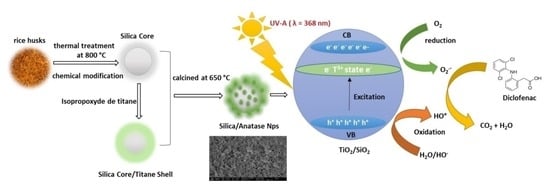
Effect of Biogenic Silica Behavior in the Incorporation of Mesoporous Anatase TiO2 for Excellent Photocatalytic Mineralization of Sodium Diclofenac
Abstract
:1. Introduction
2. Results and Discussion
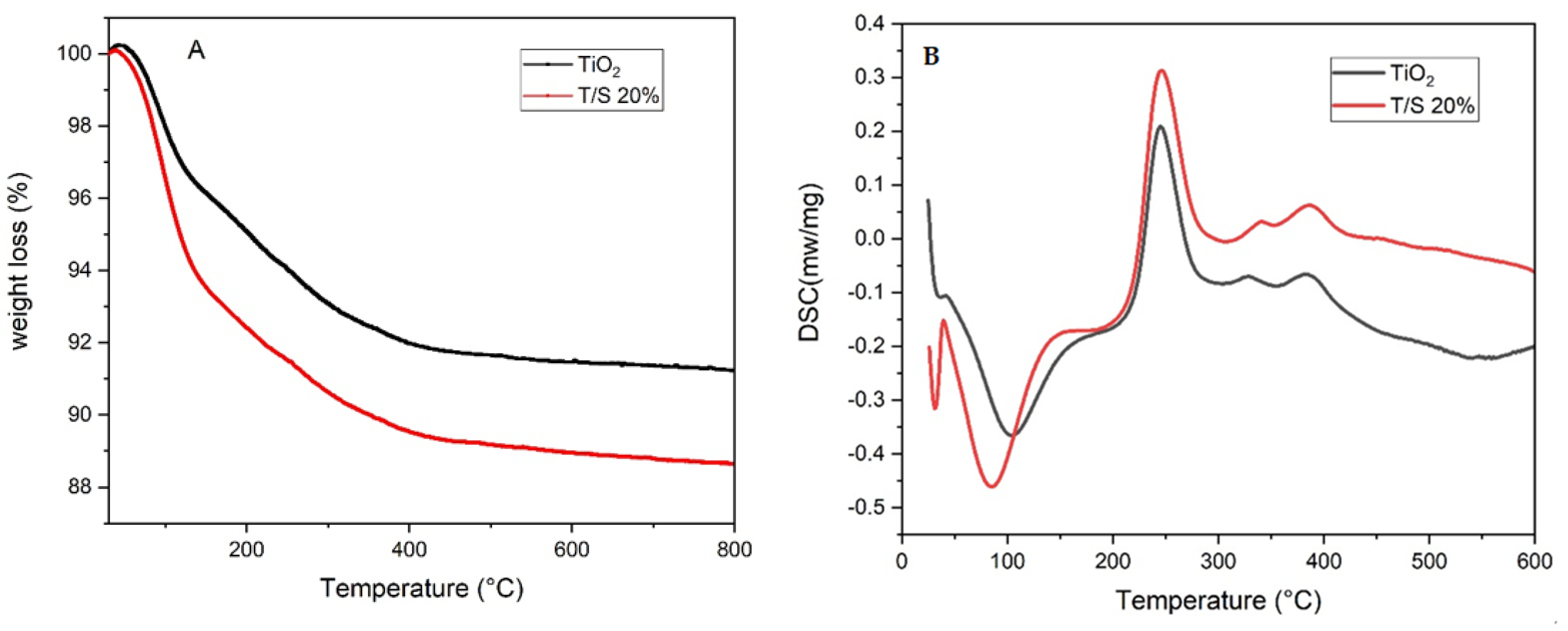
2.1. Thermal Analysis
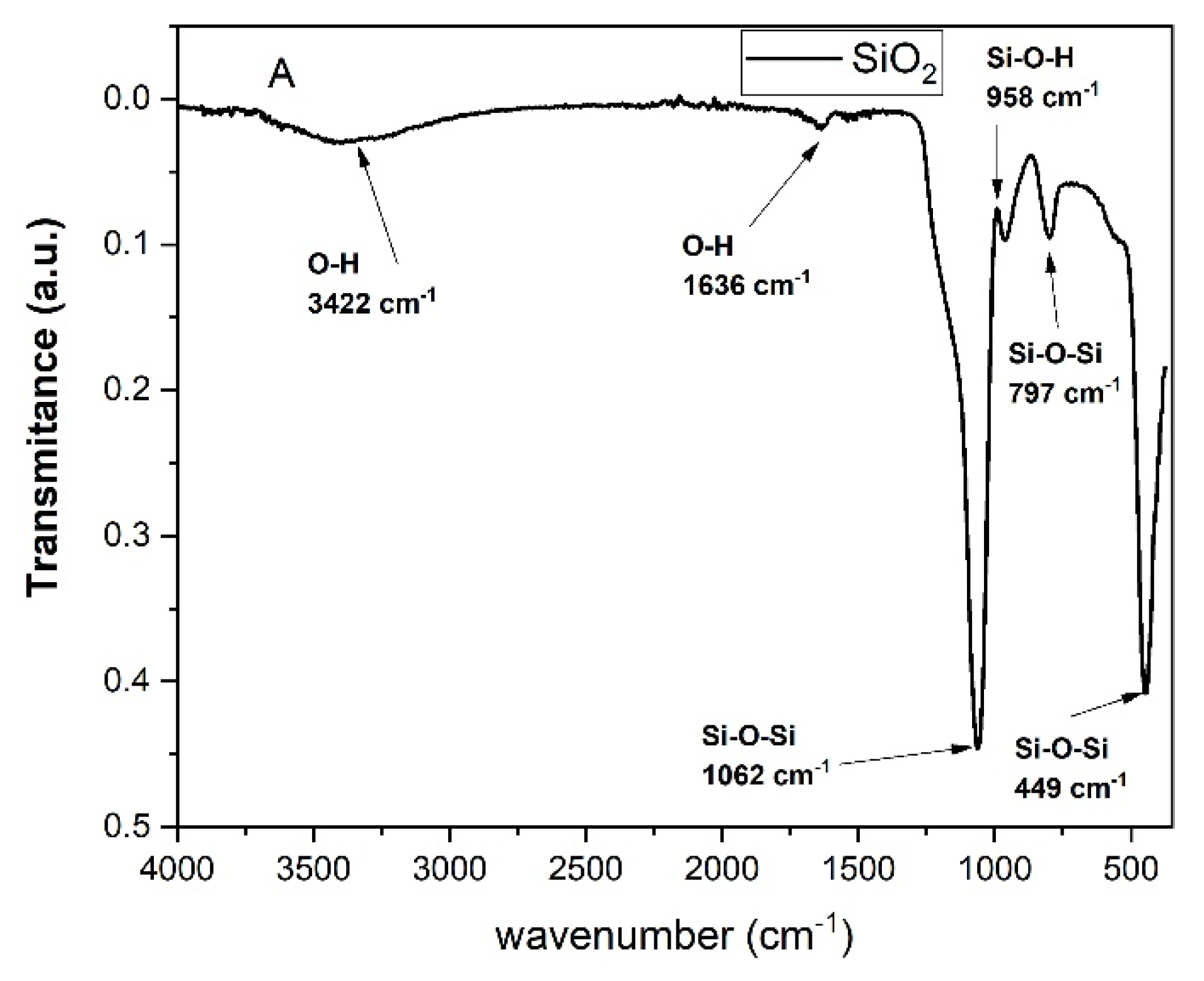
2.2. Structural Analysis
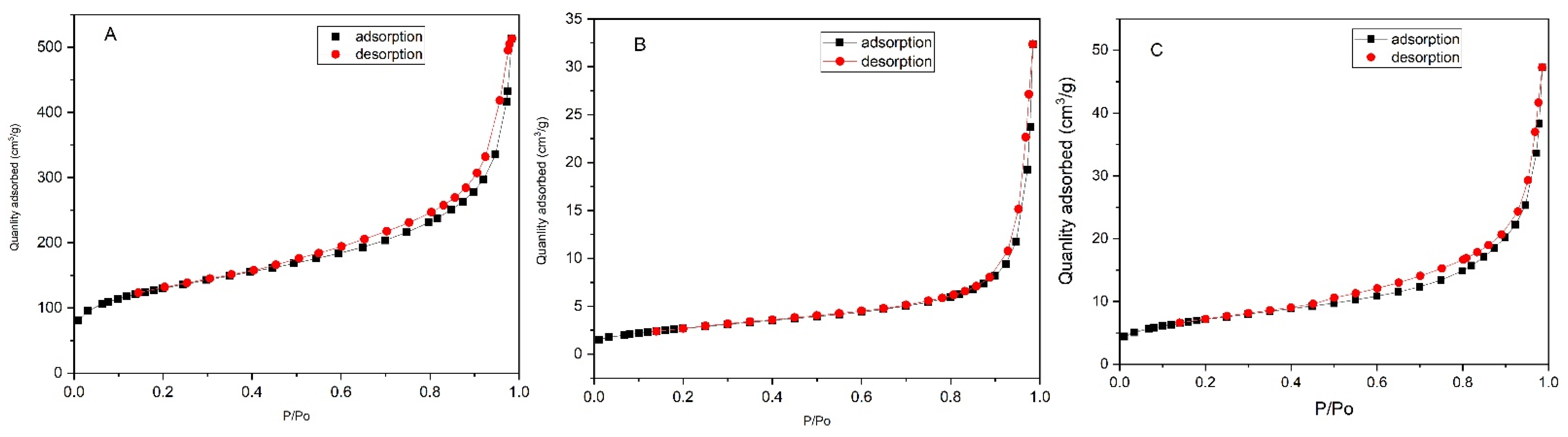
2.3. Mesoporous Structure
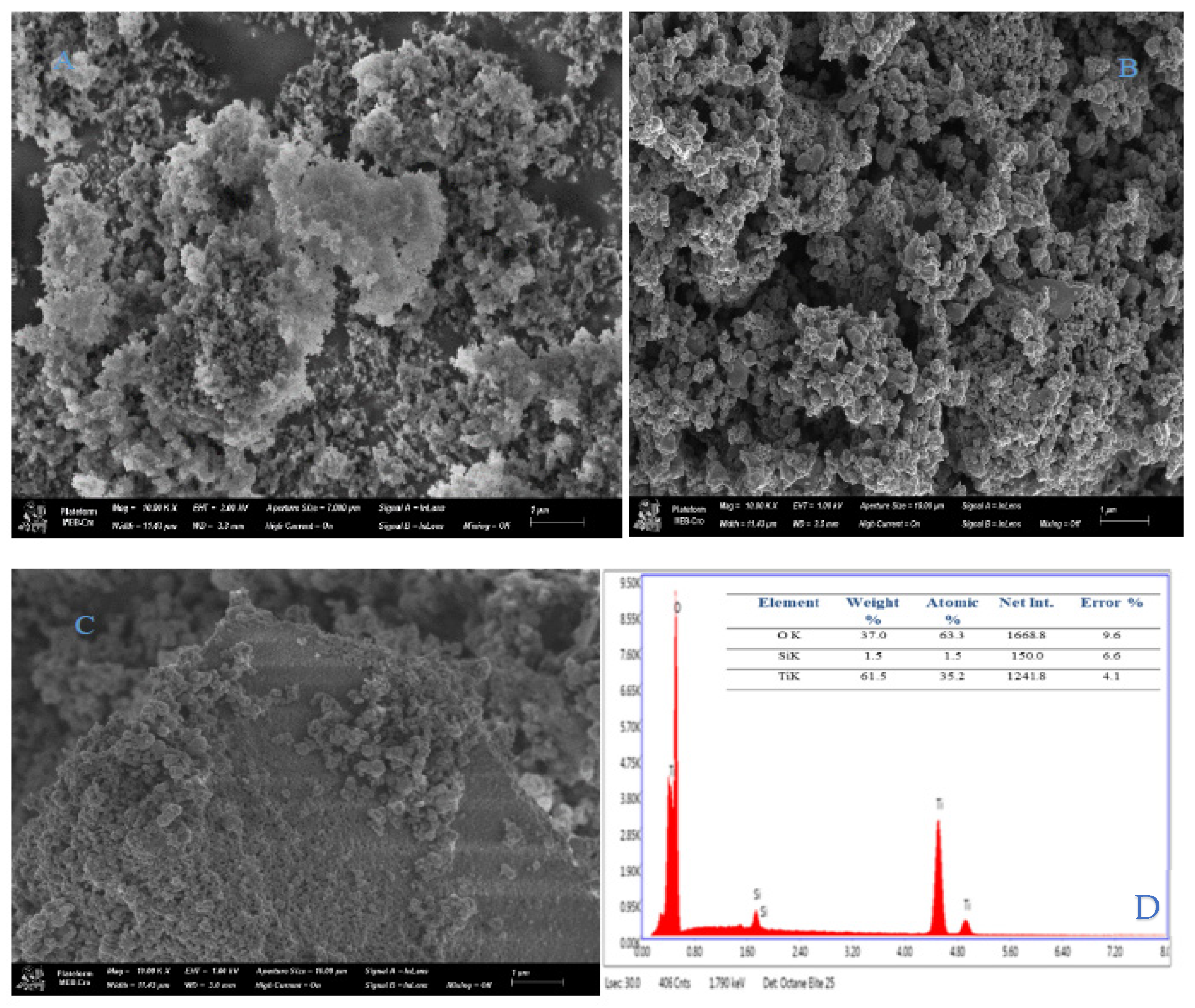
SEM and EDX Analysis
2.4. Surface Property
2.5. Surface Composition and Element Surface Oxidation State Analysis
2.6. Photocatalytic Test for SDCF Mineralization
3. Materials and Methods
3.1. Materials
3.2. Extraction of Nanosilica from Rice Husks
Synthesis of TiO2/SiO2 Nanophotocatalysts
3.3. Characterizations
3.4. Photocatalytic Measurement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Papageorgiou, M.; Kosma, C.; Lambropoulou, D. Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care products in a municipal wastewater treatment plant in Central Greece. Sci. Total Environ. 2016, 543, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geißen, S.-U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Gin, K.Y.-H.; Lin, A.Y.-C.; Reinhard, M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhou, Q. Degradation of some typical pharmaceuticals and personal care products with copper-plating iron doped Cu2O under visible light irradiation. J. Environ. Sci. 2012, 24, 827–833. [Google Scholar] [CrossRef]
- Rosales, E.; Diaz, S.; Pazos, M.; Sanromán, M.A. Comprehensive strategy for the degradation of anti-inflammatory drug diclofenac by different advanced oxidation processes. Sep. Purif. Technol. 2019, 208, 130–141. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Wang, Y.; Su, Y.; Qiao, L.; Liu, L.; Su, Q.; Zhu, C.; Liu, X. Synthesis of one-dimensional TiO2/V2O5 branched heterostructures and their visible light photocatalytic activity towards Rhodamine B. Nanotechnology 2011, 22, 225702. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kong, S.; Kim, W.-S.; Kim, J. Preparation and characterization of SiO2/TiO2 core-shell particles with controlled shell thickness. Mater. Chem. Phys. 2007, 106, 39–44. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, Y.; Nie, W.; Song, L.; Chen, P. Synthesis of highly monodispersed teardrop-shaped core–shell SiO2/TiO2 nanoparticles and their photocatalytic activities. Appl. Surf. Sci. 2015, 351, 320–326. [Google Scholar] [CrossRef]
- Abega, A.V.; Ngomo, H.M.; Nongwe, I.; Mukaya, H.E.; Sone, P.-M.A.K.; Mbianda, X.Y. Easy and convenient synthesis of CNT/TiO2 nanohybrid by in-surface oxidation of Ti3+ ions and application in the photocatalytic degradation of organic contaminants in water. Synth. Met. 2019, 251, 1–14. [Google Scholar] [CrossRef]
- Kapridaki, C.; Pinho, L.; Mosquera, M.J.; Maravelaki-Kalaitzaki, P. Producing photoactive, transparent and hydrophobic SiO2-crystalline TiO2 nanocomposites at ambient conditions with application as self-cleaning coatings. Appl. Catal. B Environ. 2014, 156–157, 416–427. [Google Scholar] [CrossRef]
- Wang, J.; Sun, S.; Ding, H.; Chen, W.; Liang, Y. Preparation of a composite photocatalyst with enhanced photocatalytic activity: Smaller TiO2 carried on SiO2 microsphere. Appl. Surf. Sci. 2019, 493, 146–156. [Google Scholar] [CrossRef]
- Ren, C.; Qiu, W.; Chen, Y. Physicochemical properties and photocatalytic activity of the TiO2/SiO2 prepared by precipitation method. Sep. Purif. Technol. 2013, 107, 264–272. [Google Scholar] [CrossRef]
- Tang, X.; Feng, Q.; Liu, K.; Tan, Y. Synthesis and characterization of a novel nanofibrous TiO2/SiO2 composite with enhanced photocatalytic activity. Mater. Lett. 2016, 183, 175–178. [Google Scholar] [CrossRef]
- He, C.; Tian, B.; Zhang, J. Thermally stable SiO2-doped mesoporous anatase TiO2 with large surface area and excellent photocatalytic activity. J. Colloid Interface Sci. 2010, 344, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, J.; He, J.; Liu, Z.; Yu, Z. Combinations of mild physical or chemical pretreatment with biological pretreatment for enzymatic hydrolysis of rice hull. Bioresour. Technol. 2009, 100, 903–908. [Google Scholar] [CrossRef]
- Ndindeng, S.A.; Wopereis, M.; Sanyang, S.; Futakuchi, K. Evaluation of fan-assisted rice husk fuelled gasifier cookstoves for application in sub-Sahara Africa. Renew. Energy 2019, 139, 924–935. [Google Scholar] [CrossRef]
- Alosaimi, E.H.; Alsohaimi, I.H.; Dahan, T.E.; Chen, Q.; Younes, A.A.; El-Gammal, B.; Melhi, S. Photocatalytic Degradation of Methylene Blue and Antibacterial Activity of Mesoporous TiO2-SBA-15 Nanocomposite Based on Rice Husk. Adsorpt. Sci. Technol. 2021, 2021, 9290644. [Google Scholar] [CrossRef]
- Efremova, S.V. Rice hull as a renewable raw material and its processing routes. Russ. J. Gen. Chem. 2012, 82, 999–1005. [Google Scholar] [CrossRef]
- Sonobe, T.; Worasuwannarak, N. Kinetic analyses of biomass pyrolysis using the distributed activation energy model. Fuel 2008, 87, 414–421. [Google Scholar] [CrossRef]
- Habeeb, G.A.; Mahmud, H.B. Study on properties of rice husk ash and its use as cement replacement material. Mater. Res. 2010, 13, 185–190. [Google Scholar] [CrossRef]
- Hong, S.-S.; Lee, M.S.; Park, S.S.; Lee, G.-D. Synthesis of nanosized TiO2/SiO2 particles in the microemulsion and their photocatalytic activity on the decomposition of p-nitrophenol. Catal. Today 2003, 87, 99–105. [Google Scholar] [CrossRef]
- Mahanta, U.; Khandelwal, M.; Deshpande, A.S. TiO2@SiO2 nanoparticles for methylene blue removal and photocatalytic degradation under natural sunlight and low-power UV light. Appl. Surf. Sci. 2022, 576, 151745. [Google Scholar] [CrossRef]
- Kominami, H.; Yukishita, K.; Kimura, T.; Matsubara, M.; Hashimoto, K.; Kera, Y.; Ohtani, B. Direct Sol-vothermal Formation of Nanocrystalline TiO2 on Porous SiO2 Adsorbent and Photocatalytic Removal of Nitrogen Oxides in Air over TiO2–SiO2 Composites. Top. Catal. 2008, 47, 155–161. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Shen, G.-Y.; He, Y.-P.; Liu, X.-X.; Yang, S.-J. Preparation of TiO2/SiO2 composite oxide and its photocatalytic degradation of rhodamine B. J. Porous Mater. 2016, 23, 589–599. [Google Scholar] [CrossRef]
- Siddiqa, A.; Sabir, S.; Hussain, S.T.; Muhammad, B. Highly active mesoporous SiO2-TiO2 based nanocomposites for photocatalytic degradation of textile dyes and phenol. Eur. J. Chem. 2013, 4, 388–395. [Google Scholar] [CrossRef]
- Florez, D.M.M.G.; Ale, R.B.G.; Idme, A.F.H.; Alarcon, L.A.L.; Huallpa, E.A.; Castro, Y.; Apestegui, P.G.R.; Rodriguez, J.M.R. SiO2-TiO2 Films Supported on Ignimbrite by Spray Coating for the Photocatalytic Degradation of NOx Gas and Methyl Orange Dye. Int. J. Photoenergy 2020, 2020, 4756952. [Google Scholar] [CrossRef]
- Cheng, J.; Zhao, L.; Li, G.; Li, F.; Yao, M. B/Co/Fe tridoped TiO2/SiO2 composite films for improved photocatalytic degradation of organic pollutants under visible light. Inorg. Chem. Commun. 2020, 119, 108089. [Google Scholar] [CrossRef]
- Yener, H.B.; Helvacı, Ş.Ş. Effect of synthesis temperature on the structural properties and photocatalytic activity of TiO2/SiO2 composites synthesized using rice husk ash as a SiO2 source. Sep. Purif. Technol. 2015, 140, 84–93. [Google Scholar] [CrossRef]
- Huang, C.; Bai, H.; Huang, Y.; Liu, S.; Yen, S.; Tseng, Y. Synthesis of Neutral SiO2/TiO2 Hydrosol and Its Application as Antireflective Self-Cleaning Thin Film. Int. J. Photoenergy 2012, 2012, 620764. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
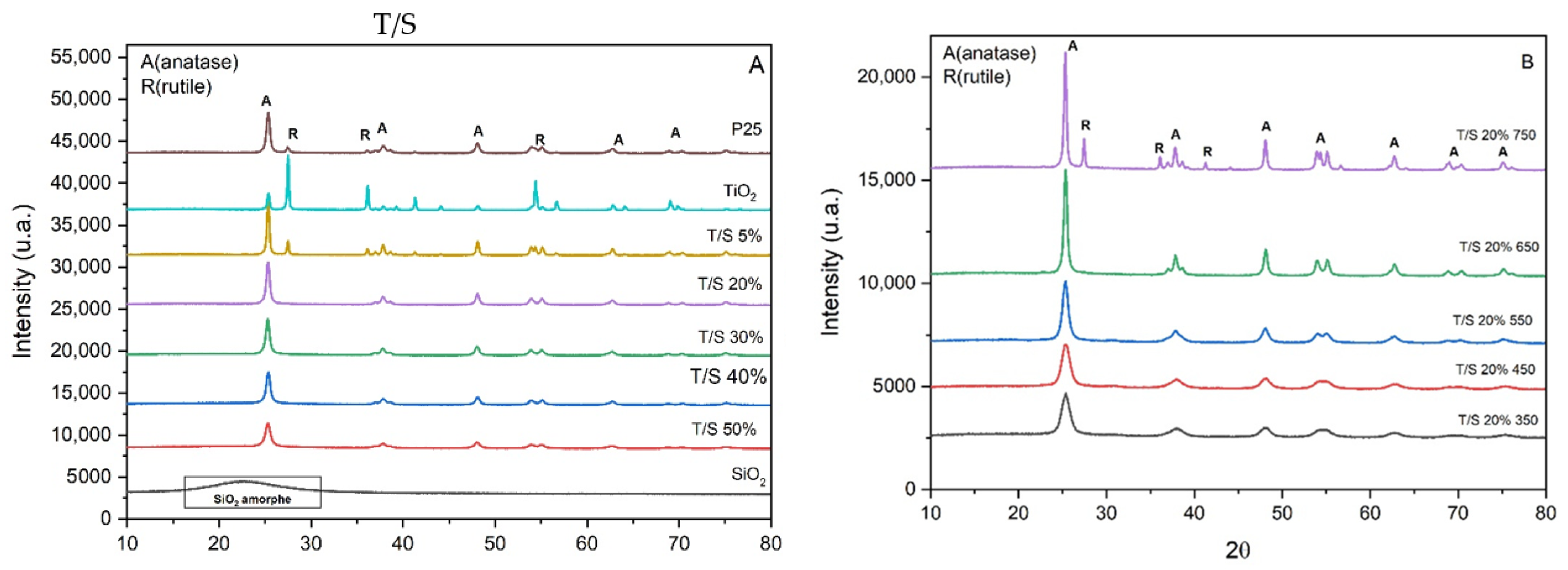





| Samples | 2θ (°) | a = b | c | c/a Ratio | Anatase Nanoparticles Size (nm) | Microstrain × 10−3 | Dislocation Density × 10−3 |
|---|---|---|---|---|---|---|---|
| T/S 20% 450 | 25.37 | 3.7850 | 9.5140 | 2.514 | 6.37 | 23.97 | 24.61 |
| T/S 20% 550 | 25.32 | 3.7858 | 9.5074 | 2.511 | 8.85 | 17.35 | 12.78 |
| T/S 20% 650 | 25.34 | 3.7842 | 9.5146 | 2.514 | 15.48 | 9.90 | 4.17 |
| T/S 20% 750 | 25.31 | 3.7850 | 9.5140 | 2.514 | 21.03 | 7.31 | 2.26 |
| TiO2 pur 650 | 25.35 | 3.7850 | 9.5140 | 2.514 | 26.03 | 5.88 | 1.48 |
| Samples | SiO2 | TiO2 | T/S5% | T/S 20% | T/S 30% | T/S 40% | T/S 50% |
|---|---|---|---|---|---|---|---|
| specific surface area (m2/g) | 455.22 | 8.99 | 25.41 | 97.72 | 144.29 | 171.75 | 205.69 |
| Pores volume (cm3/g) | 0.718 | 0.047 | 0.071 | 0.23 | 0.29 | 0.33 | 0.35 |
| Pores diameter (nm) | 9.64 | 24.112 | 13.89 | 11.19 | 9.99 | 10.03 | 8.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dantio Nguela, C.B.; Manga, N.H.; Marchal, C.; Abega, A.V.; Nsami, N.J.; Robert, D. Effect of Biogenic Silica Behavior in the Incorporation of Mesoporous Anatase TiO2 for Excellent Photocatalytic Mineralization of Sodium Diclofenac. Catalysts 2022, 12, 1001. https://doi.org/10.3390/catal12091001
Dantio Nguela CB, Manga NH, Marchal C, Abega AV, Nsami NJ, Robert D. Effect of Biogenic Silica Behavior in the Incorporation of Mesoporous Anatase TiO2 for Excellent Photocatalytic Mineralization of Sodium Diclofenac. Catalysts. 2022; 12(9):1001. https://doi.org/10.3390/catal12091001
Chicago/Turabian StyleDantio Nguela, Christian Brice, Ngomo Horace Manga, Clément Marchal, Aimé Victoire Abega, Ndi Julius Nsami, and Didier Robert. 2022. "Effect of Biogenic Silica Behavior in the Incorporation of Mesoporous Anatase TiO2 for Excellent Photocatalytic Mineralization of Sodium Diclofenac" Catalysts 12, no. 9: 1001. https://doi.org/10.3390/catal12091001
APA StyleDantio Nguela, C. B., Manga, N. H., Marchal, C., Abega, A. V., Nsami, N. J., & Robert, D. (2022). Effect of Biogenic Silica Behavior in the Incorporation of Mesoporous Anatase TiO2 for Excellent Photocatalytic Mineralization of Sodium Diclofenac. Catalysts, 12(9), 1001. https://doi.org/10.3390/catal12091001