Rutile-TiO2/PtO2 Glass Coatings Disinfects Aquatic Legionella pneumophila via Morphology Change and Endotoxin Degradation under LED Irradiation
Abstract
1. Introduction
2. Results
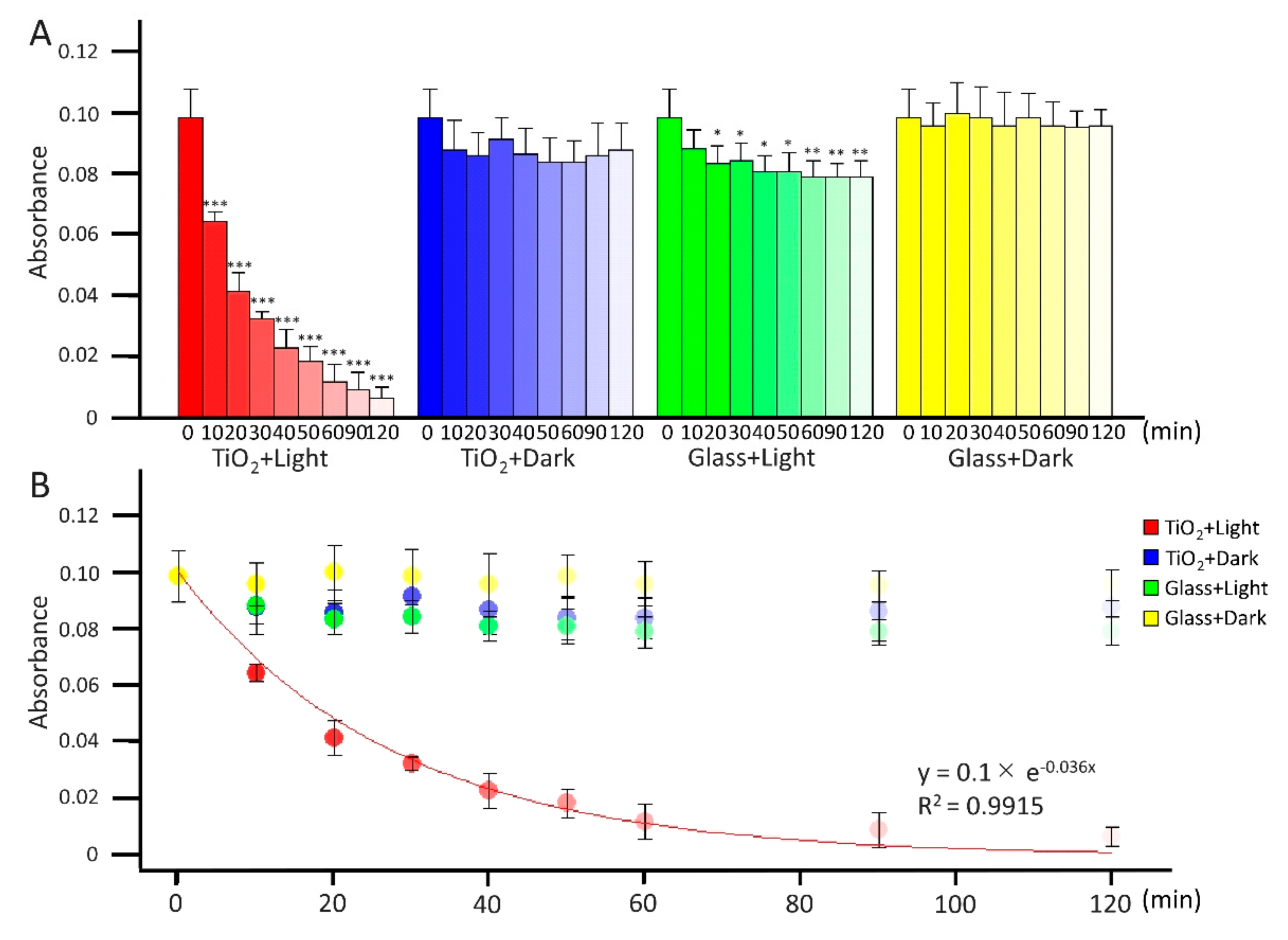
2.1. Photocatalytic Degradation of Methylene Blue in Water by TiO2 Photocatalyst
2.2. Disinfection of E. coli in Water by TiO2 Photocatalyst
2.3. Disinfection of L. pneumophila in Water by TiO2 Photocatalyst
2.4. Morphological Changes in L. pneumophila Induced by TiO2 Photocatalytic Disinfection
2.5. L. pneumophila Endotoxin Degradation by TiO2 Photocatalyst
2.6. Durability of TiO2 Photocatalyst
3. Discussion
4. Materials and Methods
4.1. Preparation of TiO2-Coated Glass or Glass Fiber Sheet
4.2. Methylene Blue Degradation
4.3. Scanning Electron Microscopy of TiO2-Coated Glass
4.4. Elemental Analysis
4.5. Microorganisms
4.6. Treatment of E. coli and L. pneumophila by the TiO2 Photocatalytic Reaction
4.7. LAL Assay
4.8. TEM
4.9. Extraction of Endotoxin from L. pneumophila
4.10. Inactivation of Endotoxin from L. pneumophila by the TiO2 Photocatalytic Reaction
4.11. Silver Stain
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boffetta, P.; Soutar, A.; Cherrie, J.W.; Granath, F.; Andersen, A.; Anttila, A.; Blettner, M.; Gaborieau, V.; Klug, S.J.; Langard, S.; et al. Mortality among workers employed in the titanium dioxide production industry in Europe. Cancer Causes Control 2004, 15, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Fryzek, J.P.; Chadda, B.; Marano, D.; White, K.; Schweitzer, S.; McLaughlin, J.K.; Blot, W.J. A cohort mortality study among titanium dioxide manufacturing workers in the United States. J. Occup. Environ. Med. 2003, 45, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Koly, F.A.; Rahman, M.A.; Islam, M.S.; Rahman, M.M. Fabrication of porous TiO2 foams by powder metallurgy technique and study of bulk crushing strength for biomedical application. Prog. Biomater. 2021, 10, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Cervantes, O.R.; Pérez-Larios, A.; Romero Arellano, V.H.; Sulbaran-Rangel, B.; Guzmán González, C.A. Effects in Band Gap for Photocatalysis in TiO2 Support by Adding Gold and Ruthenium. Processes 2020, 8, 1032. [Google Scholar] [CrossRef]
- Matsuura, R.; Lo, C.W.; Wada, S.; Somei, J.; Ochiai, H.; Murakami, T.; Saito, N.; Ogawa, T.; Shinjo, A.; Benno, Y.; et al. SARS-CoV-2 Disinfection of Air and Surface Contamination by TiO2 Photocatalyst-Mediated Damage to Viral Morphology, RNA, and Protein. Viruses 2021, 13, 942. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Choi, Y.; Park, H.; Kim, K.; Woo, G.J.; Park, J. Titanium dioxide/UV photocatalytic disinfection in fresh carrots. J. Food Prot. 2007, 70, 97–101. [Google Scholar] [CrossRef]
- Armon, R.; Weltch-Cohen, G.; Bettane, P. Disinfection of Bacillus spp. spores in drinking water by TiO2 photocatalysis as a model for Bacillus anthracis. Waterborne Pathog. 2004, 4, 7–14. [Google Scholar] [CrossRef]
- Sreeja, S.; Vidya Shetty, K. Microbial disinfection of water with endotoxin degradation by photocatalysis using Ag@TiO2 core shell nanoparticles. Environ. Sci. Pollut. Res. Int. 2016, 23, 18154–18164. [Google Scholar] [CrossRef]
- Gadgil, D.J.; Shetty Kodialbail, V. Suspended and polycaprolactone immobilized Ag @TiO2/polyaniline nanocomposites for water disinfection and endotoxin degradation by visible and solar light-mediated photocatalysis. Environ. Sci. Pollut. Res. Int. 2021, 28, 12780–12791. [Google Scholar] [CrossRef]
- Ibáñez, J.A.; Litter, M.I.; Pizarro, R.A. Photocatalytic bactericidal effect of TiO2 on Enterobacter cloacae. Comparative study with other Gram (−) bacteria. J. Photochem. Photobiol. A 2003, 157, 81–85. [Google Scholar] [CrossRef]
- Yao, K.S.; Wang, D.Y.; Chang, C.Y.; Weng, K.W.; Yang, L.Y.; Lee, S.J.; Cheng, T.C.; Hwang, C.C. Photocatalytic disinfection of phytopathogenic bacteria by dye-sensitized TiO2 thin film activated by visible light. Surf. Coat. Technol. 2007, 202, 1329–1332. [Google Scholar] [CrossRef]
- Chun, M.J.; Shim, E.; Kho, E.H.; Park, K.J.; Jung, J.; Kim, J.M.; Kim, B.; Lee, K.H.; Cho, D.L.; Bai, D.H.; et al. Surface modification of orthodontic wires with photocatalytic titanium oxide for its antiadherent and antibacterial properties. Angle Orthod. 2007, 77, 483–488. [Google Scholar] [CrossRef]
- Kim, B.; Kim, D.; Cho, D.; Cho, S. Bactericidal effect of TiO2 photocatalyst on selected food-borne pathogenic bacteria. Chemosphere 2003, 52, 277–281. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Robertson, P.K.J.; Officer, S.; Pollard, P.M.; McCullagh, C.; Robertson, J.M.C. Variables to be considered when assessing the photocatalytic destruction of bacterial pathogens. Chemosphere 2009, 74, 1374–1378. [Google Scholar] [CrossRef]
- Cho, D.L.; Min, H.; Kim, J.H.; Cha, G.S.; Kim, G.S.; Kim, B.H.; Ohk, S.H. Photocatalytic characteristics of TiO2 thin films deposited by PECVD. J. Ind. Eng. Chem. 2007, 13, 434–437. [Google Scholar]
- Hara-Kudo, Y.; Segawa, Y.; Kimura, K. Sanitation of seawater effluent from seaweed processing plants using a photo-catalytic TiO2 oxidation. Chemosphere 2006, 62, 149–154. [Google Scholar] [CrossRef]
- Fraser, D.W.; Tsai, T.R.; Orenstein, W.; Parkin, W.E.; Beecham, H.J.; Sharrar, R.G.; Harris, J.; Mallison, G.F.; Martin, S.M.; McDade, J.E.; et al. Legionnaires’ disease: Description of an epidemic of pneumonia. N. Engl. J. Med. 1977, 297, 1189–1197. [Google Scholar] [CrossRef]
- Brenner, D.J.; Steigerwalt, A.G.; McDade, J.E. Classification of the Legionnaires’ disease bacterium: Legionella pneumophila, genus novum, species nova, of the family Legionellaceae, familia nova. Ann. Intern. Med. 1979, 90, 656–658. [Google Scholar] [CrossRef]
- Dooling, K.L.; Toews, K.; Hicks, L.A.; Garrison, L.E.; Bachaus, B.; Zansky, S.; Carpenter, L.R.; Schaffner, B.; Parker, E.; Petit, S.; et al. Active Bacterial Core Surveillance for Legionellosis—United States, 2011–2013. MMWR 2015, 64, 42. [Google Scholar] [CrossRef]
- Gattuso, G.; Rizzo, R.; Lavoro, A.; Spoto, V.; Porciello, G.; Montagnese, C.; Cinà, D.; Cosentino, A.; Lombardo, C.; Mezzatesta, M.L.; et al. Overview of the Clinical and Molecular Features of Legionella Pneumophila: Focus on Novel Surveillance and Diagnostic Strategies. Antibiotics 2022, 11, 370. [Google Scholar] [CrossRef]
- Ziltener, P.; Reinheckel, T.; Oxenius, A. Neutrophil and Alveolar Macrophage-Mediated Innate Immune Control of Legionella pneumophila Lung Infection via TNF and ROS. PLoS Pathog. 2016, 12, e1005591. [Google Scholar] [CrossRef]
- Orkis, L.T.; Harrison, L.H.; Mertz, K.J.; Brooks, M.M.; Bibby, K.J.; Stout, J.E. Environmental sources of community-acquired legionnaires’ disease: A review. Int. J. Hyg. Environ. Health 2018, 221, 764–774. [Google Scholar] [CrossRef]
- Stout, J.E.; Yu, V.L.; Muraca, P.; Joly, J.; Troup, N.; Tompkins, L.S. Potable water as a cause of sporadic cases of community-acquired legionnaires’ disease. N. Engl. J. Med. 1992, 326, 151–155. [Google Scholar] [CrossRef]
- Stout, J.E.; Yu, V.L.; Yee, Y.C.; Vaccarello, S.; Diven, W.; Lee, T.C. Legionella pneumophila in residential water supplies: Environmental surveillance with clinical assessment for Legionnaires’ disease. Epidemiol. Infect. 1992, 109, 49–57. [Google Scholar]
- Rampling, A.; Butt, C.J.; West, A.A.; Tully, M.; Palmer, K.T. Community-acquired Legionnaires’ disease following minimal exposure to a contaminated source. J. Infect. 1997, 35, 300–302. [Google Scholar] [CrossRef]
- Sax, H.; Dharan, S.; Pittet, D. Legionnaires’ disease in a renal transplant recipient: Nosocomial or home-grown? Transplantation 2002, 74, 890–892. [Google Scholar] [CrossRef]
- Pinar, A.; Ramirez, J.A.; Schindler, L.L.; Miller, R.D.; Summersgill, J.T. The use of heteroduplex analysis of polymerase chain reaction products to support the possible transmission of Legionella pneumophila from a malfunctioning automobile air conditioner. Infect. Control Hosp. Epidemiol. 2002, 23, 145–147. [Google Scholar] [CrossRef]
- Moran-Gilad, J.; Lazarovitch, T.; Mentasti, M.; Harrison, T.; Weinberger, M.; Mordish, Y.; Mor, Z.; Stocki, T.; Anis, E.; Sadik, C.; et al. Humidifier-associated paediatric Legionnaires’ disease, Israel, February 2012. Eurosurveillance 2012, 17, 20293. [Google Scholar] [CrossRef]
- Ricci, M.L.; Fontana, S.; Pinci, F.; Fiumana, E.; Pedna, M.F.; Farolfi, P.; Sabattini, M.A.; Scaturro, M. Pneumonia associated with a dental unit waterline. Lancet 2012, 379, 684. [Google Scholar] [CrossRef]
- Miyamoto, H.; Jitsurong, S.; Shiota, R.; Maruta, K.; Yoshida, S.; Yabuuchi, E. Molecular determination of infection source of a sporadic Legionella pneumonia case associated with a hot spring bath. Microbiol. Immunol. 1997, 41, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Ito, I.; Naito, J.; Kadowaki, S.; Mishima, M.; Ishida, T.; Hongo, T.; Ma, L.; Ishii, Y.; Matsumoto, T.; Yamaguchi, K. Hot spring bath and Legionella pneumonia: An association confirmed by genomic identification. Intern. Med. 2002, 41, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Nozue, T.; Chikazawa, H.; Miyanishi, S.; Shimazaki, T.; Oka, R.; Shimazaki, S.; Miyamoto, S. Legionella pneumonia associated with adult respiratory distress syndrome caused by Legionella pneumophila serogroup 3. Intern. Med. 2005, 44, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, H.; Fujita, M.; Kobatake, S.; Kimura, H.; Ohshima, M.; Nagai, A.; Kaneko, S.; Iwasaki, Y.; Kozawa, K. A case of Legionella pneumonia linked to a hot spring facility in Gunma Prefecture, Japan. Jpn. J. Infect. Dis. 2010, 63, 78–79. [Google Scholar] [CrossRef]
- Matsui, M.; Fujii, S.I.; Shiroiwa, R.; Amemura-Maekawa, J.; Chang, B.; Kura, F.; Yamauchi, K. Isolation of Legionella rubrilucens from a pneumonia patient co-infected with Legionella pneumophila. J. Med. Microbiol. 2010, 59 Pt 10, 1242–1246. [Google Scholar] [CrossRef]
- Correia, A.M.; Ferreira, J.S.; Borges, V.; Nunes, A.; Gomes, B.; Capucho, R.; Gonçalves, J.; Antunes, D.M.; Almeida, S.; Mendes, A.; et al. Probable Person-to-Person Transmission of Legionnaires’ Disease. N. Engl. J. Med. 2016, 374, 497–498. [Google Scholar] [CrossRef]
- Josset, S.; Hajiesmaili, S.; Begin, D.; Edouard, D.; Pham-Huu, C.; Lett, M.C.; Keller, N.; Keller, V. UV-A photocatalytic treatment of Legionella pneumophila bacteria contaminated airflows through three-dimensional solid foam structured photocatalytic reactors. J. Hazard. Mater. 2010, 175, 372–381. [Google Scholar] [CrossRef]
- Oder, M.; Koklič, T.; Umek, P.; Podlipec, R.; Štrancar, J.; Dobeic, M. Photocatalytic biocidal effect of copper doped TiO2 nanotube coated surfaces under laminar flow, illuminated with UVA light on Legionella pneumophila. PLoS ONE 2020, 15, e0227574. [Google Scholar] [CrossRef]
- Ishiguro, H.; Yao, Y.; Nakano, R.; Hara, M.; Sunada, K.; Hashimoto, K.; Kajioka, J.; Fujishima, A.; Kubota, Y. Photocatalytic activity of Cu2+/TiO2-coated cordierite foam inactivates bacteriophages and Legionella pneumophila. Appl. Catal. B 2013, 129, 56–61. [Google Scholar] [CrossRef]
- Oana, K.; Kobayashi, M.; Yamaki, D.; Sakurada, T.; Nagano, N.; Kawakami, Y. Applicability assessment of ceramic microbeads coated with hydroxyapatite-binding silver/titanium dioxide ceramic composite earthplus™ to the eradication of Legionella in rainwater storage tanks for household use. Int. J. Nanomed. 2015, 10, 4971–4979. [Google Scholar] [CrossRef][Green Version]
- Holzheimer, R.G. Antibiotic induced endotoxin release and clinical sepsis: A review. J. Chemother. 2001, 13, 159–172. [Google Scholar] [CrossRef]
- Kiwi, J.; Nadtochenko, V. Evidence for the mechanism of photocatalytic degradation of the bacterial wall membrane at the TiO2 interface by ATR-FTIR and laser kinetic spectroscopy. Langmuir 2005, 21, 4631–4641. [Google Scholar] [CrossRef]
- Hu, C.; Lan, Y.; Qu, J.; Hu, X.; Wang, A. Ag/AgBr/TiO2 visible light photocatalyst for destruction of azodyes and bacteria. J. Phys. Chem. B 2006, 110, 4066–4072. [Google Scholar] [CrossRef]
- Leive, L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem. Biophys. Res. Commun. 1965, 21, 290–296. [Google Scholar] [CrossRef]
- Wu, P.; Imlay, J.A.; Shang, J.K. Mechanism of Escherichia coli inactivation on palladium-modified nitrogen-doped titanium dioxide. Biomaterials 2010, 31, 7526–7533. [Google Scholar] [CrossRef]
- Amézaga-Madrid, P.; Silveyra-Morales, R.; Córdoba-Fierro, L.; Nevárez-Moorillón, G.V.; Miki-Yoshida, M.; Orrantia-Borunda, E.; Solís, F.J. TEM evidence of ultrastructural alteration on Pseudomonas aeruginosa by photocatalytic TiO2 thin films. J. Photochem. Photobiol. B 2003, 70, 45–50. [Google Scholar] [CrossRef]
- Sakai, H.; Ito, E.; Cai, R.X.; Yoshioka, T.; Kubota, Y.; Hashimoto, K.; Fujishima, A. Intracellular Ca2+ concentration change of T24 cell under irradiation in the presence of TiO2 ultrafine particles. Biochim. Biophys. Acta 1994, 1201, 259–265. [Google Scholar] [CrossRef]
- Hoenes, K.; Stangl, F.; Gross, A.; Hessling, M. Improved contact lens disinfection by exposure to violet radiation. Technol. Health Care 2016, 24, 145–151. [Google Scholar] [CrossRef]
- Schmid, J.; Hoenes, K.; Vatter, P.; Hessling, M. Antimicrobial Effect of Visible Light-Photoinactivation of Legionella rubrilucens by Irradiation at 450, 470, and 620 nm. Antibiotics 2019, 8, 187. [Google Scholar] [CrossRef]
- Maclean, M.; Macgregor, S.J.; Anderson, J.G.; Woolsey, G.A. The role of oxygen in the visible-light inactivation of Staphylococcus aureus. J. Photochem. Photobiol. B 2008, 92, 180–184. [Google Scholar] [CrossRef]
- Ishihara Sangyou Kaisha, Ltd. Safety data sheet of MPT-427; Version: 2.0.; Ishihara Sangyou Kaisha, Ltd.: Osaka, Japan, 2021. [Google Scholar]
- Photopaque (R) Visible Light Activation Type MPT-623 (Powder) STS-427 (Water Dispesion). Available online: https://www.iskweb.co.jp/eng/products/pdf/MPT-623.pdf (accessed on 29 July 2022).





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuura, R.; Kawamura, A.; Matsumoto, Y.; Fukushima, T.; Fujimoto, K.; Ochiai, H.; Somei, J.; Aida, Y. Rutile-TiO2/PtO2 Glass Coatings Disinfects Aquatic Legionella pneumophila via Morphology Change and Endotoxin Degradation under LED Irradiation. Catalysts 2022, 12, 856. https://doi.org/10.3390/catal12080856
Matsuura R, Kawamura A, Matsumoto Y, Fukushima T, Fujimoto K, Ochiai H, Somei J, Aida Y. Rutile-TiO2/PtO2 Glass Coatings Disinfects Aquatic Legionella pneumophila via Morphology Change and Endotoxin Degradation under LED Irradiation. Catalysts. 2022; 12(8):856. https://doi.org/10.3390/catal12080856
Chicago/Turabian StyleMatsuura, Ryosuke, Arisa Kawamura, Yasunobu Matsumoto, Takashi Fukushima, Kazuhiro Fujimoto, Heihachiro Ochiai, Junichi Somei, and Yoko Aida. 2022. "Rutile-TiO2/PtO2 Glass Coatings Disinfects Aquatic Legionella pneumophila via Morphology Change and Endotoxin Degradation under LED Irradiation" Catalysts 12, no. 8: 856. https://doi.org/10.3390/catal12080856
APA StyleMatsuura, R., Kawamura, A., Matsumoto, Y., Fukushima, T., Fujimoto, K., Ochiai, H., Somei, J., & Aida, Y. (2022). Rutile-TiO2/PtO2 Glass Coatings Disinfects Aquatic Legionella pneumophila via Morphology Change and Endotoxin Degradation under LED Irradiation. Catalysts, 12(8), 856. https://doi.org/10.3390/catal12080856






