Degradation of Sulfamethoxazole Using a Hybrid CuOx–BiVO4/SPS/Solar System
Abstract
:1. Introduction
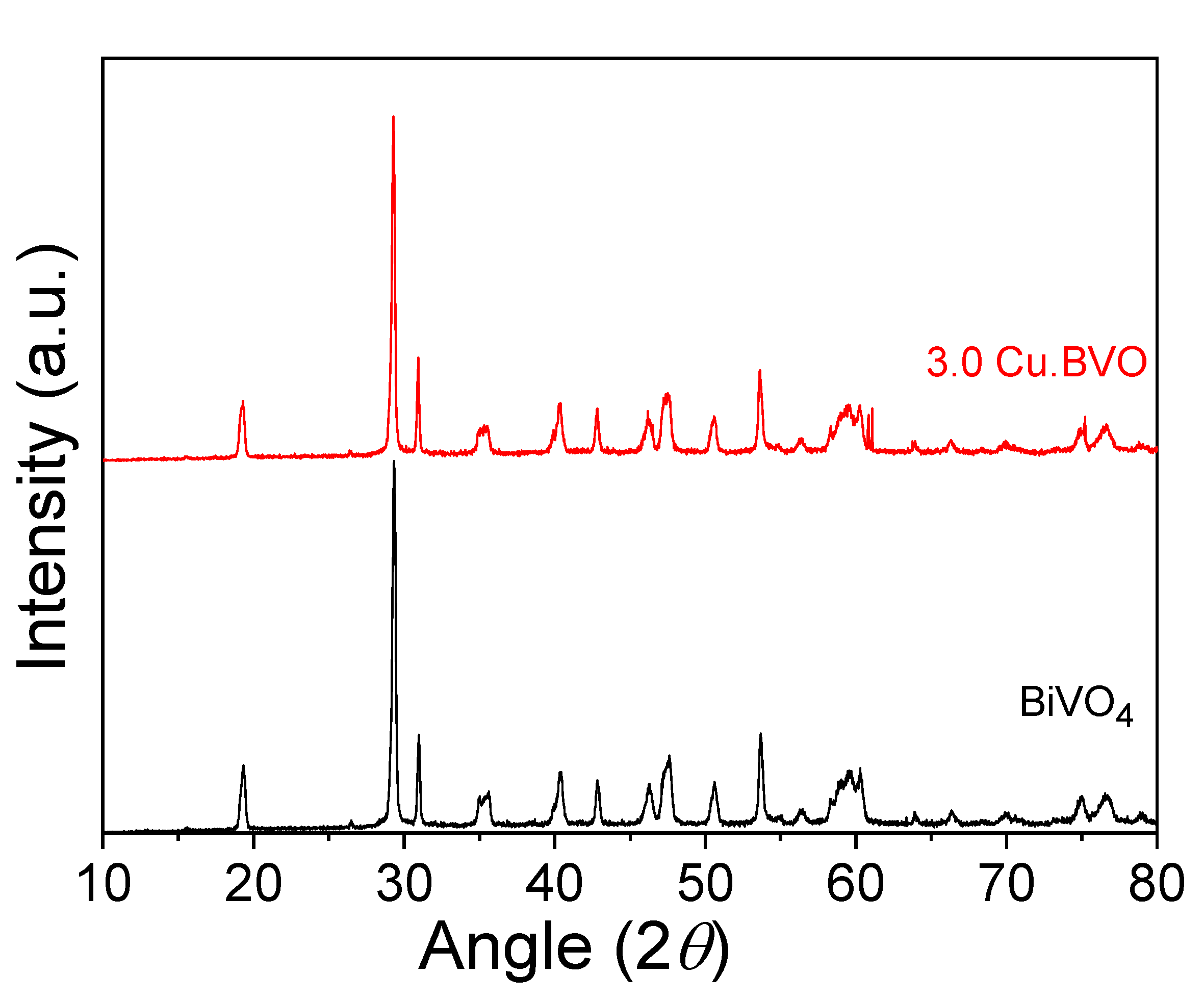
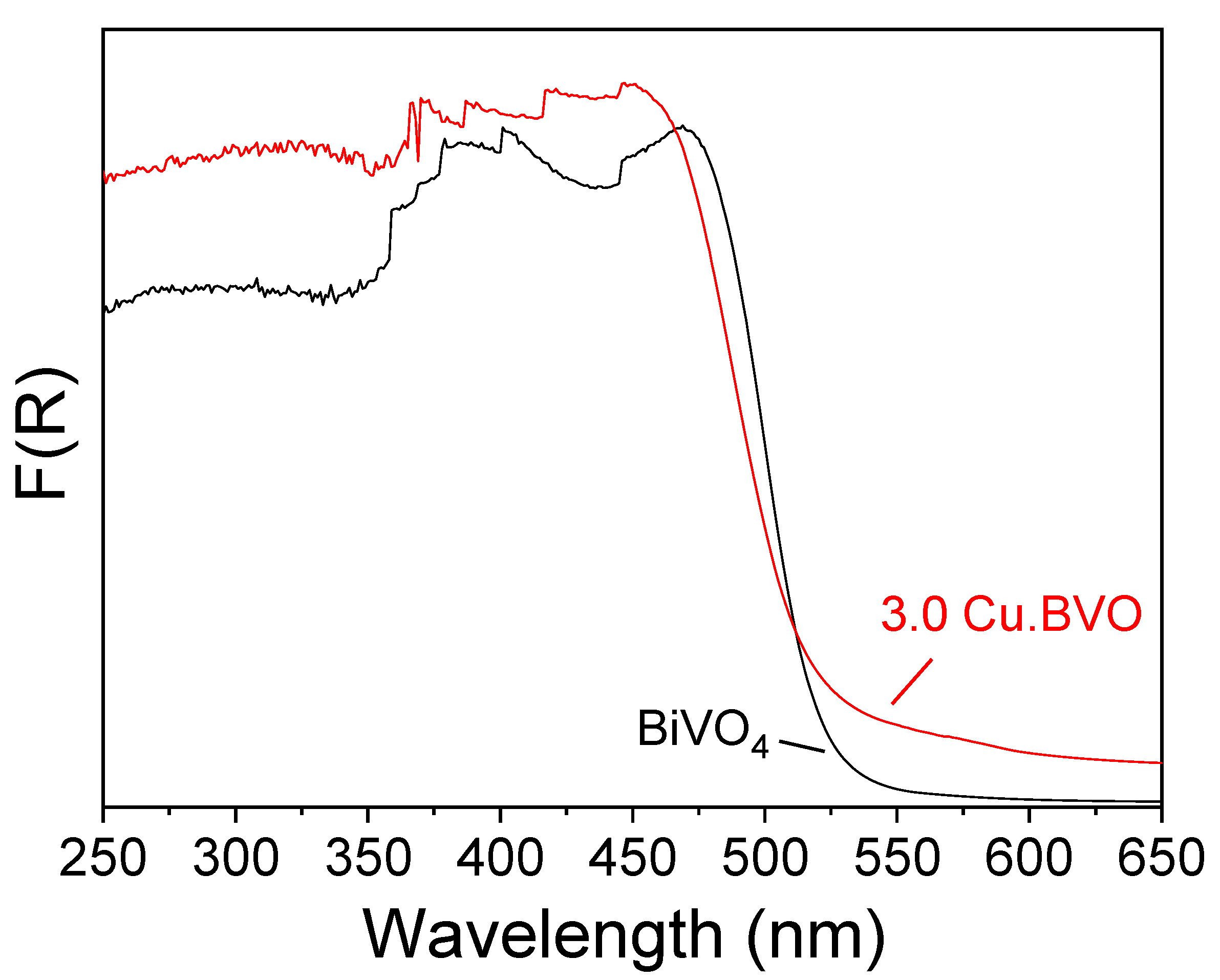
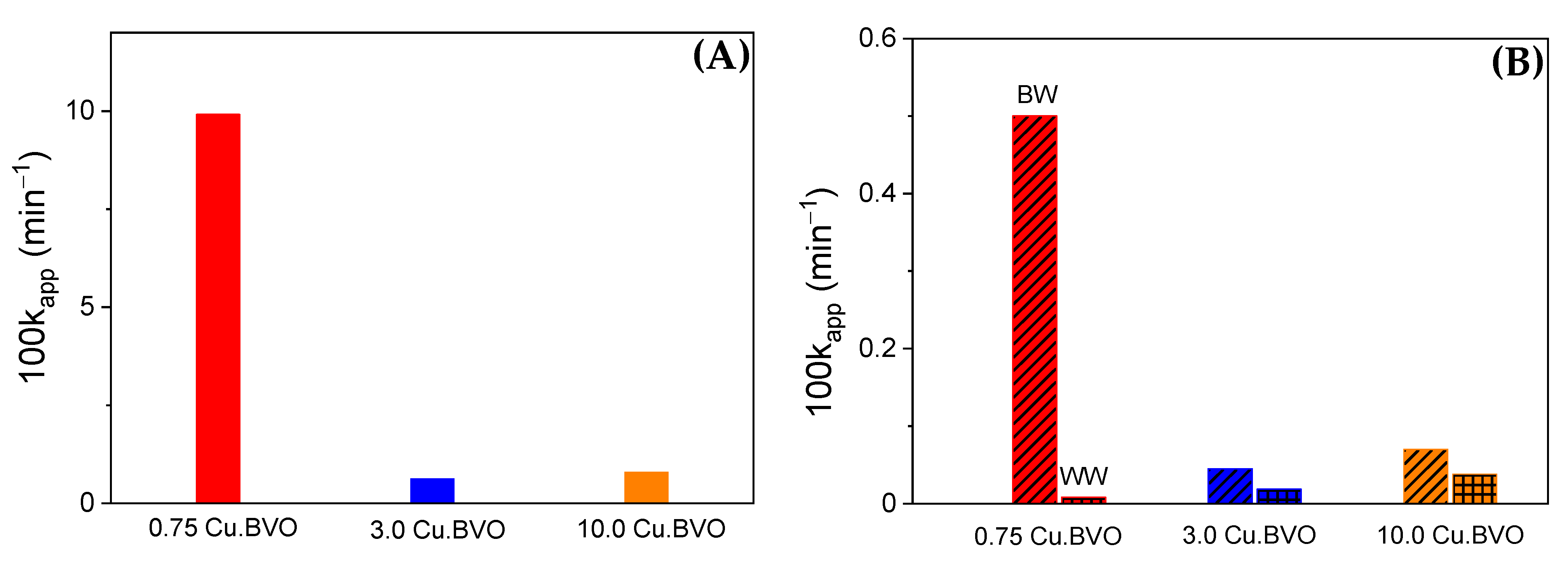
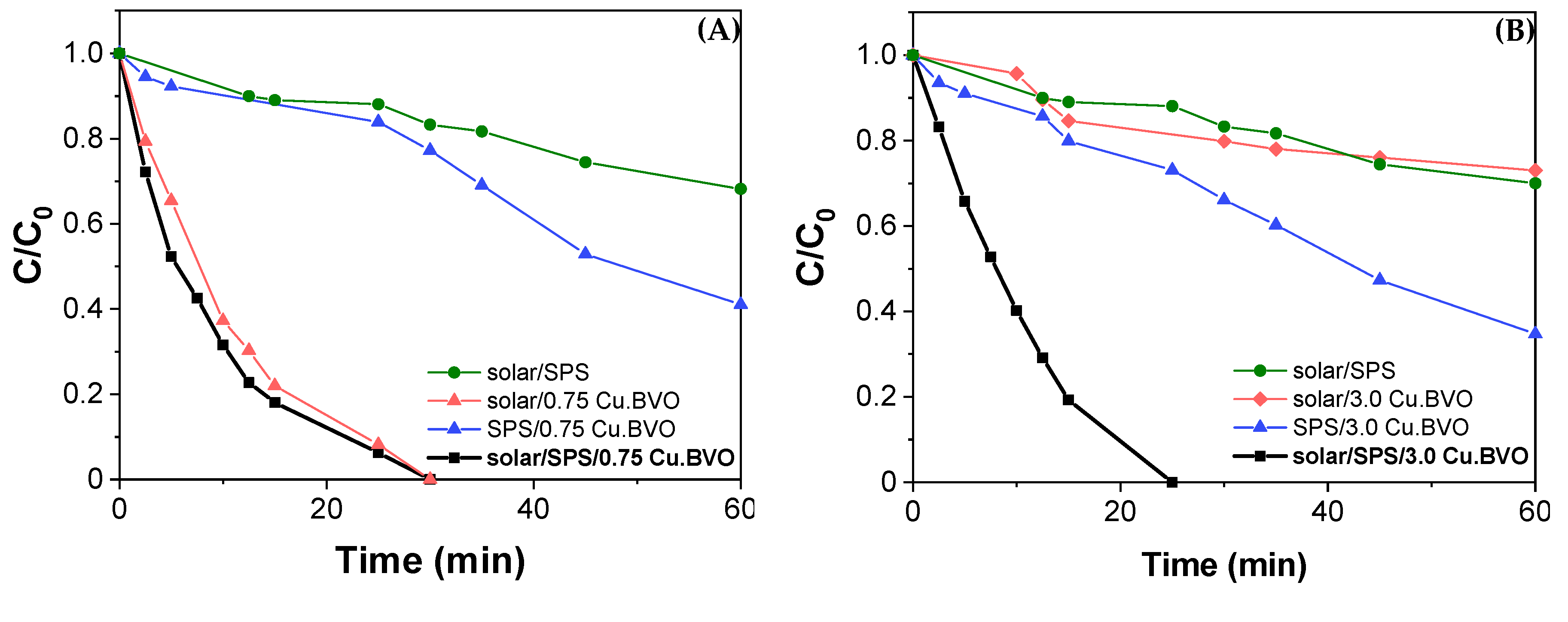
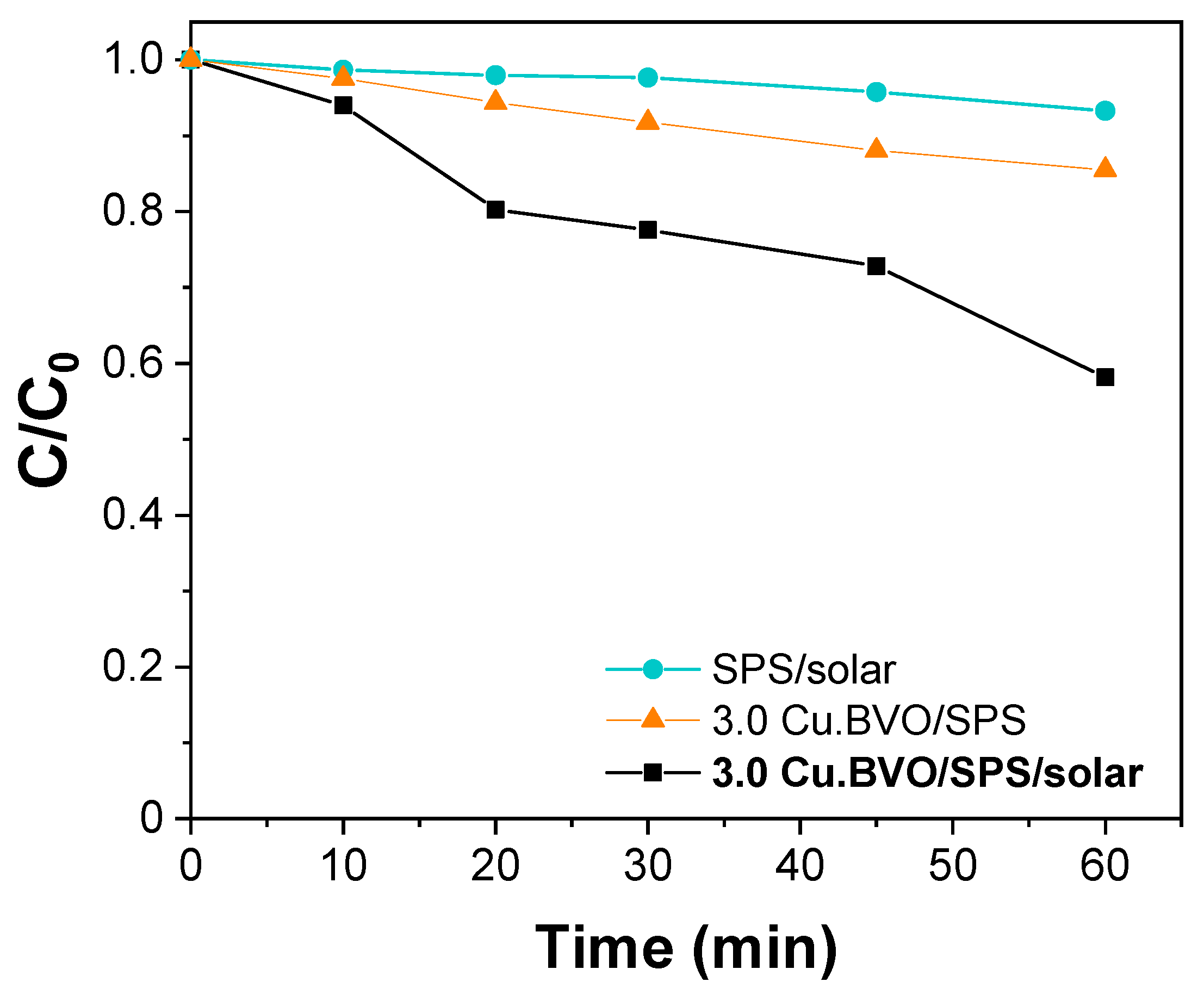
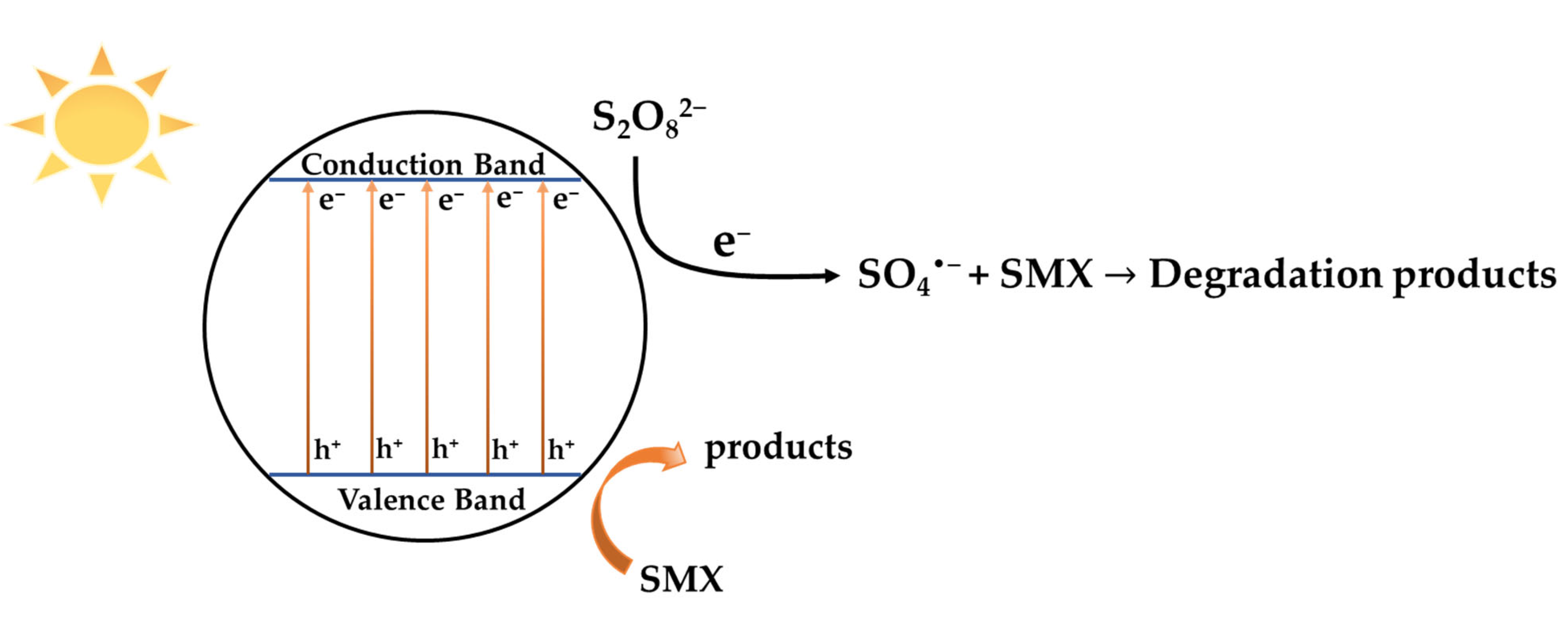
2. Results and Discussion
3. Materials and Methods
3.1. Chemical and Water Matrices
3.2. Catalyst Preparation and Characterization
3.3. Experimental Procedure and Analytical Methods
4. Conclusions
- (1)
- The hybrid system, solar/SPS/CuOx.BiVO4, leads to significantly improved photocatalytic yields than the individual systems (solar/CuOx.BiVO4 and SPS/CuOx.BiVO4), considering SMX degradation in real water matrices.
- (2)
- In real water matrices, the interaction between the photocatalytic-persulfate system and matrix components results in a synergistic rather than a cumulative effect, with a high degree of synergy. Using combined technologies seems to be preferred in very complex aqueous matrices where the competition for available oxidizing species and the catalyst surface are maximized.
- (3)
- The complexity of the water matrix determines the best performing catalytic material; 0.75 Cu.BVO showed the highest efficiency in UPW and BW, whereas 3.0 Cu.BVO and 10.0 Cu.BVO were characterized by higher activity in WW.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced Oxidation Processes in the Removal of Organic Substances from Produced Water: Potential, Configurations, and Research Needs. Chem. Eng. J. 2021, 414, 128668. [Google Scholar] [CrossRef]
- Petala, A.; Mantzavinos, D.; Frontistis, Z. Impact of Water Matrix on the Photocatalytic Removal of Pharmaceuticals by Visible Light Active Materials. Curr. Opin. Green Sustain. Chem. 2021, 28, 100445. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Y.; Liang, L.; Duan, X.; Li, R.; Lu, X.; Yan, B.; Li, N.; Wang, S. Remediation of Antibiotic Wastewater by Coupled Photocatalytic and Persulfate Oxidation System: A Critical Review. J. Hazard. Mater. 2021, 408, 124461. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.Y.; Aris, A.Z. Occurrence and Public-Perceived Risk of Endocrine Disrupting Compounds in Drinking Water. Npj Clean Water 2019, 2, 4. [Google Scholar] [CrossRef]
- Dimitrakopoulou, D.; Rethemiotaki, I.; Frontistis, Z.; Xekoukoulotakis, N.P.; Venieri, D.; Mantzavinos, D. Degradation, Mineralization and Antibiotic Inactivation of Amoxicillin by UV-A/TiO2 Photocatalysis. J. Environ. Manag. 2012, 98, 168–174. [Google Scholar] [CrossRef]
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The Technology Horizon for Photocatalytic Water Treatment: Sunrise or Sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibañez, P.; Di Somma, I. Solar Photocatalysis: Materials, Reactors, Some Commercial, and Pre-Industrialized Applications. A Comprehensive Approach. Appl. Catal. B Environ. 2015, 170–171, 90–123. [Google Scholar] [CrossRef]
- Wang, X.; Peng, Y.; Xie, T.; Zhang, Y.; Wang, Y.; Yang, H. Photocatalytic Removal of Sulfamethoxazole Using Yeast Biomass-Derived NixP/Biocarbon Composites in the Presence of Dye Sensitizer. J. Environ. Chem. Eng. 2022, 10, 107426. [Google Scholar] [CrossRef]
- Evgenidou, E.; Chatzisalata, Z.; Tsevis, A.; Bourikas, K.; Torounidou, P.; Sergelidis, D.; Koltsakidou, A.; Lambropoulou, D.A. Photocatalytic Degradation of a Mixture of Eight Antibiotics Using Cu-Modified TiO2 Photocatalysts: Kinetics, Mineralization, Antimicrobial Activity Elimination and Disinfection. J. Environ. Chem. Eng. 2021, 9, 105295. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Phan Thi, L.-A.; Chandana, P.S.; Do, H.-T.; Pham, T.-H.; Lee, T.; Nguyen, T.D.; Le Phuoc, C.; Huong, P.T. The Degradation of Paraben Preservatives: Recent Progress and Sustainable Approaches toward Photocatalysis. Chemosphere 2021, 276, 130163. [Google Scholar] [CrossRef]
- Velegraki, T.; Hapeshi, E.; Fatta-Kassinos, D.; Poulios, I. Solar-Induced Heterogeneous Photocatalytic Degradation of Methyl-Paraben. Appl. Catal. B Environ. 2015, 178, 2–11. [Google Scholar] [CrossRef]
- Wang, L.; Wang, K.; He, T.; Zhao, Y.; Song, H.; Wang, H. Graphitic Carbon Nitride-Based Photocatalytic Materials: Preparation Strategy and Application. ACS Sustain. Chem. Eng. 2020, 8, 16048–16085. [Google Scholar] [CrossRef]
- Sendão, R.M.S.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Photocatalytic Removal of Pharmaceutical Water Pollutants by TiO2–Carbon Dots Nanocomposites: A Review. Chemosphere 2022, 301, 134731. [Google Scholar] [CrossRef] [PubMed]
- Velempini, T.; Prabakaran, E.; Pillay, K. Recent Developments in the Use of Metal Oxides for Photocatalytic Degradation of Pharmaceutical Pollutants in Water—A Review. Mater. Today Chem. 2021, 19, 100380. [Google Scholar] [CrossRef]
- Shanavas, S.; Haija, M.A.; Singh, D.P.; Ahamad, T.; Roopan, S.M.; Van Le, Q.; Acevedo, R.; Anbarasan, P.M. Development of High Efficient Co3O4/Bi2O3/RGO Nanocomposite for an Effective Photocatalytic Degradation of Pharmaceutical Molecules with Improved Interfacial Charge Transfer. J. Environ. Chem. Eng. 2022, 10, 107243. [Google Scholar] [CrossRef]
- Luo, C.; Ma, J.; Jiang, J.; Liu, Y.; Song, Y.; Yang, Y.; Guan, Y.; Wu, D. Simulation and Comparative Study on the Oxidation Kinetics of Atrazine by UV/H2O2, UV/HSO5− and UV/S2O82−. Water Res. 2015, 80, 99–108. [Google Scholar] [CrossRef]
- Guo, P.-C.; Qiu, H.-B.; Yang, C.-W.; Zhang, X.; Shao, X.-Y.; Lai, Y.-L.; Sheng, G.-P. Highly Efficient Removal and Detoxification of Phenolic Compounds Using Persulfate Activated by MnOx@OMC: Synergistic Mechanism and Kinetic Analysis. J. Hazard. Mater. 2021, 402, 123846. [Google Scholar] [CrossRef]
- Shukla, P.; Wang, S.; Singh, K.; Ang, H.M.; Tadé, M.O. Cobalt Exchanged Zeolites for Heterogeneous Catalytic Oxidation of Phenol in the Presence of Peroxymonosulphate. Appl. Catal. B Environ. 2010, 99, 163–169. [Google Scholar] [CrossRef]
- Alexopoulou, C.; Petala, A.; Frontistis, Z.; Drivas, C.; Kennou, S.; Kondarides, D.I.; Mantzavinos, D. Copper Phosphide and Persulfate Salt: A Novel Catalytic System for the Degradation of Aqueous Phase Micro-Contaminants. Appl. Catal. B Environ. 2019, 244, 178–187. [Google Scholar] [CrossRef]
- Manos, D.; Papadopoulou, F.; Margellou, A.; Petrakis, D.; Konstantinou, I. Heterogeneous Activation of Persulfate by LaMO3 (M=Co, Fe, Cu, Mn, Ni) Perovskite Catalysts for the Degradation of Organic Compounds. Catalysts 2022, 12, 187. [Google Scholar] [CrossRef]
- Rao, Y.F.; Zhang, Y.; Han, F.; Guo, H.; Huang, Y.; Li, R.; Qi, F.; Ma, J. Heterogeneous Activation of Peroxymonosulfate by LaFeO3 for Diclofenac Degradation: DFT-Assisted Mechanistic Study and Degradation Pathways. Chem. Eng. J. 2018, 352, 601–611. [Google Scholar] [CrossRef]
- Guo, H.; Zhou, X.; Zhang, Y.; Yao, Q.; Qian, Y.; Chu, H.; Chen, J. Carbamazepine Degradation by Heterogeneous Activation of Peroxymonosulfate with Lanthanum Cobaltite Perovskite: Performance, Mechanism and Toxicity. J. Environ. Sci. 2020, 91, 10–21. [Google Scholar] [CrossRef]
- Kanigaridou, Y.; Petala, A.; Frontistis, Z.; Antonopoulou, M.; Solakidou, M.; Konstantinou, I.; Deligiannakis, Y.; Mantzavinos, D.; Kondarides, D.I. Solar Photocatalytic Degradation of Bisphenol A with CuOx/BiVO4: Insights into the Unexpectedly Favorable Effect of Bicarbonates. Chem. Eng. J. 2017, 318, 39–49. [Google Scholar] [CrossRef]
- Lalas, K.; Petala, A.; Frontistis, Z.; Konstantinou, I.; Mantzavinos, D. Sulfamethoxazole Degradation by the CuOx/Persulfate System. Catal. Today 2021, 361, 139–145. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Wang, Y.; Le Roux, J.; Yang, Y.; Croué, J.P. Efficient peroxydisulfate activation process not relying on sulfate radical generation for water pollutant degradation. Environ. Sci. Technol. 2014, 48, 5868–5875. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Chen, C.S.; Tu, Y.J.; Huang, Y.H.; Zhang, H. Heterogeneous degradation of organic pollutants by persulfate activated by CuO-Fe3O4: Mechanism, stability, and effects of pH and bicarbonate ions. Environ. Sci. Technol. 2015, 49, 6838–6845. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, D.; Jin, B.; Jiao, Z. The enhanced photocatalytic properties of BiOCl/BiVO4 p–n heterojunctions via plasmon resonance of metal Bi. RSC Adv. 2015, 5, 75947–75952. [Google Scholar] [CrossRef]
- Hirota, K.; Komatsu, G.; Yamashita, M.; Takemura, H.; Yamaguchi, O. Formation, characterization and sintering of alkoxy-derived bismuth vanadate. Mater. Res. Bull. 1992, 27, 823–830. [Google Scholar] [CrossRef]
- Sayama, K.; Nomura, A.; Zou, Z.; Abe, R.; Abe, Y.; Arakawa, H. Photoelectrochemical decomposition of water on nanocrystalline BiVO4 film electrodes under visible light. Chem. Commun. 2003, 3, 2908–2909. [Google Scholar] [CrossRef]
- Park, Y.; Mc Donald, K.J.; Choi, K.S. Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem. Soc. Rev. 2013, 42, 2321–2337. [Google Scholar] [CrossRef]
- Lu, Y.; Shang, H.; Shi, F.; Chao, C.; Zhang, X.; Zhang, B. Preparation and Efficient Visible Light-Induced Photocatalytic Activity of m-BiVO4 with Different Morphologies. J. Phys. Chem. Solids 2015, 85, 44–50. [Google Scholar] [CrossRef]
- Petala, A.; Bontemps, R.; Spartatouille, A.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Solar light-induced degradation of ethyl paraben with CuOx/BiVO4: Statistical evaluation of operating factors and transformation by-products. Catal. Today 2017, 280, 122–131. [Google Scholar] [CrossRef]
- Aguilera-Ruiz, E.; García-Pérez, U.M.; De La Garza-Galván, M.; Zambrano-Robledo, P.; Bermúdez-Reyes, B.; Peral, J. Efficiency of Cu2O/BiVO4 Particles Prepared with a New Soft Procedure on the Degradation of Dyes under Visible-Light Irradiation. Appl. Surf. Sci. 2015, 328, 361–367. [Google Scholar] [CrossRef]
- Xu, H.; Li, H.; Wu, C.; Chu, J.; Yan, Y.; Shu, H.; Gu, Z. Preparation, Characterization and Photocatalytic Properties of Cu-Loaded BiVO4. J. Hazard. Mater. 2008, 153, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-Q.; Endo, H.; Natori, H.; Nagai, M.; Kobayashi, K. Fabrication and Efficient Photocatalytic Degradation of Methylene Blue over CuO/BiVO4 Composite under Visible-Light Irradiation. Mater. Res. Bull. 2009, 44, 700–706. [Google Scholar] [CrossRef]
- Lado Ribeiro, A.R.; Moreira, N.F.F.; Li Puma, G.; Silva, A.M.T. Impact of Water Matrix on the Removal of Micropollutants by Advanced Oxidation Technologies. Chem. Eng. J. 2019, 363, 155–173. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Frontistis, Z.; Mantzavinos, D.; Silva, A.M.T.; Faria, J.L.; Gomes, H.T. Screening of Heterogeneous Catalysts for the Activated Persulfate Oxidation of Sulfamethoxazole in Aqueous Matrices. Does the Matrix Affect the Selection of Catalyst? J. Chem. Technol. Biotechnol. 2019, 94, 2425–2432. [Google Scholar] [CrossRef]
- Kemmou, L.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of antibiotic sulfamethoxazole by biochar-activated persulfate: Factors affecting the activation and degradation processes. Catal. Today 2018, 313, 128–133. [Google Scholar] [CrossRef]
- Yin, R.; Chen, Y.; He, S.; Li, W.; Zeng, L.; Guo, W.; Zhu, M. In situ photoreduction of structural Fe(III) in a metal–organic framework for peroxydisulfate activation and efficient removal of antibiotics in real wastewater. J. Hazard. Mater. 2020, 388, 121996. [Google Scholar] [CrossRef]
- Song, Y.; Huang, L.; Zhang, X.; Zhang, H.; Wang, L.; Zhang, H.; Liu, Y. Synergistic effect of persulfate and g-C3N4 under simulated solar light irradiation: Implication for the degradation of sulfamethoxazole. J. Hazard. Mater. 2020, 393, 122379. [Google Scholar] [CrossRef]
- Min, S.; Wang, F.; Jin, Z.; Xu, J. Cu2O Nanoparticles Decorated BiVO4 as an Effective Visible-Light-Driven p-n Heterojunction Photocatalyst for Methylene Blue Degradation. Superlattices Microstruct. 2014, 74, 294–307. [Google Scholar] [CrossRef]
- Petala, A.; Tsikritzis, D.; Kollia, M.; Ladas, S.; Kennou, S.; Kondarides, D.I. Synthesis and Characterization of N-Doped TiO2 Photocatalysts with Tunable Response to Solar Radiation. Appl. Surf. Sci. 2014, 305, 281–291. [Google Scholar] [CrossRef]
- Abellán, M.N.; Giménez, J.; Esplugas, S. Photocatalytic Degradation of Antibiotics: The Case of Sulfamethoxazole and Trimethoprim. Catal. Today 2009, 144, 131–136. [Google Scholar] [CrossRef]
- Liang, C.; Huang, C.F.; Mohanty, N.; Kurakalva, R.M. A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere 2008, 73, 1540–1543. [Google Scholar] [CrossRef] [PubMed]
- Metheniti, M.; Frontistis, Z.; Ribeiro, R.; Silva, A.; Faria, J.; Gomes, H.; Mantzavinos, D. Degradation of Propyl Paraben by Activated Persulfate Using Iron-Containing Magnetic Carbon Xerogels: Investigation of Water Matrix and Process Synergy Effects. Environ. Sci. Pollut. Res. 2018, 25, 34801–34810. [Google Scholar] [CrossRef]


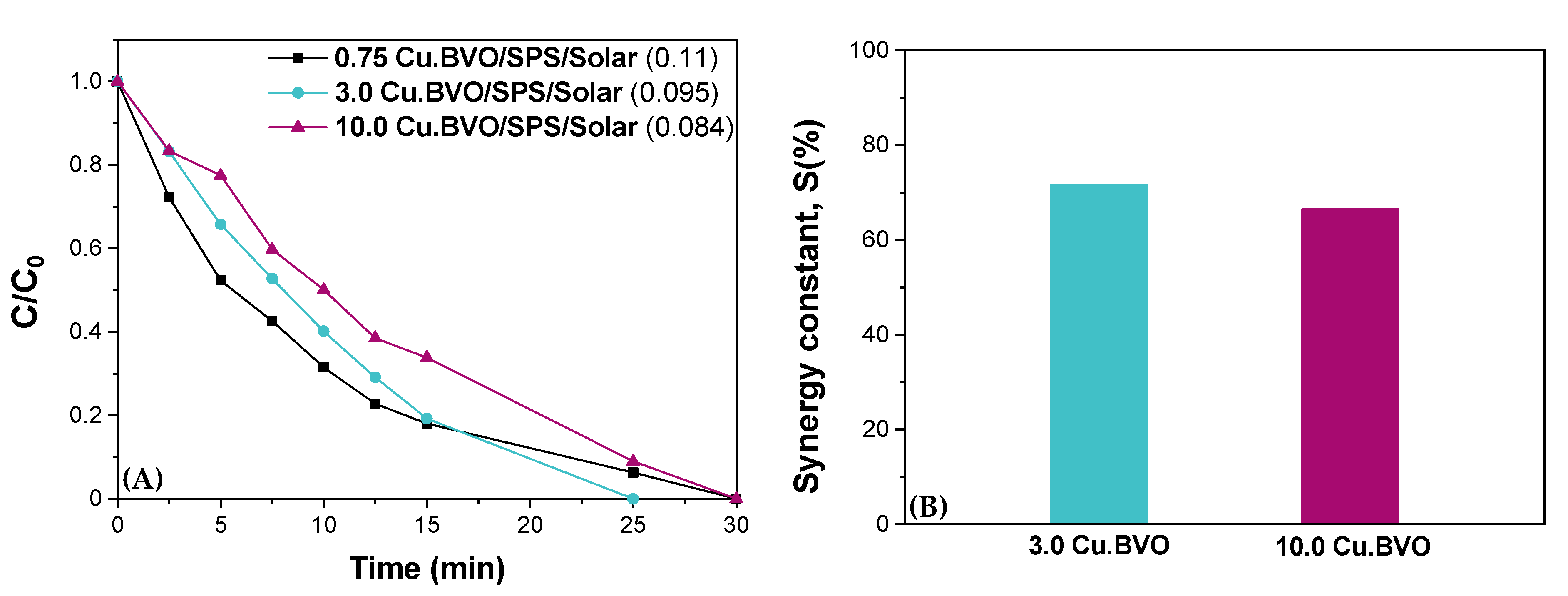
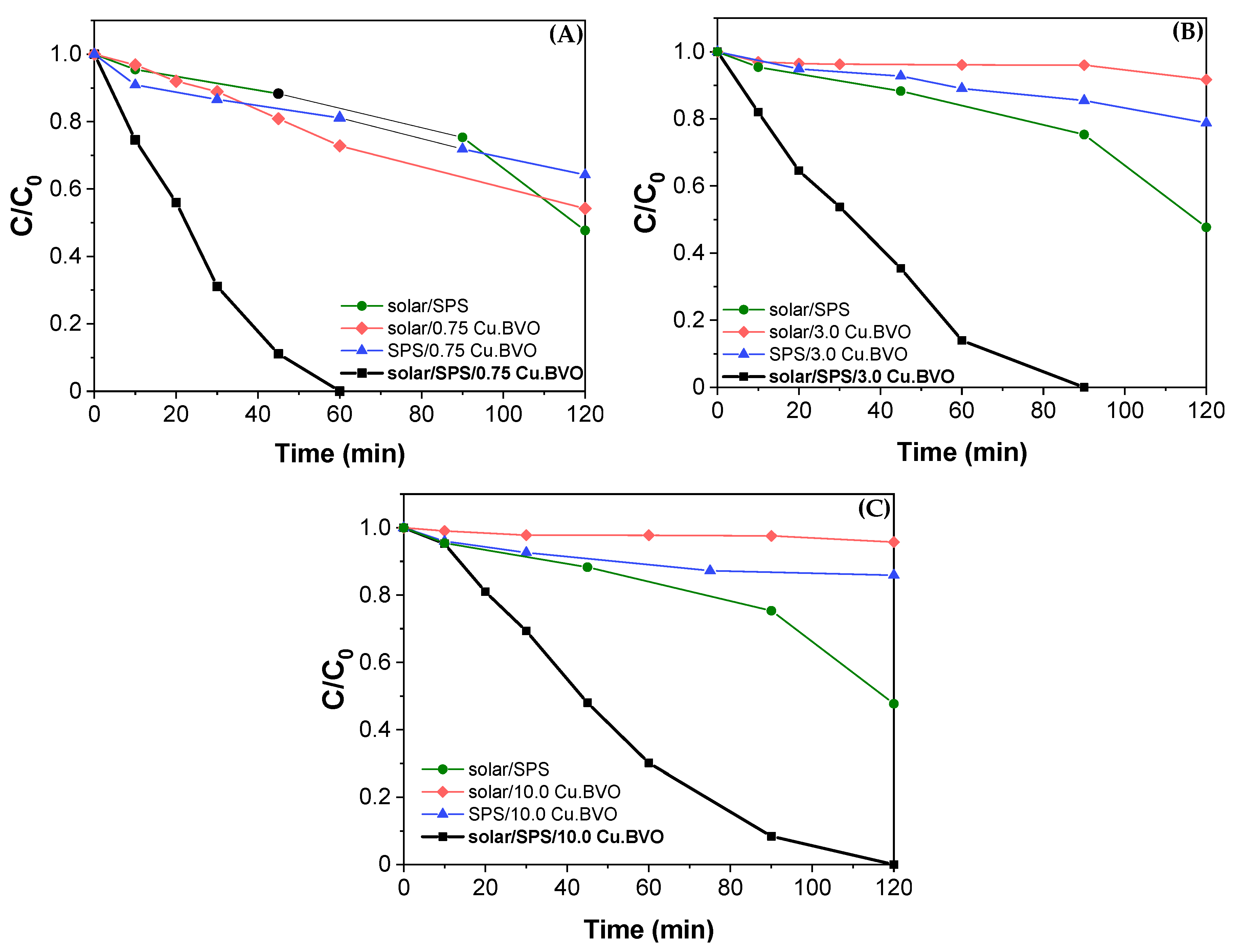
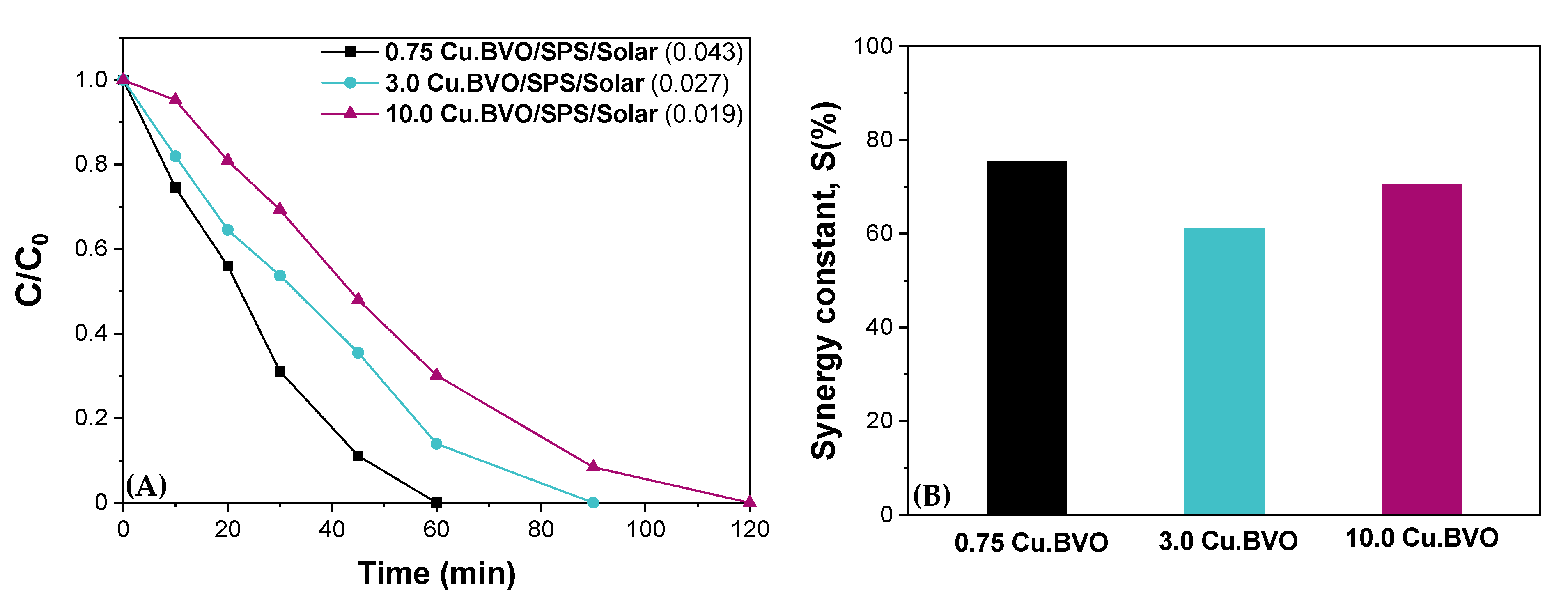

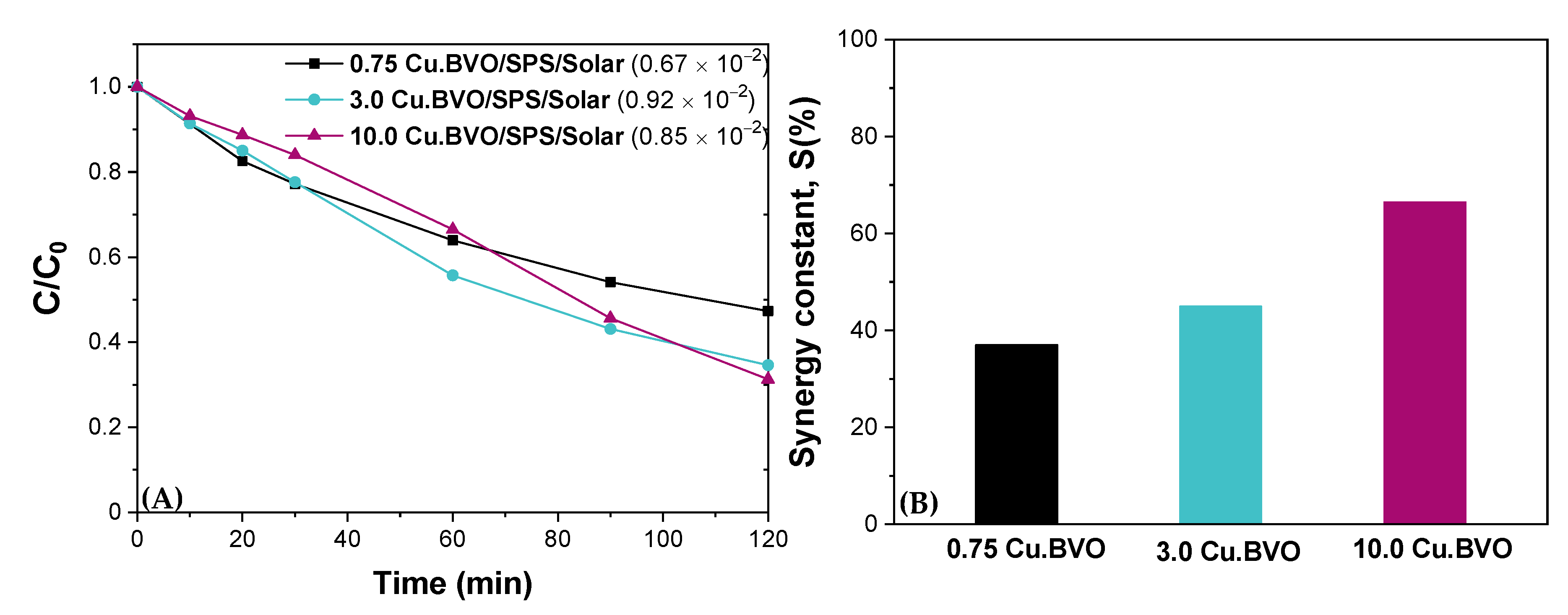

| Matrix | ktotal, 10−2 min−1 | S (%) | R2 | |
|---|---|---|---|---|
| 0.75 Cu.BVO | UPW | 11.33 | 0 | 0.998 |
| BW | 4.33 | 75.5 | 0.966 | |
| WW | 0.67 | 37 | 0.984 | |
| 3.0 Cu.BVO | UPW | 9.95 | 71.6 | 0.989 |
| BW | 2.27 | 61.1 | 0.968 | |
| WW | 0.92 | 45 | 0.997 | |
| 10.0 Cu.BVO | UPW | 8.45 | 68.2 | 0.975 |
| BW | 1.91 | 70.4 | 0.932 | |
| WW | 0.85 | 66.5 | 0.951 |
| SMX Concentration (Mg/L) | Catalyst Concentration (Mg/L) | Persulfate Concentration (Mg/L) | k (Min−1) | Time Period for Complete Degradation (Min) | Ref. | |
|---|---|---|---|---|---|---|
| Solar/SPS/Biochar (BC) | 0.25 | 90 | 250 | 0.065 | 90 | [38] |
| Solar/SPS/Cu3P | 0.5 | 40 | 100 | 0.114 | 20 | [19] |
| vis/PDS/MIF-100(Fe) | 10 | 500 | 1000 | 0.012 | 180 | [39] |
| solar/PDS/g-C3N4 | 1 | 500 | 120 | 0.068 | 60 | [40] |
| Solar/SPS/3.0 Cu.BVO | 0.5 | 500 | 100 | 0.099 | 25 | Present study |
| Parameter | WW | BW |
|---|---|---|
| pH | 8.0 | 7.4 |
| Conductivity (μs/cm) | 300 | 350 |
| Sulfate (mg/L) | 35 | 10 |
| Chloride (mg/L) | 75 | 8 |
| Nitrate (mg/L) | 60 | 10 |
| Bicarbonate (mg/L) | 180 | 240 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouvelis, K.; Kampioti, A.A.; Petala, A.; Frontistis, Z. Degradation of Sulfamethoxazole Using a Hybrid CuOx–BiVO4/SPS/Solar System. Catalysts 2022, 12, 882. https://doi.org/10.3390/catal12080882
Kouvelis K, Kampioti AA, Petala A, Frontistis Z. Degradation of Sulfamethoxazole Using a Hybrid CuOx–BiVO4/SPS/Solar System. Catalysts. 2022; 12(8):882. https://doi.org/10.3390/catal12080882
Chicago/Turabian StyleKouvelis, Konstantinos, Adamantia A. Kampioti, Athanasia Petala, and Zacharias Frontistis. 2022. "Degradation of Sulfamethoxazole Using a Hybrid CuOx–BiVO4/SPS/Solar System" Catalysts 12, no. 8: 882. https://doi.org/10.3390/catal12080882
APA StyleKouvelis, K., Kampioti, A. A., Petala, A., & Frontistis, Z. (2022). Degradation of Sulfamethoxazole Using a Hybrid CuOx–BiVO4/SPS/Solar System. Catalysts, 12(8), 882. https://doi.org/10.3390/catal12080882







