, C.D.; He, H.; Yang, S.G.; Li, S.Y.; Yan, S.C.; et al. Understanding spatial effects of tetrahedral and octahedral cobalt cations on peroxymonosulfate activation for efficient pollution degradation. Appl. Catal. B-Environ. 2021, 291, 120072. [Google Scholar] [CrossRef]
- Bao, Y.P.; Oh, W.D.; Lim, T.T.; Wang, R.; Webster, R.D.; Hu, X. Surface-nucleated heterogeneous growth of zeolitic imidazolate framework—A unique precursor towards catalytic ceramic membranes: Synthesis, characterization and organics degradation. Chem. Eng. J. 2018, 353, 69–79. [Google Scholar] [CrossRef]
- Duan, X.L.; Pan, M.; Yu, F.P.; Yuan, D.R. Synthesis, structure and optical properties of CoAl2O4 spinel nanocrystals. J. Alloys Compd. 2011, 509, 1079–1083. [Google Scholar] [CrossRef]
- Deng, J.; Xu, M.Y.; Qiu, C.G.; Chen, Y.; Ma, X.Y.; Gao, N.Y.; Li, X.Y. Magnetic MnFe2O4 activated peroxymonosulfate processes for degradation of bisphenol A: Performance, mechanism and application feasibility. Appl. Surf. Sci. 2018, 459, 138–147. [Google Scholar] [CrossRef]
- Kang, S.; Hwang, J. CoMn2O4 embedded hollow activated carbon nanofibers as a novel peroxymonosulfate activator. Chem. Eng. J. 2021, 406, 127158. [Google Scholar] [CrossRef]
- Zhong, X.; Cai, Y.H.; Bai, H.P.; Huang, W.; Zhou, B.X. Visible light driven spherical CuBi2O4 with surface oxygen vacancy enhanced photocatalytic activity: Catalyst fabrication, performance, and reaction mechanism. Catalysts 2020, 10, 945. [Google Scholar] [CrossRef]
- Okoye, P.U.; Wang, S.; Xu, L.L.; Li, S.X.; Wang, J.Y.; Zhang, L.N. Promotional effect of calcination temperature on structural evolution, basicity, and activity of oil palm empty fruit bunch derived catalyst for glycerol carbonate synthesis. Energy Convers. Manag. 2019, 179, 192–200. [Google Scholar] [CrossRef]
- Ren, J.; Jiang, L.S.; Li, Y.; Zhang, G.K. Cobalt doped bismuth oxysulfide with abundant oxygen vacancies towards tetracycline degradation through peroxymonosulfate activation. Sep. Purif. Technol. 2021, 275, 119100. [Google Scholar] [CrossRef]
- Hung, C.M.; Chen, C.W.; Huang, C.P.; Dong, C.D. Activation of peroxymonosulfate by nitrogen-doped carbocatalysts derived from brown algal (Sargassum duplicatum) for the degradation of polycyclic aromatic hydrocarbons in marine sediments. J. Environ. Chem. Eng. 2021, 9, 106420. [Google Scholar] [CrossRef]
- Ji, R.C.; Chen, J.B.; Liu, T.C.; Zhou, X.F.; Zhang, Y.L. Critical review of perovskites-based advanced oxidation processes for wastewater treatment: Operational parameters, reaction mechanisms, and prospects. Chin. Chem. Lett. 2022, 33, 643–652. [Google Scholar] [CrossRef]
- Hu, P.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B-Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Xu, M.; Li, J.; Yan, Y.; Zhao, X.G.; Yan, J.F.; Zhang, Y.H.; Lai, B.; Chen, X.; Song, L.P. Catalytic degradation of sulfamethoxazole through peroxymonosulfate activated with expanded graphite loaded CoFe2O4 particles. Chem. Eng. J. 2019, 369, 403–413. [Google Scholar] [CrossRef]
- Sun, B.J.; Ma, W.J.; Wang, N.; Xu, P.; Zhang, L.J.; Wang, B.N.; Zhao, H.H.; Lin, K.Y.A.; Du, Y.C. Polyaniline: A new metal-free catalyst for peroxymonosulfate activation with highly efficient and durable removal of organic pollutants. Environ. Sci. Technol. 2019, 53, 9771–9780. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yang, Z.X.; Zhang, H.L.; Yang, W.; Li, J.; Zhou, K. Enhanced photocatalytic degradation of organic contaminants over CaFe2O4 under visible LED light irradiation mediated by peroxymonosulfate. J. Mater. Sci. Technol. 2020, 62, 34–43. [Google Scholar] [CrossRef]
- Wang, K.; Ma, E.H.; Wang, H. Biotemplated shell micromotors for efficient degradation of antibiotics via enhanced peroxymonosulfate activation. Adv. Mater. Interfaces 2022, 9, 2200271. [Google Scholar] [CrossRef]
- Wang, Y.T.; Xue, Y.D.; Zhang, C.H. Generation and application of reactive chlorine species by electrochemical process combined with UV irradiation: Synergistic mechanism for enhanced degradation performance. Sci. Total Environ. 2020, 712, 136501. [Google Scholar] [CrossRef]
- Hong, Y.C.; Zhou, H.Y.; Xiong, Z.K.; Liu, Y.; Yao, G.; Lai, B. Heterogeneous activation of peroxymonosulfate by CoMgFe-LDO for degradation of carbamazepine: Efficiency, mechanism and degradation pathways. Chem. Eng. J. 2019, 391, 123604. [Google Scholar] [CrossRef]
- Chen, G.F.; Qiao, Y.X.; Liu, F.; Zhang, X.B.; Liao, H.; Zhang, R.Y.; Dong, J.N. Effects of fertilization on the triafamone photodegradation in aqueous solution: Kinetic, identification of photoproducts and degradation pathway. Ecotox. Environ. Saf. 2020, 194, 110363. [Google Scholar] [CrossRef]
- Liao, S.; Zhu, F.W.; Zhao, X.Y.; Yang, H.; Chen, X.Q. A reusable P, N-doped carbon quantum dot fluorescent sensor for cobalt ion. Sens. Actuator B-Chem. 2018, 260, 156–164. [Google Scholar] [CrossRef]
- Cai, C.; Liu, J.; Zhang, Z.Y.; Zheng, Y.Y.; Zhang, H. Visible light enhanced heterogeneous photo-degradation of Orange II by zinc ferrite (ZnFe2O4) catalyst with the assistance of persulfate. Sep. Purif. Technol. 2016, 165, 42–52. [Google Scholar] [CrossRef]
- Peng, G.L.; You, W.Q.; Zhou, W.; Zhou, G.M.; Qi, C.D.; Hu, Y. Activation of peroxymonosulfate by phosphite: Kinetics and mechanism for the removal of organic pollutants. Chemosphere 2020, 266, 129016. [Google Scholar] [CrossRef]
- Li, J.Q.; Zhao, S.Y.; Zhang, L.J.; Jiang, S.P.; Yang, S.Z.; Wang, S.B.; Sun, H.Q.; Liu, S.M. Cobalt single atoms embedded in Nitrogen-doped graphene for selective oxidation of benzyl alcohol by activated peroxymonosulfate. Small 2021, 17, 2004579. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S.; Fan, W.C.; Tian, Y.; Zhao, X. The synthesis of novel Co-Al2O3 nanofibrous membranes with efficient activation of peroxymonosulfate for bisphenol A degradation. Environ. Sci. Nano 2018, 5, 1933–1942. [Google Scholar] [CrossRef]
- Peng, J.L.; Zhou, H.Y.; Liu, W.; Ao, Z.M.; Ji, H.D.; Liu, Y.; Su, S.J.; Yao, G.; Lai, B. Insights into heterogeneous catalytic activation of peroxymonosulfate by natural chalcopyrite: pH-dependent radical generation, degradation pathway and mechanism. Chem. Eng. J. 2020, 397, 125387. [Google Scholar] [CrossRef]
- Sun, Y.H.; Wang, W.J.; Zheng, F.Y.; Zhang, S.W.; Liu, S.W. Phytotoxicity of iron-bed materials in mung bean: Seed germination tests. Chemosphere 2020, 251, 126432. [Google Scholar] [CrossRef]
- Kannan, A.; Upreti, R.K. Influence of distillery effluent on germination and growth of mung bean (Vigna radiata) seeds. J. Hazard. Mater. 2008, 153, 609–615. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).