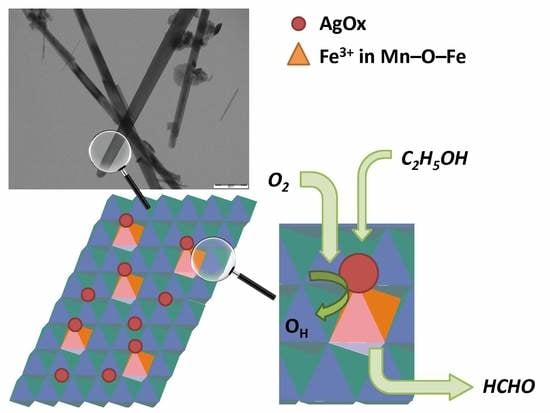
Synergistic Effect in Ag/Fe–MnO2 Catalysts for Ethanol Oxidation
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD and XPS Results
2.2. Study of Texture Properties and Morphology
2.3. H2–TPR and TPO Results
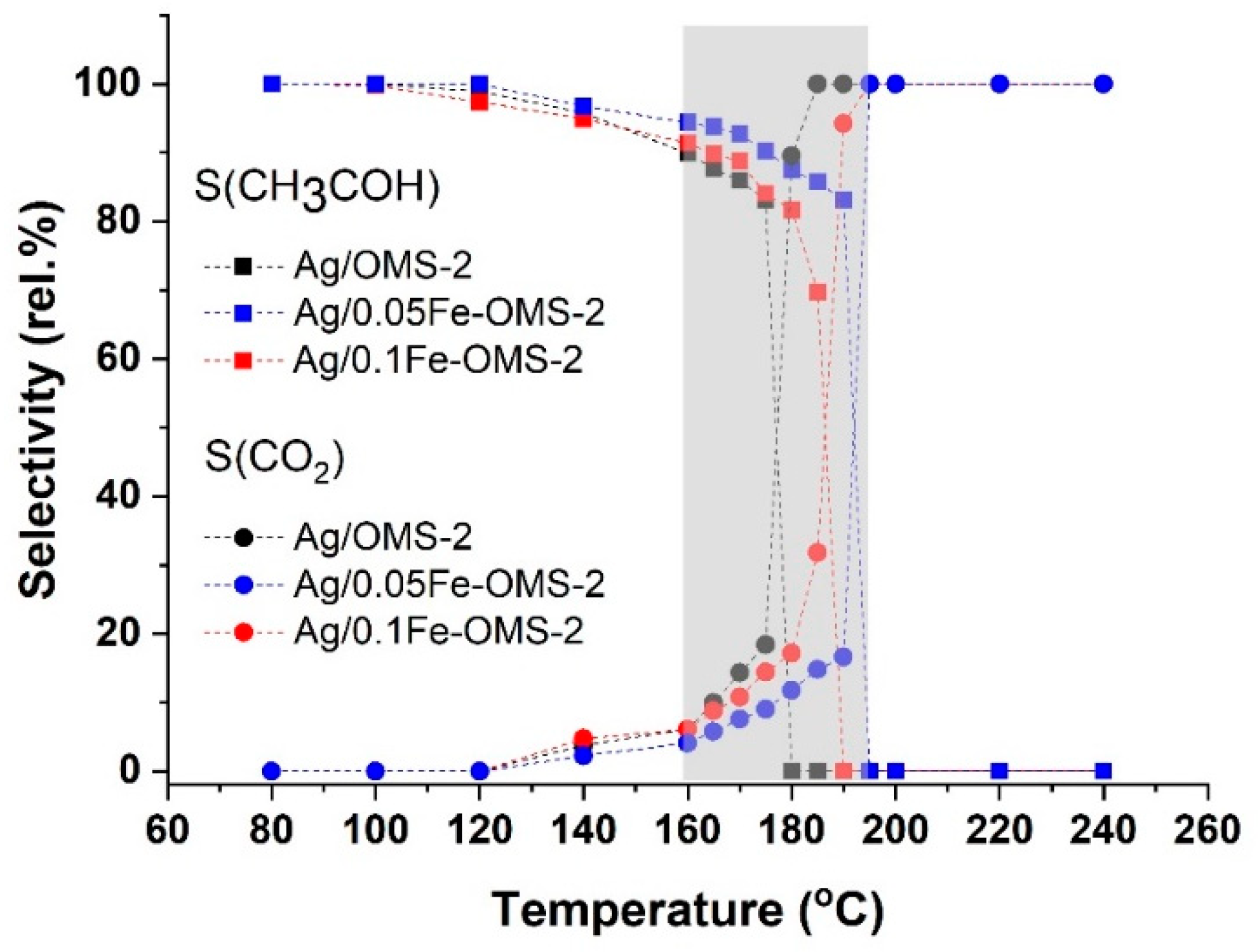
2.4. Catalytic Properties of the Ag-Containing Catalysts
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Assal, M.; Shaik, M.R.; Kuniyil, M.; Khan, M.; Kumar, J.V.S.; Alzahrani, A.Y.; Al-Warthan, A.; Al-Tamrah, S.A.; Siddiqui, M.R.H.; Hashmi, S.A.; et al. Silver-doped manganese based nanocomposites for aerial oxidation of alcohols. Mater. Express 2018, 8, 35–54. [Google Scholar] [CrossRef]
- Li, D.; Yang, G.; Li, P.; Wang, J.; Zhang, P. Promotion of formaldehyde oxidation over Ag catalyst by Fe doped MnOx support at room temperature. Catal. Today 2016, 277, 257–265. [Google Scholar] [CrossRef]
- Ochoa, J.V.; Cavani, F. Gas-Phase Oxidation of Alcohols: Innovation in Industrial Technologies and Recent Developments. RSC Green Chem. 2015, 28, 203–230. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Chesalov, Y.A.; Saraev, A.A.; Klyushin, A.Y.; Knop-Gericke, A.; Andrushkevich, T.V.; Bukhtiyarov, V.I. Redox mechanism for selective oxidation of ethanol over monolayer V2O5/TiO2 catalysts. J. Catal. 2016, 338, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Hensen, E.J.M. Highly Efficient and Robust Au/MgCuCr2O4 Catalyst for Gas-Phase Oxidation of Ethanol to Acetaldehyde. J. Am. Chem. Soc. 2013, 135, 14032–14035. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Luo, H.; Wang, J.; Han, B.; Mei, F.; Liu, P. Synergistic effect between gold nanoparticles and Fe-doped γ-MnO2 toward enhanced aerobic selective oxidation of ethanol. Catal. Sci. Technol. 2020, 10, 4332–4339. [Google Scholar] [CrossRef]
- Grabchenko, M.V.; Mamontov, G.V.; Zaikovskii, V.I.; La Parola, V.; Liotta, L.F.; Vodyankina, O.V. Design of Ag-CeO2/SiO2 catalyst for oxidative dehydrogenation of ethanol: Control of Ag–CeO2 interfacial interaction. Catal. Today 2019, 333, 2–9. [Google Scholar] [CrossRef]
- Vodyankina, O.V.; Blokhina, A.S.; Kurzina, I.A.; Sobolev, V.I.; Koltunov, K.Y.; Chukhlomina, L.N.; Dvilis, E.S. Selective oxidation of alcohols over Ag-containing Si3N4 catalysts. Catal. Today 2013, 203, 127–132. [Google Scholar] [CrossRef]
- Son, Y.C.; Makwana, V.D.; Howell, A.R.; Suib, S.L. Efficient, Catalytic, Aerobic Oxidation of Alcohols with Octahedral Molecular Sieves. Angew. Chem. Int. Ed. 2001, 40, 4280–4283. [Google Scholar] [CrossRef]
- Jia, X.; Ma, J.; Xia, F.; Gao, J.; Xu, J. Switching acidity on manganese oxide catalyst with acetylacetones for selectivity-tunable amines oxidation. Nat. Commun. 2019, 10, 2338. [Google Scholar] [CrossRef] [Green Version]
- Elmaci, G.; Erturk, A.S.; Sevim, M.; Metin, O. MnO2 nanowires anchored on mesoporous graphitic carbon nitride (MnO2@mpg-C3N4) as a highly efficient electrocatalyst for the oxygen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 17995–18006. [Google Scholar] [CrossRef]
- Elmaci, G.; Cerci, S.; Sunar-Cerci, D. The evaluation of the long-term stability of α-MnO2 based OER electrocatalyst in neutral medium by using data processing approach. J. Mol. Struct. 2019, 1195, 632–640. [Google Scholar] [CrossRef]
- de Guzman, R.N.; Shen, Y.-F.; Neth, E.J.; Suib, S.L.; O’Young, C.L.; Levine, J.M.; Newsam, S. Synthesis and Characterization of Octahedral Molecular Sieves (OMS-2) Having the Hollandite Structure. Chem. Mater. 1994, 6, 815–821. [Google Scholar] [CrossRef]
- Rasul, S.; Suzuki, S.; Yamaguchi, S.; Miyayama, M. Manganese oxide octahedral molecular sieves as insertion electrodes for rechargeable Mg batteries. Electrochim. Acta 2013, 110, 247–252. [Google Scholar] [CrossRef]
- Jiang, C.H.; Dou, S.X.; Liu, H.K.; Ichihara, M.; Zhou, H.S. Synthesis of spinel LiMn2O4 nanoparticles through one-step hydrothermal reaction. J. Power Sources 2007, 172, 410–415. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, J.; Chen, S.; Hammond, P.T.; Shao-Horn, Y. Carbon Nanotube/Manganese Oxide Ultrathin Film Electrodes for Electrochemical Capacitors. ACS Nano 2010, 4, 3889–3896. [Google Scholar] [CrossRef]
- Zhang, L.L.; Tu, J.J.; Lyu, L.; Hu, C. Enhanced catalytic degradation of ciprofloxacin over Ce-doped OMS-2 microspheres. Appl. Catal. B 2016, 181, 561–569. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, J.; Zhang, N.; Zheng, K.; Hu, Y.; Liu, X.; Meng, X. The Effect of Metal Ions as Dopants on OMS-2 in the Catalytic Degradation. Catal. Lett. 2020, 150, 2021–2026. [Google Scholar] [CrossRef]
- Deng, H.; Kang, S.; Ma, J.; Zhang, C.; He, H. Silver incorporated into cryptomelane-type Manganese oxide boosts the catalytic oxidation of benzene. Appl. Catal. B 2018, 239, 214–222. [Google Scholar] [CrossRef]
- He, B.; Cheng, G.; Zhao, S.; Zeng, X.; Li, Y.; Yang, R.; Sun, M.; Yu, L. Controlled synthesis of tunnel-structured MnO2 through hydrothermal transformation of δ-MnO2 and their catalytic combustion of dimethyl ether. J. Sol. State Chem. 2019, 269, 305–311. [Google Scholar] [CrossRef]
- Sun, M.; Li, W.; Zhang, B.; Cheng, G.; Lan, B.; Ye, F.; Zheng, Y.; Cheng, X.; Yu, L. Enhanced catalytic performance by oxygen vacancy and active interface originated from facile reduction of OMS-2. Chem. Eng. J. 2018, 331, 626–635. [Google Scholar] [CrossRef]
- Özacara, M.; Poyraz, A.S.; Genuino, H.C.; Kuo, C.-H.; Meng, Y.; Sui, S.L. Influence of silver on the catalytic properties of the cryptomelane and Ag-hollandite types manganese oxides OMS-2 in the low-temperature CO oxidation. Appl. Catal. A 2013, 462–463, 64–74. [Google Scholar] [CrossRef]
- Ma, J.; Wang, C.; He, H. Transition metal doped cryptomelane-type manganese oxide catalysts for ozone decomposition. Appl. Catal. B 2017, 201, 503–510. [Google Scholar] [CrossRef]
- Liu, P.; Duan, J.; Ye, Q.; Mei, F.; Shu, Z.; Chen, H. Promoting effect of unreducible metal doping on OMS-2 catalysts for gas-phase selective oxidation of ethanol. J. Catal. 2018, 367, 115–125. [Google Scholar] [CrossRef]
- Dutov, V.V.; Mamontov, G.V.; Sobolev, V.I.; Vodyankina, O.V. Silica-supported silver-containing OMS-2 catalysts for ethanol oxidative dehydrogenation. Catal. Today 2016, 278, 164–173. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, J.Y.; Chen, X.; O’Young, C.L.; Sib, S.L. Studies of oxidative dehydrogenation of ethanol over manganese oxide octahedral molecular sieve catalysts. Microporous Mesoporous Mater. 1998, 21, 315–324. [Google Scholar] [CrossRef]
- Shen, X.; Morey, A.M.; Liu, J.; Ding, Y.; Cai, J.; Durand, J.; Wang, Q.; Wen, W.; Hines, W.A.; Hanson, J.C.; et al. Characterization of the Fe-Doped Mixed-Valent Tunnel Structure Manganese Oxide KOMS-2. J. Phys. Chem. C 2011, 115, 21610–21619. [Google Scholar] [CrossRef]
- Sun, M.; Yu, L.; Ye, F.; Diao, G.; Yu, Q.; Hao, Z.; Zheng, Y.; Yuan, L. Transition metal doped cryptomelane-type manganese oxide for low-temperature catalytic combustion of dimethyl ether. Chem. Eng. J. 2013, 220, 320–327. [Google Scholar] [CrossRef]
- Wang, H.; Jin, B.; Wang, H.; Ma, N.; Liu, W.; Weng, D.; Wu, X.; Liu, S. Study of Ag promoted Fe2O3@CeO2 as superior soot oxidation catalysts: The role of Fe2O3 crystal plane and tandem oxygen delivery. Appl. Catal. B 2018, 237, 251–262. [Google Scholar] [CrossRef]
- Durán, F.G.; Barbero, B.P.; Cadús, L.E.; Rojas, C.; Centeno, M.A.; Odriozola, J.A. Manganese and iron oxides as combustion catalysts of volatile organic compounds. Appl. Cat. B 2009, 92, 194–201. [Google Scholar] [CrossRef]
- Sun, W.; Li, X.; Zhao, Q.; Mu, J.; Chen, J. Fe–Mn Mixed Oxide Catalysts Synthesized by One-Step Urea-Precipitation Method for the Selective Catalytic Reduction of NOx with NH3 at Low Temperatures. Catal. Lett. 2018, 148, 227–234. [Google Scholar] [CrossRef]
- Vodyankina, O.V.; Mamontov, G.V.; Dutov, V.V.; Kharlamova, T.S.; Salaev, M.A. Ag-containing nanomaterials in heterogeneous catalysis: Advances and recent trends. In Advanced Nanomaterials for Catalysis and Energy, 1st ed.; Sadykov, V.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 5; pp. 143–175. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C.; Mohan, D.; Prasad, R. Synthesis of silver promoted CuMnOx catalyst for ambient temperature oxidation of carbon monoxide. J. Sci. Adv. Mater. Devices 2019, 4, 47–56. [Google Scholar] [CrossRef]
- Dutov, V.V.; Mamontov, G.V.; Zaikovskii, V.I.; Liotta, L.F.; Vodyankina, O.V. Low-temperature CO oxidation over Ag/SiO2 catalysts: Effect of OH/Ag ratio. Appl. Catal. B 2018, 221, 598–609. [Google Scholar] [CrossRef]
- Chen, D.; Qu, Z.; Sun, Y.; Gao, K.; Wang, Y. Identification of reaction intermediates and mechanism responsible for highly active HCHO oxidation on Ag/MCM-41 catalysts. Appl. Catal. B 2013, 142–143, 838–848. [Google Scholar] [CrossRef]
- Kharlamova, T.; Mamontov, G.; Salaev, M.; Zaikovskii, V.; Popova, G.; Sobolev, V.; Knyazev, A.; Vodyankina, O. Silica-supported silver catalysts modified by cerium/manganese oxides for total oxidation of formaldehyde. Appl. Catal. A 2013, 467, 519–529. [Google Scholar] [CrossRef]
- Ye, Q.; Zhao, J.; Huo, F.; Wang, J.; Cheng, S.; Kang, T.; Dai, H. Nanosized Ag/α-MnO2 catalysts highly active for the low-temperature oxidation of carbon monoxide and benzene. Catal. Today 2011, 175, 603–609. [Google Scholar] [CrossRef]
- Montaña, M.; Aparicio, M.S.L.; Ocsachoque, M.A.; Navas, M.B.; Barros, I.C.L.; Rodriguez-Castellón, E.; Casella, M.L.; Lick, I.D. Zirconia-Supported Silver Nanoparticles for the Catalytic Combustion of Pollutants Originating from Mobile Sources. Catalysts 2019, 9, 297. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Li, J.; Liu, Q.; Huang, X.; Shen, W. Chin Facile Synthesis of Ag-Hollandite Nanofibers and Their Catalytic Activity for Ethanol Selective Oxidation. J. Catal. 2007, 28, 1034–1036. [Google Scholar] [CrossRef]
- Khan, I.A.; Sajid, N.; Badshah, A.; Wattoo, M.H.S.; Anjum, D.H. CO Oxidation Catalyzed by Ag Nanoparticles Supported on SnO/CeO2. Chem. Soc. 2015, 26, 695–704. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Gao, Z.-W.; Yang, K.-F.; Zhang, W.-Q.; Xu, L.-W. Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catal. Sci. Technol. 2015, 5, 2554–2574. [Google Scholar] [CrossRef]
- Wen, C.; Yin, A.; Dai, W.-L. Recent advances in silver-based heterogeneous catalysts for green chemistry processes. Appl. Catal. B 2014, 160–161, 730–741. [Google Scholar] [CrossRef]
- Ho, P.H.; Lee, S.C.; Kim, J.; Lee, D.; Woo, H.C. Properties of a manganese oxide octahedral molecular sieve (OMS-2) for adsorptive desulfurization of fuel gas for fuel cell applications. Fuel Process. Technol. 2015, 131, 238–246. [Google Scholar] [CrossRef]
- Munoz-Rojas, D.; Oro-Sole, J.; Ayyad, O.; Gómez-Romero, P. Shaping hybrid nanostructures with polymer matrices: The formation mechanism of silver–polypyrrole core/shell nanostructures. J. Mater. Chem. 2011, 21, 2078–2086. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Makimizu, Y.; Nguyen, N.T.; Ahn, H.-J.; Yoo, J.E.; Hwang, I.; Kmentcand, S.; Schmuki, P.J. Effects of low oxygen annealing on the photoelectrochemical water splitting properties of α-Fe2O3. Mater. Chem. A 2020, 8, 1315–1325. [Google Scholar] [CrossRef]
- Flak, D.; Chen, Q.; Mun, B.S.; Liu, Z.; Rękas, M.; Braun, A. In situ ambient pressure XPS observation of surface chemistry and electronic structure of α-Fe2O3 and γ-Fe2O3 nanoparticles. Appl. Surf. Sci. 2018, 455, 1019–1028. [Google Scholar] [CrossRef]
- Santos, L.M.; Machado, W.A.; France, M.D.; Borges, K.A.; Paniago, R.M.; Patrocino, A.O.T.; Machado, A.E.H. Structural characterization of Ag-doped TiO2 with enhanced photocatalytic activity. RSC Adv. 2015, 5, 103752–103759. [Google Scholar] [CrossRef]
- Ren, H.; Jia, S.; Zoou, J.; Wu, S.; Han, X. A facile preparation of Ag2O/P25 photocatalyst for selective reduction of nitrate. Appl. Catal. B 2015, 176, 53–61. [Google Scholar] [CrossRef]
- Han, Q.; Liu, Z.; Xu, Y.; Chen, Z.; Wang, T.; Zhang, H. Growth and Properties of Single-Crystalline γ-Fe2O3 Nanowires. J. Phys. Chem. C 2007, 111, 5034–5038. [Google Scholar] [CrossRef]
- Idriss, H. On the wrong assignment of the XPS O1s signal at 531–532 eV attributed to oxygen vacancies in photo- and electro-catalysts for water splitting and other materials applications. Surf. Sci. 2021, 712, 121894. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Li, J.; Jiang, C.; Yunus, R.; Kim, J. Room-temperature oxidation of formaldehyde by layered manganese oxide: Effect of water. Environ. Sci. Technol. 2015, 49, 12372–12379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Wang, Y.; He, H. Sodium-Promoted Pd/TiO2 for Catalytic Oxidation of Formaldehyde at Ambient Temperature. Environ. Sci. Technol. 2014, 48, 5816–5822. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.G.; Jenne, E.A.; Chao, T.T. The sorption of silver by poorly crystallized manganese oxides. Geochim. Cosmochim. Acta 1978, 37, 611–622. [Google Scholar] [CrossRef]
- Dutov, V.V.; Mamontov, G.V.; Zaikovskii, V.I.; Vodyankina, O.V. The effect of support pretreatment on activity of Ag/SiO2 catalysts in low-temperature CO oxidation. Catal. Today 2016, 278, 150–156. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Bastos, S.S.T.; Orfao, J.J.M.; Pereira, M.F.R.; Delgado, J.J.; Fifueiredo, J.L. Carbon Monoxide Oxidation Catalysed by Exotemplated Manganese Oxides. Catal. Lett. 2010, 134, 217–227. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Ivanova, I.I.; Taarning, E. Mechanistic Study of Ethanol Dehydrogenation over Silica-Supported Silver. ChemCatChem 2013, 5, 2367–2373. [Google Scholar] [CrossRef]
- Liu, Q.; Bie, Y.; Qiu, S.; Zhang, Q.; Sainio, J.; Wang, T. Lehtonen, Hydrogenolysis of methyl heptanoate over Co based catalysts: Mediation of support property on activity and product distribution. J. Appl. Catal. B 2014, 147, 236–245. [Google Scholar] [CrossRef]
- Liang, S.; Teng, F.; Bulgan, G.; Zong, R.; Zhu, Y. Effect of Phase Structure of MnO2 Nanorod Catalyst on the Activity for CO Oxidation. J. Phys. Chem. C 2008, 112, 5307–5315. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, P.; Chen, L. The effect of morphology of α-MnO2 on catalytic decomposition of gaseous ozone. Catal. Sci. Technol. 2016, 6, 5841–5847. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zhang, Y.; Chen, M.; Wang, L.; Zhang, C.; He, H. Effect of Support on the Activity of Ag-based Catalysts for Formaldehyde Oxidation. Sci. Rep. 2015, 5, 12950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lup, N.K.; Abnisa, F.; Ashri, W.M.; Daud, W.; Aroua, M.K. Temperature-programmed reduction of silver(I) oxide using a titania-supported silver catalyst under a H2 atmosphere. J. Chin. Chem. Soc. 2019, 66, 1443–1455. [Google Scholar] [CrossRef]
- Savel’eva, A.S.; Vodyankina, O.V. Formation of the active surface of Ag/SiO2 catalysts in the presence of FeOx additives. Russ J. Phys. Chem. A 2014, 88, 2203–2208. [Google Scholar] [CrossRef]
- Santos, V.P.; Carabineiro, S.A.C.; Bakker, J.J.W.; Soares, O.S.G.P.; Chen, X.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L.; Gascon, J.; Kapteijn, F. Stabilized gold on cerium-modified cryptomelane: Highly active in low-temperature CO oxidation. J. Catal. 2014, 309, 58–65. [Google Scholar] [CrossRef]
- Dotsenko, S.S.; Verkhov, V.A.; Svetlichnyi, V.A.; Liotta, L.F.; La Parola, V.; Izaak, T.I.; Vodyankina, O.V. Oxidative dehydrogenation of ethanol on modified OMS-2 catalysts. Catal. Today 2020, 357, 503–510. [Google Scholar] [CrossRef]
- Idriss, H.; Seebauer, E.G. Effect of oxygen electronic polarisability on catalytic reactions over oxides. Catal. Lett. 2000, 66, 139–145. [Google Scholar] [CrossRef]
- Hu, S.; Wang, Y.; Wang, W.; Han, Y.; Fan, Q.; Feng, X.; Xu, Q.; Zhu, J. Ag Nanoparticles on Reducible CeO2(111) Thin Films: Effect of Thickness and Stoichiometry of Ceria. J. Phys. Chem. C 2015, 119, 3579–3588. [Google Scholar] [CrossRef]
- Hoh, S.W.; Thomas, L.; Jones, G.; Willock, D.J. A density functional study of oxygen vacancy formation on α-Fe2O3(0001) surface and the effect of supported Au nanoparticles. Res. Chem. Intermed. 2015, 41, 9587–9601. [Google Scholar] [CrossRef]
- An, N.; Wu, P.; Li, S.; Jia, M.; Zhang, W. Catalytic oxidation of formaldehyde over Pt/Fe2O3 catalysts prepared by different method. Appl. Surf. Sci. 2013, 285, 805–809. [Google Scholar] [CrossRef]
- Wang, P.; Duan, J.; Wang, J.; Mei, F.; Liu, P. Elucidating structure-performance correlations in gas-phase selective ethanol oxidation and CO oxidation over metal-doped γ-MnO2. Chin. J. Catal. 2020, 41, 1298. [Google Scholar] [CrossRef]
- Chen, J.; Tang, X.; Liu, J.; Zhan, E.; Li, J.; Huang, X.; Shen, W. Synthesis and Characterization of Ag−Hollandite Nanofibers and Its Catalytic Application in Ethanol Oxidation. Chem. Mater. 2007, 19, 4292–4299. [Google Scholar] [CrossRef]
- Wang, J.; Luo, H.; Liu, P. Highly dispersed gold nanoparticles on metal-doped α-MnO2 catalysts for aerobic selective oxidation of ethanol. Catal. Commun. 2020, 142, 106030. [Google Scholar] [CrossRef]
- Koltunov, K.Y.; Sobolev, V.I. Selective gas-phase oxidation of ethanol by molecular oxygen over oxide and gold-containing catalysts. Catal. Ind. 2012, 4, 247–252. [Google Scholar] [CrossRef]



| Sample | Ag/OMS-2 | Ag/0.05Fe–OMS-2 | Ag/0.1Fe–OMS-2 |
|---|---|---|---|
| Mn 2p3/2 | 640.6 | 640.4 | 641.0 |
| (eV) | 641.6 | 641.5 | 641.9 |
| Mn (III)/Mn(IV) | 0.13 | 0.17 | 0.28 |
| Fe 2p3/2 (eV) | – | 711.0 | 711.0 |
| Ag 3d5/2 (eV) | 368.1 | 368.0 | 368.0 |
| O1s-Lattice (eV) | 529.7 (59%) * | 529.6 (49%) | 529.8 (66%) |
| O1s-OH (eV) | 531.9 (41%) | 532.2 (51%) | 531.9 (34%) |
| Sample | K/Mn XPS (XRF) | Ag/Mn XPS (XRF) | Fe/Mn XPS (XRF) |
|---|---|---|---|
| Ag/OMS-2 | 0.14 (0.13) | 0.10 (0.052) | – |
| Ag/0.05Fe–OMS-2 | 0.10 (0.13) | 0.12 (0.056) | 0.052 (0.054) |
| Ag/0.1Fe–OMS-2 | 0.04 (0.10) | 0.08 (0.051) | 0.125 (0.098) |
| Sample | SBET (m2/g) | Vp (cm3/g) | dp (nm) |
|---|---|---|---|
| Ag/OMS-2 | 49 | 0.30 | 27.3 |
| Ag/0.05Fe–OMS-2 | 41 | 0.27 | 26.9 |
| Ag/0.1Fe–OMS-2 | 33 | 0.27 | 30.6 |
| Sample | T50 (°C) | SAc50 (%) | T80 (°C) | SAc80 (%) | SCO280 (%) |
|---|---|---|---|---|---|
| Ag/OMS-2 | 131 | 97 | 150 | 93 | 5.0 |
| Ag/0.05Fe–OMS-2 | 135 | 96 | 155 | 93 | 3.6 |
| Ag/0.1Fe–OMS-2 | 166 | 93 | 190 | 82 | 9.2 |
| OMS-2 [67] | 132 | 87 | 152 | 69 | 17 |
| Catalyst | Ethanol/O2 (vol./vol) | SV (ml/gcat/h) | T50 (°C) | SAc50 (%) | T80 (°C) | SAc80 (%) | Ref. |
|---|---|---|---|---|---|---|---|
| K–OMS-2 | 1/1 | 100,000 | 190 | 100 | 220 | 82 | [24] |
| OMS-2 | 2/18 | 7200 | 132 | 87 | 152 | 69 | [67] |
| Fe–γ-MnO2 (M/Mn = 1/9) | 1/1 | 100,000 | 185 | 97 | 220 | 90 | [72] |
| Fe–OMS-2 (M/Mn = 1/30) | 1/1 | 100,000 | 180 | 95 | 212 | 65 | [72] |
| Ag/OMS-2 (5 wt.% Ag) | 2/18 | 7200 | 131 | 97 | 150 | 93 | This work |
| Ag-hollandite nanofibers AgMnO4/Mn(NO3)2 = 2/3 | 5/10 | 36,000 | 220 | 90 | 235 | 80 | [73] |
| Ag/Fe–OMS-2 (Mn/Fe = 1/20, 5 wt.% Ag) | 2/18 | 7200 | 135 | 96 | 155 | 93 | This work |
| Ag/Fe–OMS-2 (Mn/Fe = 1/10, 5 wt.% Ag) | 2/18 | 7200 | 166 | 93 | 190 | 82 | This work |
| Au/Fe–OMS-2 (1 wt.% Au) | 2.5/2.5 | 100,000 | – | – | 200 | 65 | [74] |
| V2O5–TiO2 | 2/18 | 3600 1/h | 110 | 60 * | 130 | 80 * | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulchakovskaya, E.V.; Dotsenko, S.S.; Liotta, L.F.; La Parola, V.; Galanov, S.I.; Sidorova, O.I.; Vodyankina, O.V. Synergistic Effect in Ag/Fe–MnO2 Catalysts for Ethanol Oxidation. Catalysts 2022, 12, 872. https://doi.org/10.3390/catal12080872
Kulchakovskaya EV, Dotsenko SS, Liotta LF, La Parola V, Galanov SI, Sidorova OI, Vodyankina OV. Synergistic Effect in Ag/Fe–MnO2 Catalysts for Ethanol Oxidation. Catalysts. 2022; 12(8):872. https://doi.org/10.3390/catal12080872
Chicago/Turabian StyleKulchakovskaya, Ekaterina V., Svyatoslav S. Dotsenko, Leonarda F. Liotta, Valeria La Parola, Sergey I. Galanov, Olga I. Sidorova, and Olga V. Vodyankina. 2022. "Synergistic Effect in Ag/Fe–MnO2 Catalysts for Ethanol Oxidation" Catalysts 12, no. 8: 872. https://doi.org/10.3390/catal12080872