Modified Mn/ZSM-5 for Non-Thermal Plasma Mineralization of VOCs and DFT Simulation Using a Novel Y-Type ZSM-5 Model
Abstract
:1. Introduction
2. Results and Discussion
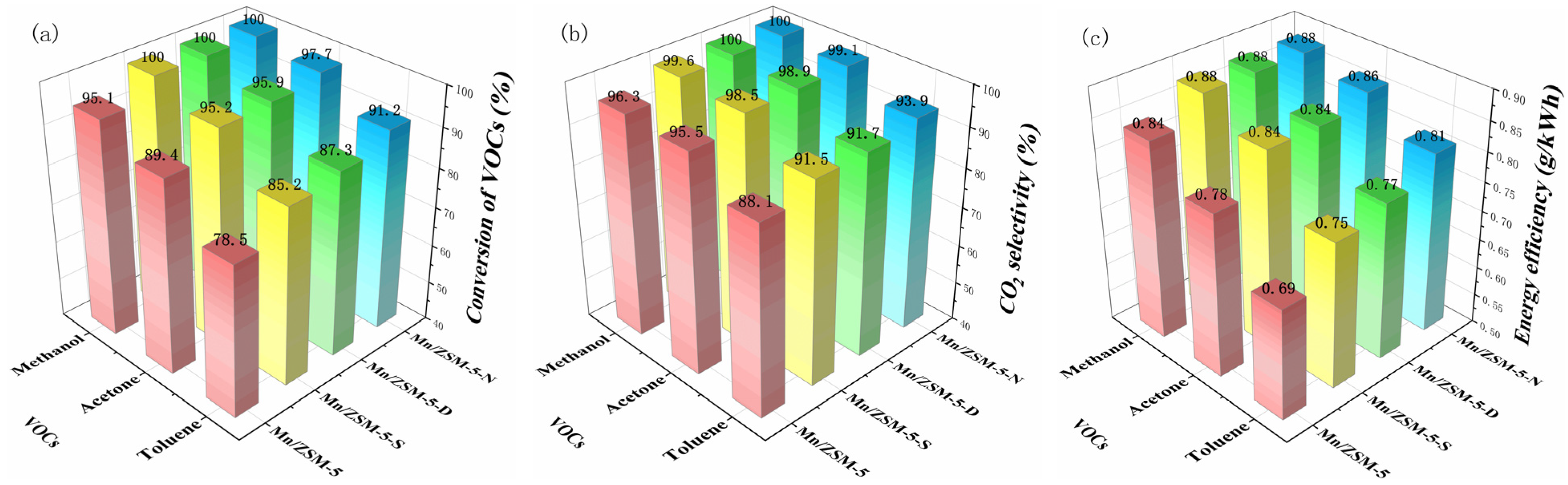
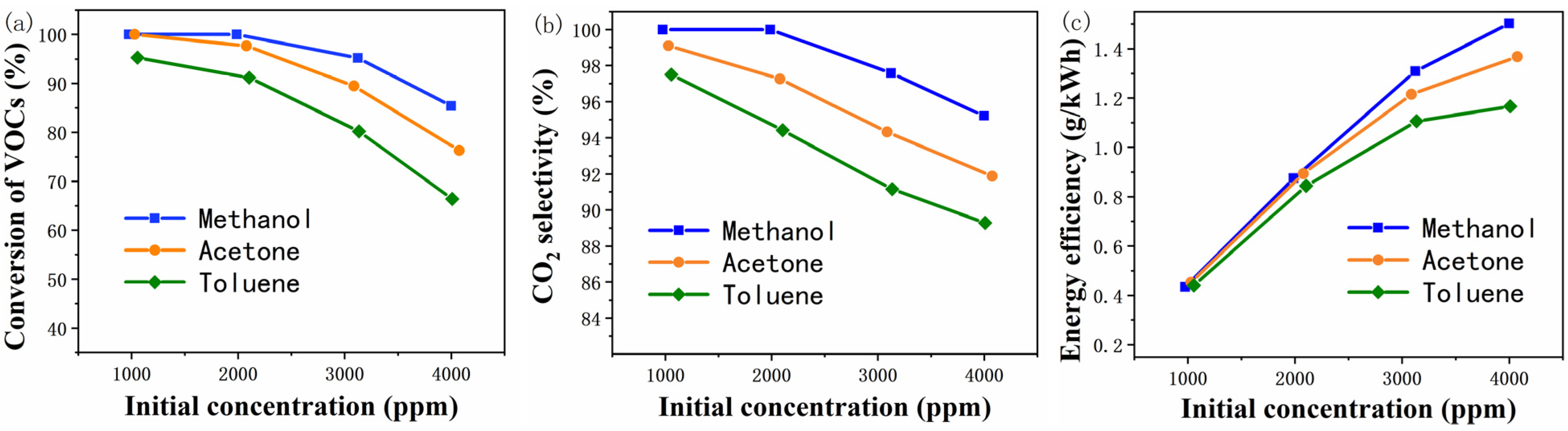
2.1. Catalytic Evaluation
2.2. Phase Analysis of Catalysts
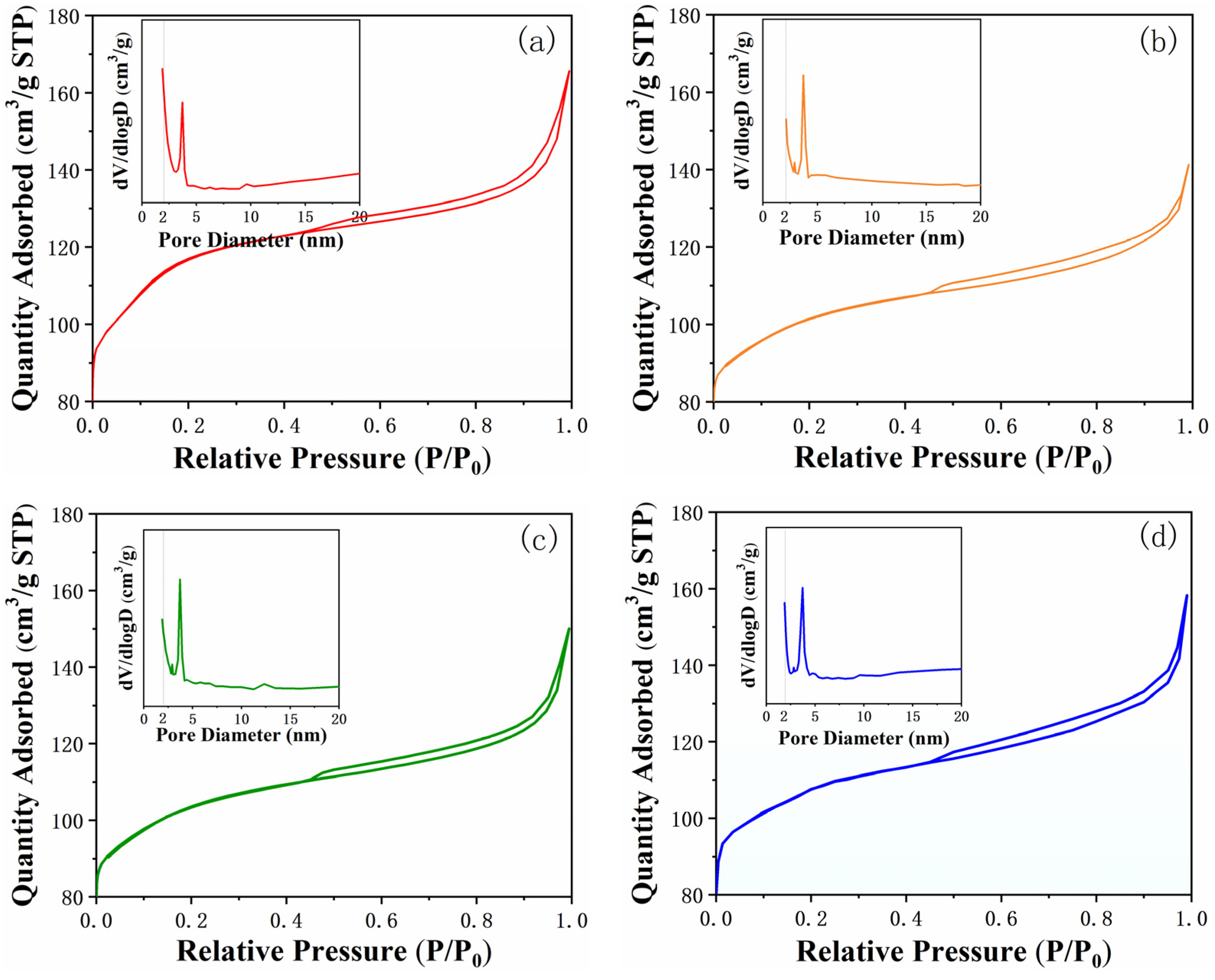
2.3. Pore Structure Study of Samples
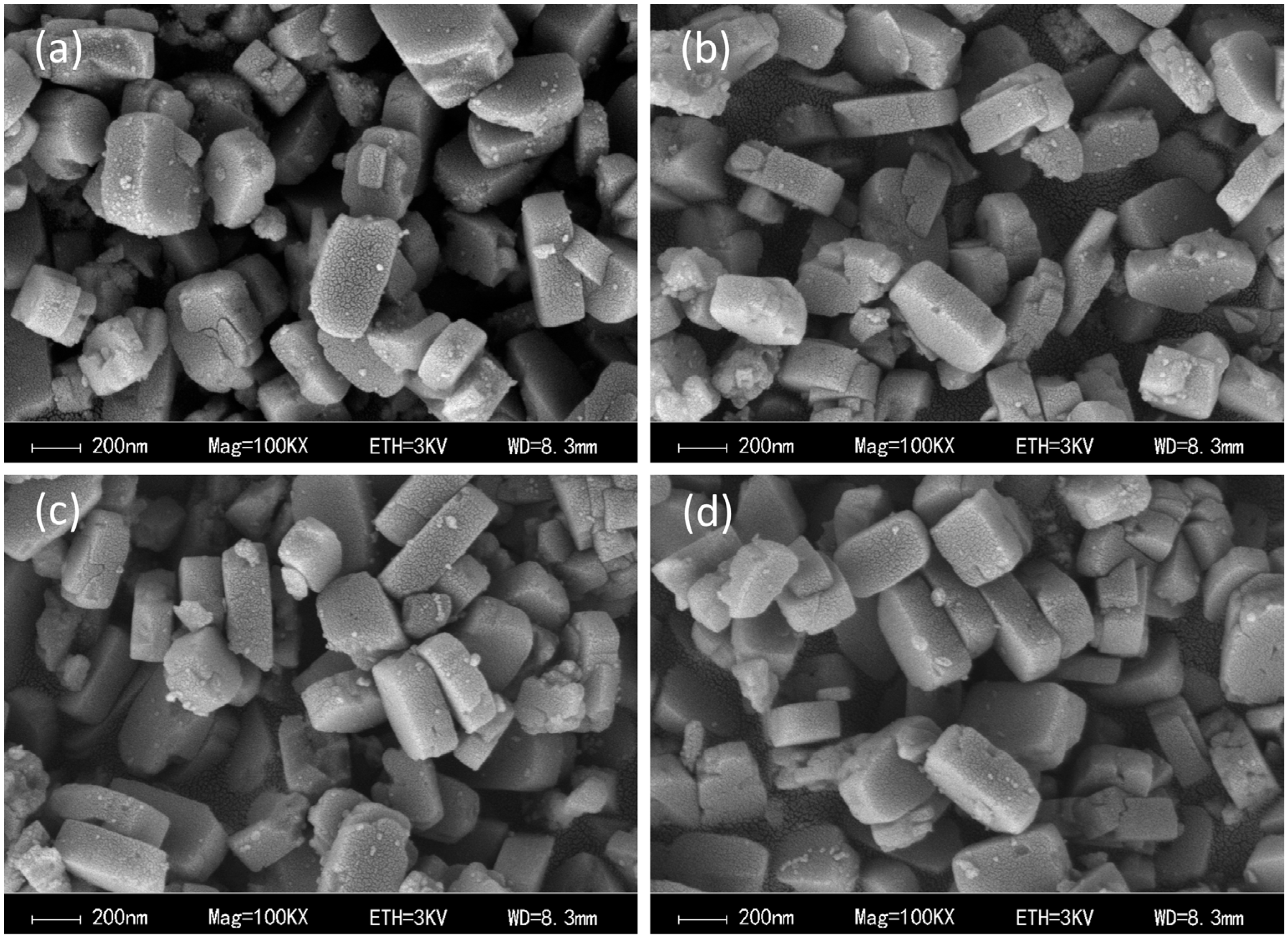
2.4. Catalyst Morphology
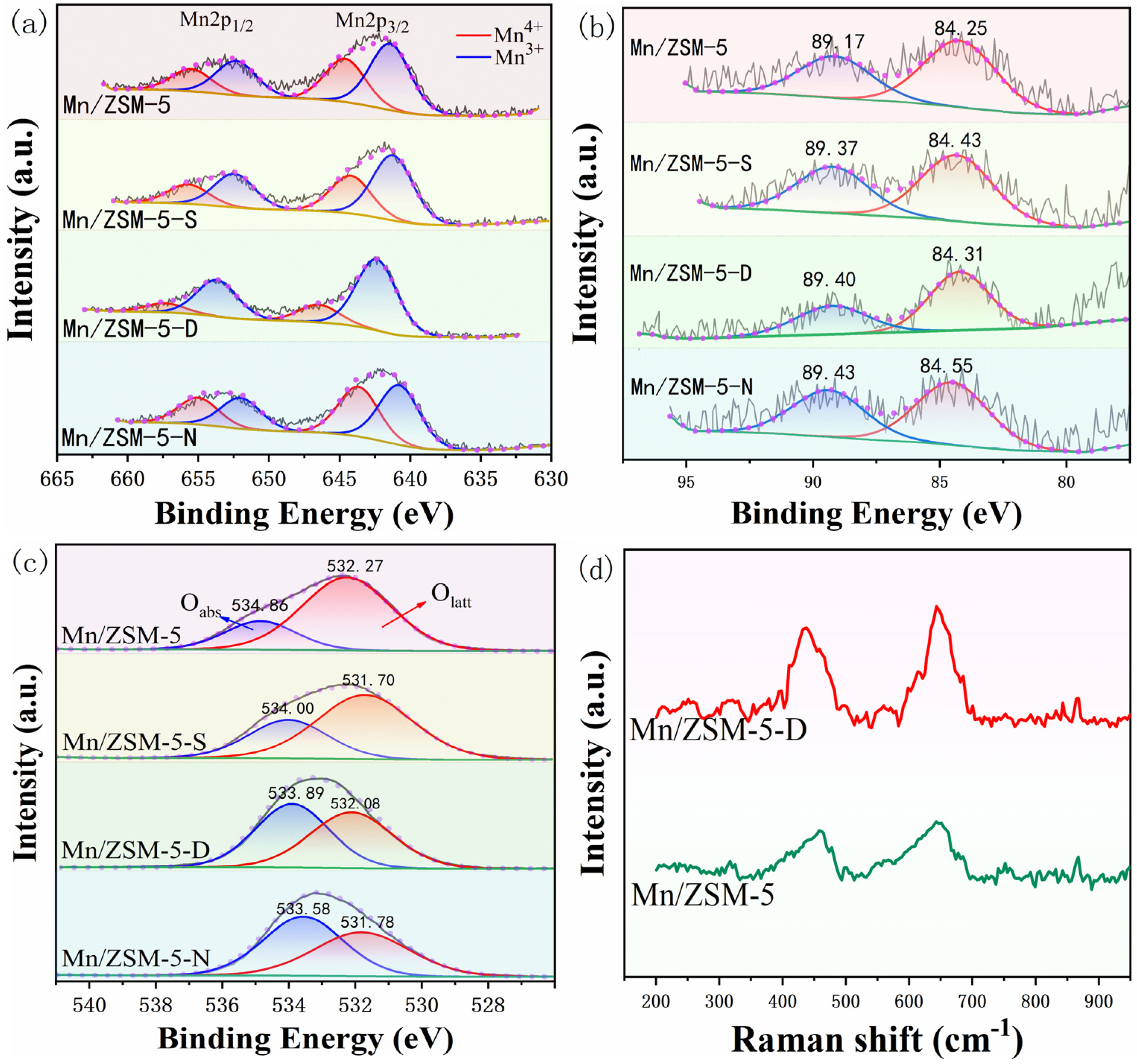
2.5. Surface Chemistry Analysis
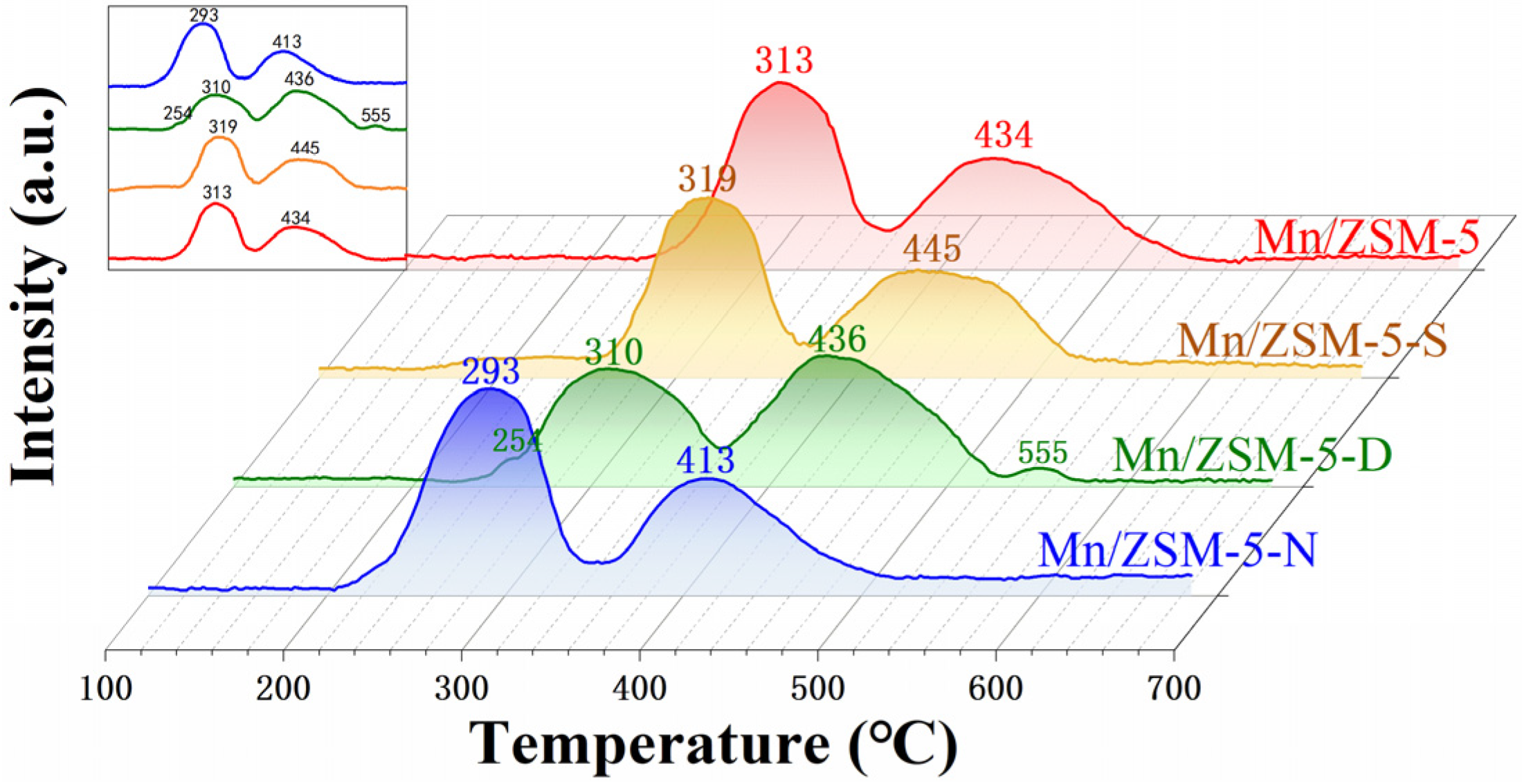
2.6. Redox Properties of the Materials
2.7. Computational Analyses by DFT
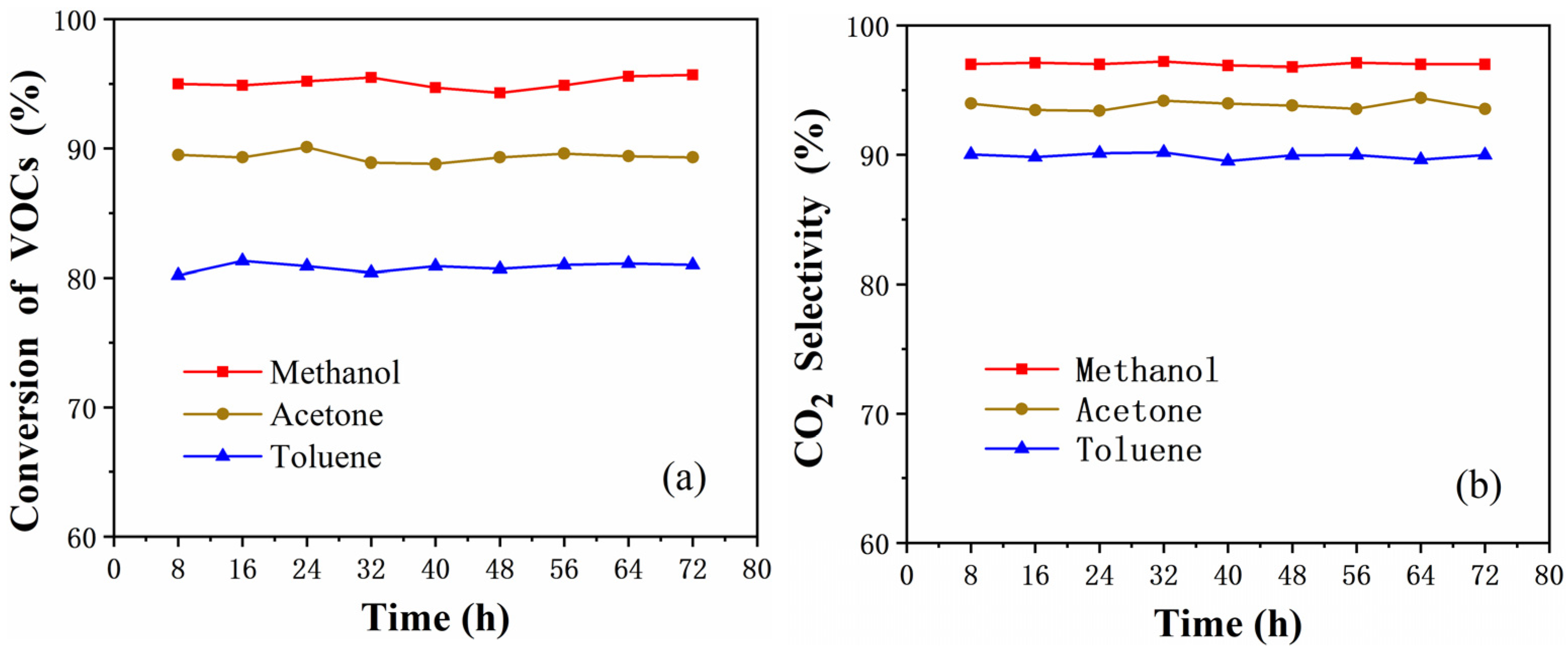
2.8. Durability Test
3. Experimental
3.1. Experimental Setup
3.2. Synthesis of Catalysts
3.3. Characterization and Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blasin-Aubé, V.; Belkouch, J.; Monceaux, L. General study of catalytic oxidation of various VOCs over La0.8Sr0.2MnO3+x perovskite catalyst-influence of mixture. Appl. Catal. B Environ. 2003, 43, 175–186. [Google Scholar] [CrossRef]
- Rostami, R.; Moussavi, G.; Jafari, A.J.; Darbari, S. A modeling concept on removal of VOCs in wire-tube non-thermal plasma, considering electrical and structural factors. Environ. Monit. Assess. 2020, 192, 280. [Google Scholar] [CrossRef] [PubMed]
- Padilla, O.; Munera, J.; Gallego, J.; Santamaria, A. Approach to the Characterization of Monolithic Catalysts Based on La Perovskite-like Oxides and Their Application for VOC Oxidation under Simulated Indoor Environment Conditions. Catalysts 2022, 12, 168. [Google Scholar] [CrossRef]
- Odum, J.R.; Jungkamp, T.; Griffin, R.J.; Forstner, H.; Flagan, C.; Seinfeld, J.H. Aromatics, Reformulated Gasoline, and Atmospheric Organic Aerosol Formation. Environ. Sci. Technol. 1997, 31, 1890–1897. [Google Scholar] [CrossRef]
- Yang, K.; Liu, Y.; Deng, J.; Zhao, X.; Yang, J.; Han, Z.; Hou, Z.; Dai, H. Three-dimensionally ordered mesoporous iron oxide-supported single-atom platinum: Highly active catalysts for benzene combustion. Appl. Catal. B. 2018, 244, 650–659. [Google Scholar] [CrossRef]
- Chen, Y.S.; Hsu, Y.C.; Lin, C.C.; Tai, Y.D.; Liu, H.S. Volatile Organic Compounds Absorption in a Cross-Flow Rotating Packed Bed. Environ. Sci. Technol. 2008, 42, 2631–2636. [Google Scholar] [CrossRef]
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: A critical review. Appl. Catal. B Environ. 2021, 281, 0926–3373. [Google Scholar] [CrossRef]
- Woellner, M.; Hausdorf, S.; Klein, N.; Mueller, P.; Smith, M.W.; Kaskel, S. Adsorption and Detection of Hazardous Trace Gases by Metal-Organic Frameworks. Adv. Mater. 2018, 30, 1704679. [Google Scholar] [CrossRef]
- Xiao, G.; Xu, W.; Wu, R.; Ni, M.; Du, C.; Gao, X.; Luo, Z.; Cen, K. Non-Thermal Plasmas for VOCs Abatement. Plasma Chem. Plasma Process. 2014, 34, 1033–1065. [Google Scholar] [CrossRef]
- Hassan, A.A.; Sorial, G.A. Removal of benzene under acidic conditions in a controlled Trickle Bed Air Biofilter. J. Hazard. Mater. 2010, 184, 345–349. [Google Scholar] [CrossRef]
- Harling, A.M.; Demidyuk, V.; Fischer, S.J.; Whitehead, J.C. Plasma-catalysis destruction of aromatics for environmental clean-up: Effect of temperature and configuration. Appl. Catal. B Environ. 2008, 82, 180–189. [Google Scholar] [CrossRef]
- Kim, H.H.; Oh, S.M.; Ogata, A.; Futamura, S. Decomposition of gas-phase benzene using plasma-driven catalyst (PDC) reactor packed with Ag/TiO2 catalyst. Appl. Catal. B Environ. 2005, 56, 213–220. [Google Scholar] [CrossRef]
- Caroline, N.; Jean-Michel, T.; Catherine, B.; Elodie, F. Non-thermal plasma assisted catalysis of methanol oxidation on Mn, Ce and Cu oxides supported on γ-Al2O3. Chem. Eng. J. 2016, 304, 563–572. [Google Scholar]
- Roland, U.; Holzer, F.; Kopinke, F.D. Combination of non-thermal plasma and heterogeneous catalysis for oxidation of volatile organic compounds: Part 2. Ozone decomposition and deactivation of γ-Al2O3. Appl. Catal. B Environ. 2002, 58, 217–226. [Google Scholar] [CrossRef]
- Yan, K.; Heesch, E.; Pemen, A. A high-voltage pulse generator for corona plasma generation. IEEE Transact. Ind. Appl. 2002, 38, 866–872. [Google Scholar] [CrossRef]
- Dou, B.J.; Li, S.M.; Liu, D.L.; Zhao, R.Z.; Liu, J.G.; Hao, Q.L. Catalytic oxidation of ethyl acetate and toluene over Cu-Ce-Zr supported ZSM-5/TiO2 catalysts. RSC Adv. 2016, 6, 53852–53859. [Google Scholar]
- Shu, Y.; He, M.; Ji, J.; Huang, H.; Liu, S.; Leung, D.Y.C. Synergetic degradation of VOCs by vacuum ultraviolet photolysis and catalytic ozonation over Mn-xCe/ZSM-5. J. Hazard. Mater. 2019, 364, 770–779. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, T.; Zhang, Y. Enhancing Electrocatalytic Oxygen Reduction on MnO2 with Vacancies. Angew. Chem. Int. Edit. 2013, 52, 2474–2477. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, X.Y.; Wang, H.; Ji, L.; Zhang, Y.; Chen, H.; Li, T.S.; Luo, Y.; Cui, G.; Sun, X. Boosting electrocatalytic N2 reduction by MnO2 with oxygen vacancies. Chem. Commun. 2019, 55, 4627–4630. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Zhang, Y.Y.; Han, X.X.; Guo, L.; Wang, D.J.; Lv, K.L. Assembly of CaIn2S4 on Defect-Rich BiOCl for Acceleration of Interfacial Charge Separation and Photocatalytic Phenol Degradation via S-Scheme Electron Transfer Mechanism. Catalysts 2021, 11, 1130. [Google Scholar] [CrossRef]
- Hu, Z.; Li, K.; Wu, X.; Wang, N.; Li, X.; Qin, L.; Li, L.; Lv, K.L. Dramatic promotion of visible-light photoreactivity of TiO2 hollow microspheres towards NO oxidation by introduction of oxygen vacancy. Appl. Catal. B Environ. 2019, 256, 117860. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, J.; Liu, W.; Zhang, T. Removal of Toluene in Air by a Non-thermal Plasma-Catalytic Reactor Using MnOx/ZSM-5. Catal. Lett. 2021, 152, 239–253. [Google Scholar] [CrossRef]
- Roy, P.K.; Prins, R.; Pirngruber, G.D. The effect of pretreatment on the reactivity of Fe-ZSM-5 catalysts for N2O decomposition: Dehydroxylation vs. steaming. Appl. Catal. B Environ. 2008, 80, 226–236. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Z.; Shi, J. Modified manganese oxide octahedral molecular sieves M´-OMS-2 (M´ = Co, Ce, Cu) as catalysts in post plasma-catalysis for acetaldehyde degradation. Catal. Today 2015, 256, 178–185. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.E.; Lee, H.W.; Jeon, J.K.; Park, Y.K. Catalytic ozonation of toluene using Mn-M bimetallic HZSM-5 (M: Fe, Cu, Ru, Ag) catalysts at room temperature. J. Hazard. Mater. 2020, 397, 122577. [Google Scholar] [CrossRef]
- Jiang, N.; Zhao, Y.; Qiu, C.; Shang, K.; Lu, N.; Li, J.; Wu, Y.; Zhang, Y. Enhanced catalytic performance of CoO-CeO2 for synergetic degradation of toluene in multistage sliding plasma system through response surface methodology (RSM). Appl. Catal. B Environ. 2019, 259, 118061. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, G.; Wu, Y.; Zhang, P.; Kang, X. Manganese oxide with rich oxygen vacancies based on Plasma modification for high performance supercapacitors. Colloids Surf. A Phys. Eng. Asp. 2020, 603, 125254. [Google Scholar] [CrossRef]
- Lee, C.J.; Lee, D.H.; Kim, T. Modification of Catalyst Surface from Interaction Between Catalysts and Dielectric Barrier Discharge Plasma. J. Nanosci. Nanotechno. 2017, 17, 2707–2710. [Google Scholar] [CrossRef]
- Zhu, F.; Li, X.; Zhang, H.; Wu, A.; Yan, J. Destruction of toluene by rotating gliding arc discharge. Fuel 2016, 176, 78–85. [Google Scholar] [CrossRef]
- Petitpas, G. A comparative study of non-thermal plasma assisted reforming technologies. Int. J. Hydrogen Energ. 2007, 32, 2848–2867. [Google Scholar] [CrossRef]
- Tu, X.; Verheyde, B.; Corthals, S.; Paulussen, S.; Sels, B.F. Effect of packing solid material on characteristics of helium dielectric barrier discharge at atmospheric pressure. Phys. Plasmas 2011, 18, 1–68. [Google Scholar] [CrossRef]
- Saleem, F.; Zhang, K.; Harvey, A. Role of CO2 in the Conversion of Toluene as a Tar Surrogate in a Nonthermal Plasma Dielectric Barrier Discharge Reactor. Energ. Fuel. 2018, 32, 5164–5170. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Gillot, B.; Buguet, S.; Kester, E. Cation valencies and distribution in the spinels CoxCuyMnzFeuO4+δ (δ≥0) thin films studied by X-ray photoelectron spectroscopy. Thin Solid Films. 1999, 357, 223–231. [Google Scholar] [CrossRef]
- Wang, F.; Deng, J.; Peng, S.; Shen, Y.; Zhang, D. Unraveling the effects of the coordination number of Mn over α-MnO2 catalysts for toluene oxidation. Chem. Eng. J. 2020, 396, 125192. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Liu, D.; Li, L.; Feng, C. Construction of Z-scheme CuFe2O4/MnO2 photocatalyst and activating peroxymonosulfate for phenol degradation: Synergistic effect, degradation pathways, and mechanism. Environ. Res. 2021, 200, 111736. [Google Scholar] [CrossRef]
- Li, H.; Lu, G.; Dai, Q.; Wang, Y.; Yun, G.; Guo, Y. Efficient low-temperature catalytic combustion of trichloroethylene over flower-like mesoporous Mn-doped CeO2 microspheres. Appl. Catal. B Environ. 2011, 102, 475–483. [Google Scholar] [CrossRef]
- Yang, S.; Yang, H.; Yang, J.; Qi, H.; Tu, X. Three-dimensional hollow urchin α-MnO2 for enhanced catalytic activity towards toluene decomposition in post-plasma catalysis. Chem. Eng. J. 2020, 402, 126154. [Google Scholar] [CrossRef]
- Yang, W.; Peng, Y.; Wang, Y.; Wang, Y.; Li, J. Controllable Redox-induced In-situ Growth of MnO2 over Mn2O3 for Toluene Oxidation: Active Heterostructure Interfaces. Appl. Catal. B Environ. 2020, 278, 119279. [Google Scholar] [CrossRef]
- Chang, T.; Lu, J.; Shen, Z.; Zhang, B.; Morent, R. Post Plasma Catalysis for the Removal of Acetaldehyde Using Mn-Co/HZSM-5 Catalysts. Ind. Eng. Chem. Res. 2019, 58, 14719–14728. [Google Scholar] [CrossRef]
- Verezia, A.M.; Floriano, M.A.; Deganello, G. The Structure of Pumice: An XPS and 27Al MAS NMR Study. Surf. Interface Anal. 1992, 18, 532–538. [Google Scholar]
- Yang, X.; Yu, X.; Jing, M. Defective MnxZr1-xO2 Solid Solution for the Catalytic Oxidation of Toluene: Insights into the Oxygen Vacancy Contribution. ACS Appl. Mat. Inter. 2019, 11, 730–739. [Google Scholar] [CrossRef]
- Gang, L.; Feng, B.; Song, C.; Wang, K.; Song, J. Promoting effect of zirconium doping on Mn/ZSM-5 for the selective catalytic reduction of NO with NH3. Fuel 2013, 107, 217–224. [Google Scholar]
- Li, H.; Wu, Y.; Li, C.; Gong, Y.; Niu, L.; Liu, X. Design of Pt/t-ZrO2/g-C3N4 efficient photocatalyst for the hydrogen evolution reaction. Appl. Catal. B Environ. 2019, 251, 305–312. [Google Scholar] [CrossRef]






| Catalysts | SBET a (m2g−1) | SMicro b (m2g−1) | SExter b (m2g−1) | Vp c (cm3g−1) | VMicro b (cm3g−1) | VMeso d (cm3g−1) | DP e (nm) |
|---|---|---|---|---|---|---|---|
| Mn/ZSM-5 | 424 | 268 | 156 | 0.254 | 0.111 | 0.143 | 2.39 |
| Mn/ZSM-5-S | 363 | 254 | 109 | 0.218 | 0.109 | 0.109 | 2.41 |
| Mn/ZSM-5-D | 370 | 256 | 114 | 0.231 | 0.110 | 0.121 | 2.39 |
| Mn/ZSM-5-N | 402 | 261 | 141 | 0.247 | 0.111 | 0.136 | 2.40 |
| Catalysts | Mn3+ (%) | Mn4+ (%) | ΔE (eV) | AOS of Mn | Ovac (%) | Olat (%) |
|---|---|---|---|---|---|---|
| Mn/ZSM-5 | 60.5 | 39.5 | 4.92 | 3.41 | 23.9 | 76.1 |
| Mn/ZSM-5-S | 63.3 | 36.7 | 4.94 | 3.39 | 33.1 | 66.9 |
| Mn/ZSM-5-D | 79.9 | 20.1 | 5.09 | 3.22 | 51.3 | 48.7 |
| Mn/ZSM-5-N | 54.5 | 45.5 | 4.87 | 3.47 | 53.7 | 46.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhou, J.; Liu, D.; Du, K. Modified Mn/ZSM-5 for Non-Thermal Plasma Mineralization of VOCs and DFT Simulation Using a Novel Y-Type ZSM-5 Model. Catalysts 2022, 12, 906. https://doi.org/10.3390/catal12080906
Liu S, Zhou J, Liu D, Du K. Modified Mn/ZSM-5 for Non-Thermal Plasma Mineralization of VOCs and DFT Simulation Using a Novel Y-Type ZSM-5 Model. Catalysts. 2022; 12(8):906. https://doi.org/10.3390/catal12080906
Chicago/Turabian StyleLiu, Su, Jiabin Zhou, Dan Liu, and Ke Du. 2022. "Modified Mn/ZSM-5 for Non-Thermal Plasma Mineralization of VOCs and DFT Simulation Using a Novel Y-Type ZSM-5 Model" Catalysts 12, no. 8: 906. https://doi.org/10.3390/catal12080906