Development and Investigation of Photoactive WO3 Nanowire-Based Hybrid Membranes
Abstract
:1. Introduction
2. Results
2.1. HRTEM and EDS Analysis of WO3 NW@Fe2O3 and WO3 NW@CuO Nanocomposites
2.2. SEM and EDS Analysis of WO3 NW@Fe2O3/Cellulose and WO3 NW@CuO/Cellulose Membranes
2.3. XRD and Specific Surface Area Analysis of Hybrid Membranes
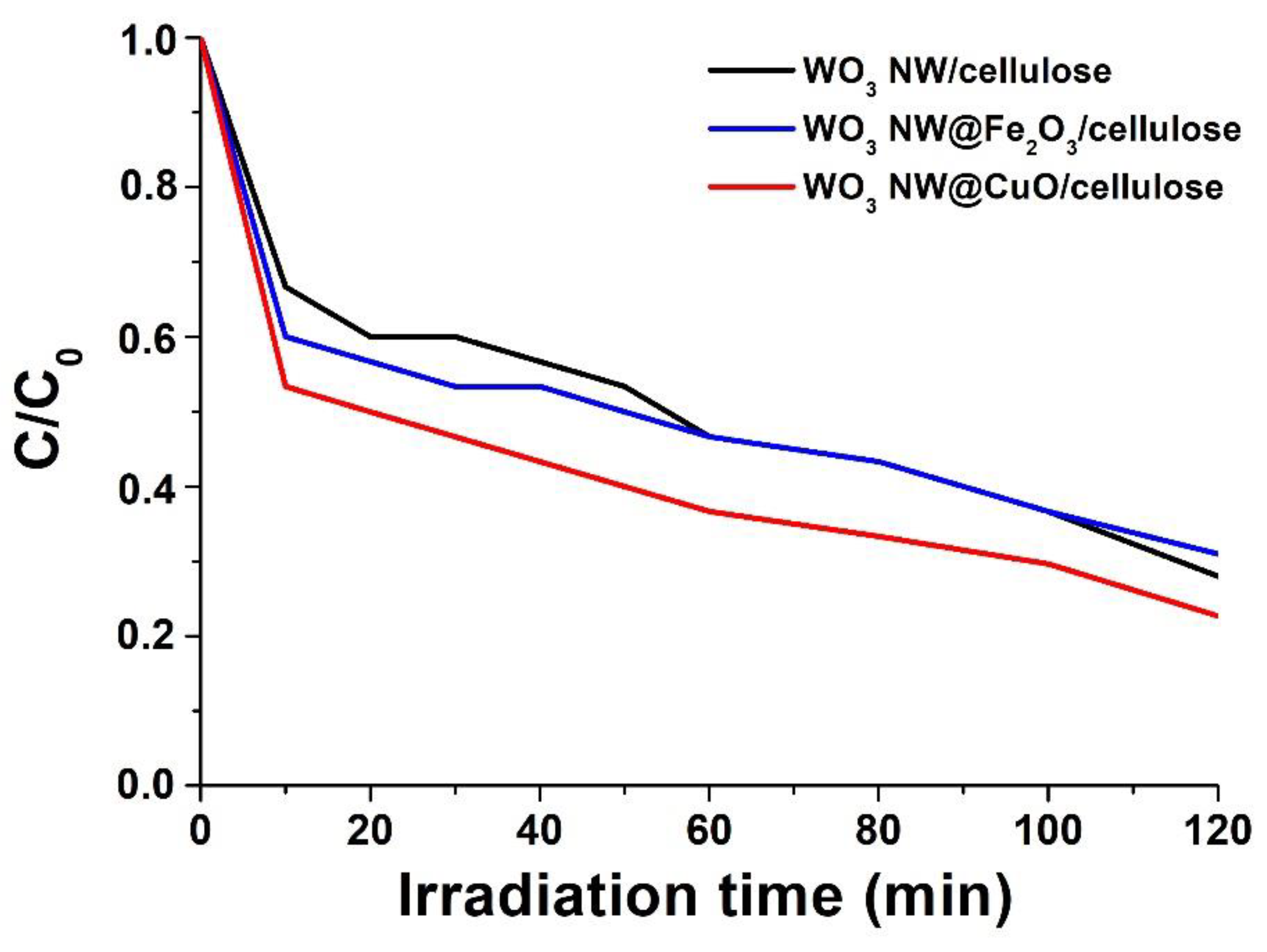
2.4. Photocatalytic Efficiency of Hybrid Membranes
3. Materials and Methods
3.1. Materials
3.2. Synthesis of WO3 NanoWires (WO3 NW)
3.3. Synthesis of WO3 NW@Fe2O3/Cellulose Membranes
3.4. Synthesis of WO3 NW@CuO/Cellulose Membranes
3.5. Characterization Techniques
3.6. Photocatalytic Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bexfield:, L.M.; Toccalino, P.L.; Belitz, K.; Foreman, W.T.; Furlong, E.T. Hormones and Pharmaceuticals in Groundwater Used As a Source of Drinking Water Across the United States. Environ. Sci. Technol. 2019, 53, 2950–2960. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2019, 701, 135023. [Google Scholar] [CrossRef] [PubMed]
- Poerio, T.; Piacentini, E.; Mazzei, R. Membrane Processes for Microplastic Removal. Molecules 2019, 24, 4148. [Google Scholar] [CrossRef] [PubMed]
- Karpińska, J.; Kotowska, U. Removal of Organic Pollution in the Water Environment. Water 2019, 11, 2017. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent Advances and Applications of Semiconductor Photocatalytic Technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef]
- Santos, R.K.; Martins, T.A.; Silva, G.N.; Conceição, M.V.S.; Nogueira, I.C.; Longo, E.; Botelho, G. Ag3PO4 /NiO composites with enhanced photocatalytic activity under visible light. ACS Omega 2020, 5, 21651–21661. [Google Scholar] [CrossRef]
- Liao, C.; Ma, Z.; Chen, X.; He, X.; Qiu, J. Controlled synthesis of bismuth oxyiodide toward optimization of photocatalytic performance. Appl. Surf. Sci. 2016, 387, 1247–1256. [Google Scholar] [CrossRef]
- Sharma, N.; Pap, Z.; Garg, S.; Hernadi, K. Photocatalyst Composites from Bi-based and Carbon Materials for Visible Light Photodegradation. In Green Photocatalytic Semiconductors; Springer: Cham, Switzerland, 2022; pp. 145–178. [Google Scholar] [CrossRef]
- Castillo-Cabrera, G.X.; Espinoza-Montero, P.J.; Alulema-Pullupaxi, P.; Mora, J.R.; Villacís-García, M.H. Bismuth Oxyhalide-Based Materials (BiOX: X = Cl, Br, I) and Their Application in Photoelectrocatalytic Degradation of Organic Pollutants in Water: A Review. Front. Chem. 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, S.; Gu, J.; Liu, R.; Wang, Z.; Chen, H.; Jiang, F. Visible-light photocatalytic degradation of bisphenol A using cobalt-to-oxygen doped graphitic carbon nitride with nitrogen vacancies via metal-to-ligand charge transfer. J. Hazard. Mater. 2020, 384, 121247. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lü, H.; Yu, C.; Ding, B.; Ye, R.; Ji, Y.; Dai, B.; Liu, W. Novel FeWO4/WO3 nanoplate with p-n heterostructure and its enhanced mechanism for organic pollutants removal under visible-light illumination. J. Environ. Chem. Eng. 2020, 8, 104044. [Google Scholar] [CrossRef]
- Fakhri, A.; Behrouz, S. Photocatalytic properties of tungsten trioxide (WO3) nanoparticles for degradation of Lidocaine under visible and sunlight irradiation. Sol. Energy 2015, 112, 163–168. [Google Scholar] [CrossRef]
- Samuel, O.; Othman, M.H.D.; Kamaludin, R.; Sinsamphanh, O.; Abdullah, H.; Puteh, M.H.; Kurniawan, T.A. WO3-based photocatalysts: A review on synthesis, performance enhancement and photocatalytic memory for environmental applications. Ceram. Int. 2021, 48, 5845–5875. [Google Scholar] [CrossRef]
- Zhou, J.; Ding, Y.; Deng, S.Z.; Gong, L.; Xu, N.S.; Wang, Z.L. Three-Dimensional Tungsten Oxide Nanowire Networks. Adv. Mater. 2005, 17, 2107–2110. [Google Scholar] [CrossRef]
- Szilágyi, I.M.; Fórizs, B.; Rosseler, O.; Szegedi, Á.; Németh, P.; Király, P.; Tárkányi, G.; Vajna, B.; Varga-Josepovits, K.; László, K.; et al. WO3 photocatalysts: Influence of structure and composition. J. Catal. 2012, 294, 119–127. [Google Scholar] [CrossRef]
- Zheng, H.; Ou, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-Zadeh, K. Nanostructured Tungsten Oxide-Properties, Synthesis, and Applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Yao, S.; Qu, F.; Wang, G.; Wu, X. Facile hydrothermal synthesis of WO3 nanorods for photocatalysts and supercapacitors. J. Alloys Compd. 2017, 724, 695–702. [Google Scholar] [CrossRef]
- Jamali, M.; Tehrani, F.S. Effect of synthesis route on the structural and morphological properties of WO3 nanostructures. Mater. Sci. Semicond. Process. 2019, 107, 104829. [Google Scholar] [CrossRef]
- Wang, H.-R.; Zhang, G.-Y.; Xu, Y.-Y.; Wei, X.-M.; Shen, X.-Q.; Sun, Y.-Q. Facile ethanol/water solvothermal synthesis of {001} facet oriented WO3architectures with superior simulated sunlight photocatalytic activities. CrystEngComm 2016, 18, 8089–8100. [Google Scholar] [CrossRef]
- Parthibavarman, M.; Karthik, M.; Prabhakaran, S. Facile and one step synthesis of WO3 nanorods and nanosheets as an efficient photocatalyst and humidity sensing material. Vacuum 2018, 155, 224–232. [Google Scholar] [CrossRef]
- Ahmed, B.; Kumar, S.; Ojha, A.K.; Donfack, P.; Materny, A. Facile and controlled synthesis of aligned WO3 nanorods and nanosheets as an efficient photocatalyst material. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 175, 250–261. [Google Scholar] [CrossRef]
- Navarrete-Magaña, M.; Estrella-González, A.; May-Ix, L.; Cipagauta-Díaz, S.; Gómez, R. Improved photocatalytic oxidation of arsenic (III) with WO3/TiO2 nanomaterials synthesized by the sol-gel method. J. Environ. Manag. 2021, 282, 111602. [Google Scholar] [CrossRef]
- Martínez-de la Cruz, A.; Martínez, D.S.; Cuéllar, E.L. Synthesis and characterization of WO3 nanoparticles prepared by the precipitation method: Evaluation of photocatalytic activity under vis-irradiation. Solid State Sci. 2010, 12, 88–94. [Google Scholar] [CrossRef]
- Mohammadi, S.; Sohrabi, M.; Golikand, A.N.; Fakhri, A. Preparation and characterization of zinc and copper co-doped WO3 nanoparticles: Application in photocatalysis and photobiology. J. Photochem. Photobiol. B Biol. 2016, 161, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, V.; Poli, N.; D’Arco, A.; Macis, S.; Lupi, S.; Comini, E. A novel approach for green synthesis of WO3 nanomaterials and their highly selective chemical sensing properties. J. Mater. Chem. A 2020, 8, 20373–20385. [Google Scholar] [CrossRef]
- Nayak, A.K.; Sohn, Y.; Pradhan, D. Facile green synthesis of WO3·H2O nanoplates and WO3 nanowires with enhanced photoelectrochemical performance. Cryst. Growth Des. 2017, 17, 4949–4957. [Google Scholar] [CrossRef]
- Manjunatha, A.; Pavithra, N.; Shivanna, M.; Nagaraju, G.; Ravikumar, C. Synthesis of Citrus Limon mediated SnO2-WO3 nanocomposite: Applications to photocatalytic activity and electrochemical sensor. J. Environ. Chem. Eng. 2020, 8, 104500. [Google Scholar] [CrossRef]
- Nagy, D.; Szilágyi, I.M.; Fan, X. Effect of the morphology and phases of WO3 nanocrystals on their photocatalytic efficiency. RSC Adv. 2016, 6, 33743–33754. [Google Scholar] [CrossRef]
- Senthil, R.A.; Priya, A.; Theerthagiri, J.; Selvi, A.; Nithyadharseni, P.; Madhavan, J. Facile synthesis of α-Fe2O3/WO3 composite with an enhanced photocatalytic and photo-electrochemical performance. Ionics 2018, 24, 3673–3684. [Google Scholar] [CrossRef]
- Ikram, M.; Umar, E.; Raza, A.; Haider, A.; Naz, S.; Ul-Hamid, A.; Haider, J.; Shahzadi, I.; Hassan, J.; Ali, S. Dye degradation performance, bactericidal behavior and molecular docking analysis of Cu-doped TiO2 nanoparticles. RSC Adv. 2020, 10, 24215–24233. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Song, J.; Pan, L.; Zhang, X.; Wang, L.; Zou, J.-J. Tungsten Oxides for Photocatalysis, Electrochemistry, and Phototherapy. Adv. Mater. 2015, 27, 5309–5327. [Google Scholar] [CrossRef] [PubMed]
- Dursun, S.; Koyuncu, S.N.; Kaya, I.C.; Kaya, G.G.; Kalem, V.; Akyildiz, H. Production of CuO-WO3 hybrids and their dye removal capacity/performance from wastewater by adsorption/photocatalysis. J. Water Process Eng. 2020, 36, 101390. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, Z.; Liu, H.; Zhang, S.; Wang, P.; Lu, J.; Tong, Y. Heterojunction Architecture of N-Doped WO3 Nanobundles with Ce2S3 Nanodots Hybridized on a Carbon Textile Enables a Highly Efficient Flexible Photocatalyst. Adv. Funct. Mater. 2019, 29, 1903490. [Google Scholar] [CrossRef]
- Hitam, C.; Jalil, A. A review on exploration of Fe2O3 photocatalyst towards degradation of dyes and organic contaminants. J. Environ. Manag. 2020, 258, 110050. [Google Scholar] [CrossRef] [PubMed]
- Dulta, K.; Ağçeli, G.K.; Chauhan, P.; Jasrotia, R.; Ighalo, J.O. Multifunctional CuO nanoparticles with enhanced photocatalytic dye degradation and antibacterial activity. Sustain. Environ. Res. 2022, 32, 2. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Arunachalam, P.; Ramachandran, K.; Al-Mayouf, A.M.; Karuppuchamy, S. Recent advances in semiconductor metal oxides with enhanced methods for solar photocatalytic applications. J. Alloys Compd. 2020, 828, 154281. [Google Scholar] [CrossRef]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Pang, X.; Xue, S.; Zhou, T.; Xu, Q.; Lei, W. 2D/2D nanohybrid of Ti3C2 MXene/WO3 photocatalytic membranes for efficient water purification. Ceram. Int. 2021, 48, 3659–3668. [Google Scholar] [CrossRef]
- Kim, J.; Van der Bruggen, B. The use of nanoparticles in polymeric and ceramic membrane structures: Review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef] [PubMed]
- Asiri, A.M.; Pugliese, V.; Petrosino, F.; Khan, S.B.; Alamry, K.A.; Alfifi, S.Y.; Marwani, H.M.; Alotaibi, M.M.; Mukherjee, D.; Chakraborty, S. Photocatalytic Degradation of Textile Dye on Blended Cellulose Acetate Membranes. Polymers 2022, 14, 636. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.N.; Ágoston, Á.; Kertész, S.; Hodúr, C.; László, Z.; Pap, Z.; Kása, Z.; Alapi, T.; Krishnan, S.G.; Arthanareeswaran, G.; et al. Investigation of the applicability of TiO2, BiVO4, and WO3 nanomaterials for advanced photocatalytic membranes used for oil-in-water emulsion separation. Asia-Pac. J. Chem. Eng. 2020, 15, e2549. [Google Scholar] [CrossRef]
- Warsi, A.-Z.; Aziz, F.; Zulfiqar, S.; Haider, S.; Shakir, I.; Agboola, P.O. Synthesis, Characterization, Photocatalysis, and Antibacterial Study of WO3, MXene and WO3/MXene Nanocomposite. Nanomaterials 2022, 12, 713. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Chen, L.; Jing, Z.; Luna, P.D.; Wen, L.; Zhang, L.; Zhao, L.; Xu, J.; Li, Z.; Yang, Z.; et al. Robust antibacterial activity of tungsten oxide (WO3-x ) nanodots. Chem. Res. Toxicol. 2019, 32, 1357–1366. [Google Scholar] [CrossRef]
- Asenath-Smith, E.; Ambrogi, E.K.; Barnes, E.; Brame, J.A. CuO enhances the photocatalytic activity of Fe2O3 through synergistic reactive oxygen species interactions. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 603, 125179. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, K.; Sun, J.; Luo, R.; Li, D.; Chen, A. Surface decoration of WO3 architectures with Fe2O3 nanoparticles for visible-light-driven photocatalysis. CrystEngComm 2014, 16, 3289–3295. [Google Scholar] [CrossRef]
- Lima, M.S.; Cruz-Filho, J.F.; Noleto, L.F.; Silva, L.J.; Costa, T.M.; Luz, G.E. Synthesis, characterization and catalytic activity of Fe3O4@WO3/SBA-15 on photodegradation of the acid dichlorophenoxyacetic (2,4-D) under UV irradiation. J. Environ. Chem. Eng. 2020, 8, 104145. [Google Scholar] [CrossRef]
- Shehab, M.A.; Sharma, N.; Valsesia, A.; Karacs, G.; Kristály, F.; Koós, T.; Leskó, A.K.; Nánai, L.; Hernadi, K.; Németh, Z. Preparation and Photocatalytic Performance of TiO2 Nanowire-Based Self-Supported Hybrid Membranes. Molecules 2022, 27, 2951. [Google Scholar] [CrossRef]

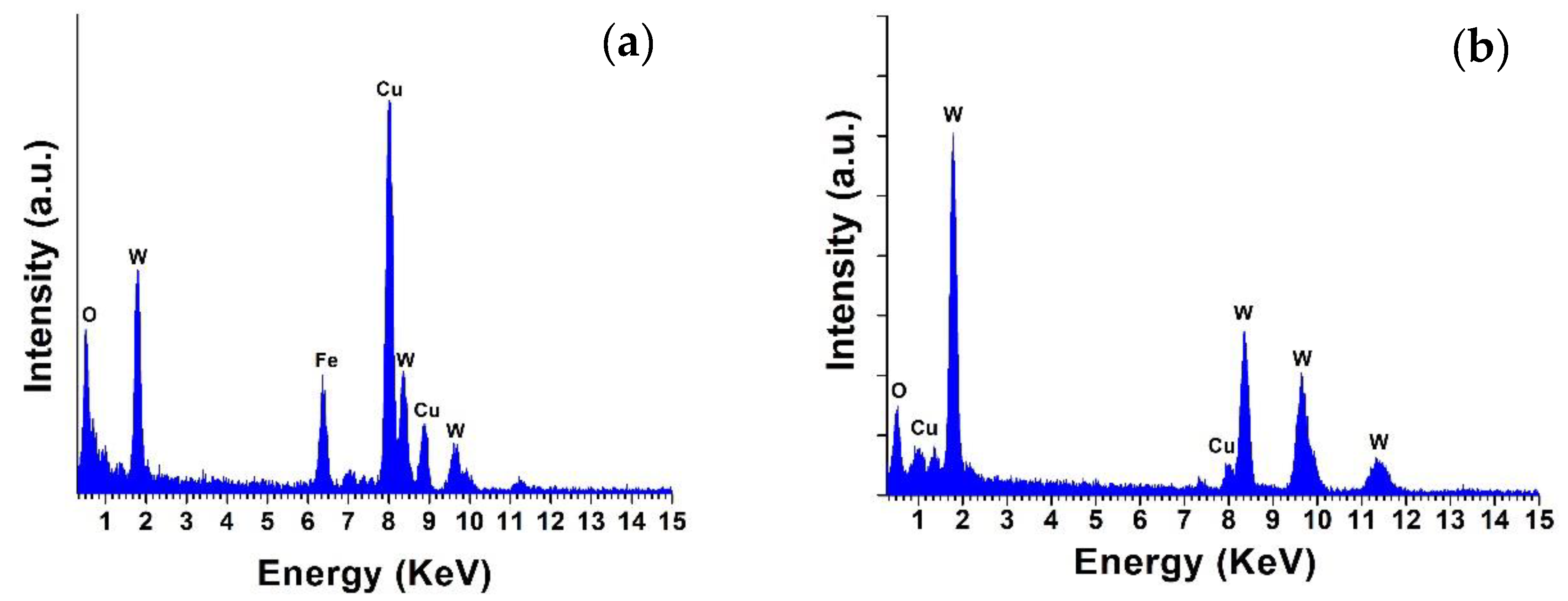
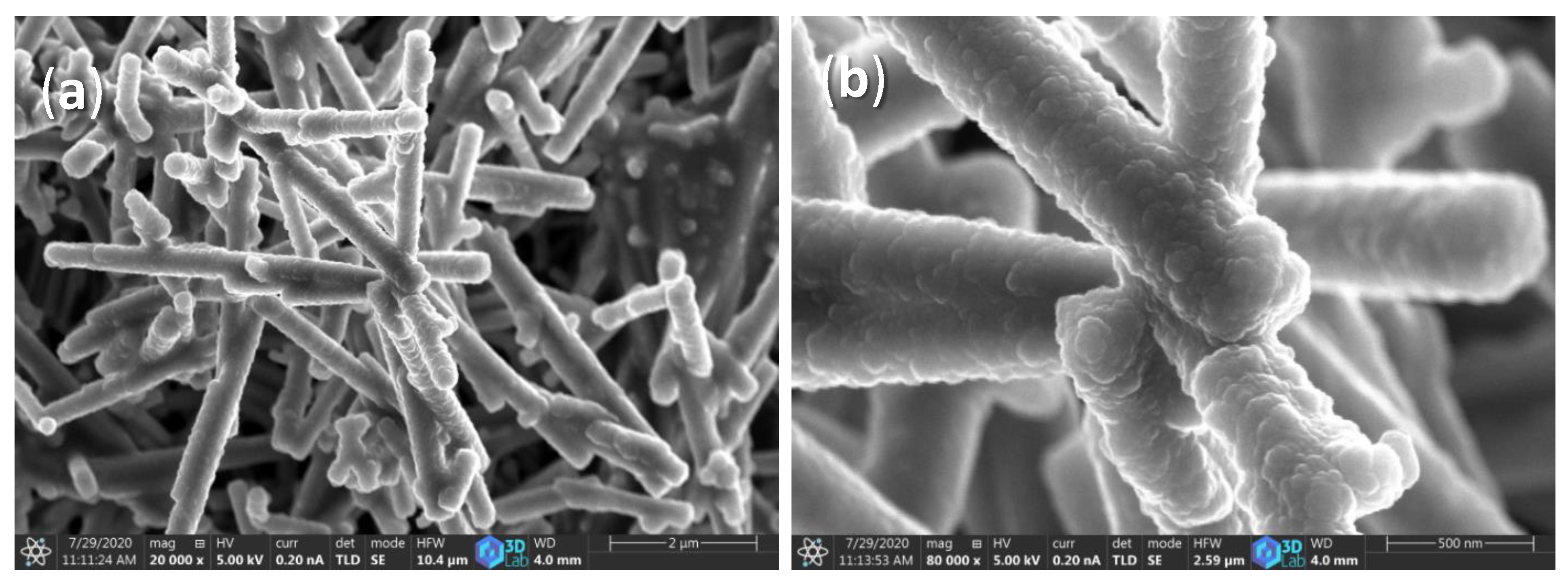

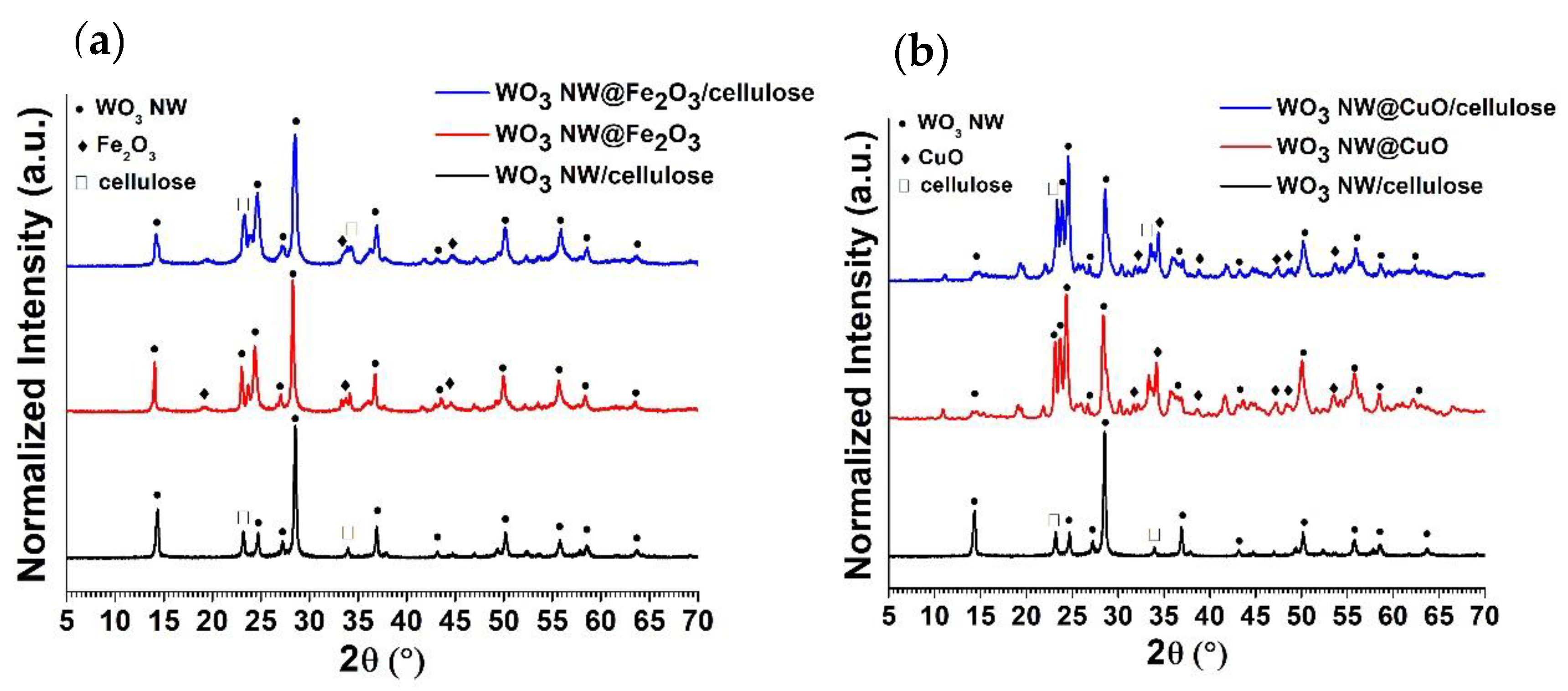

| Sample Name | C | O | W | Na | Fe | Cu |
|---|---|---|---|---|---|---|
| Cellulose | 45 | 55 | — | — | — | — |
| WO3 NW | — | 76 | 24 | — | — | |
| WO3 NW/cellulose | 36 | 48 | 14 | 2 | — | — |
| WO3 NW@Fe2O3/cellulose | 33 | 48 | 16 | 2 | 1 | — |
| WO3 NW@CuO/cellulose | 31 | 49 | 14 | 2 | — | 4 |
| Sample Name | Surface Area (m2/g) | Pore Diameter (nm) |
|---|---|---|
| WO3 NW | 14 | 11 |
| cellulose membrane | 6 | 18 |
| Fe2O3 | 62 | — |
| CuO | 141 | — |
| WO3 NW/cellulose | 9 | 15 |
| WO3 NW@Fe2O3 | 7 | 17 |
| WO3 NW@CuO | 4 | 17 |
| WO3 NW@Fe2O3/cellulose | 8 | 17 |
| WO3 NW@CuO/cellulose | 5 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shehab, M.A.; Sharma, N.; Karacs, G.; Nánai, L.; Kocserha, I.; Hernadi, K.; Németh, Z. Development and Investigation of Photoactive WO3 Nanowire-Based Hybrid Membranes. Catalysts 2022, 12, 1029. https://doi.org/10.3390/catal12091029
Shehab MA, Sharma N, Karacs G, Nánai L, Kocserha I, Hernadi K, Németh Z. Development and Investigation of Photoactive WO3 Nanowire-Based Hybrid Membranes. Catalysts. 2022; 12(9):1029. https://doi.org/10.3390/catal12091029
Chicago/Turabian StyleShehab, Mohammed Ahmed, Nikita Sharma, Gábor Karacs, Lilla Nánai, István Kocserha, Klara Hernadi, and Zoltán Németh. 2022. "Development and Investigation of Photoactive WO3 Nanowire-Based Hybrid Membranes" Catalysts 12, no. 9: 1029. https://doi.org/10.3390/catal12091029
APA StyleShehab, M. A., Sharma, N., Karacs, G., Nánai, L., Kocserha, I., Hernadi, K., & Németh, Z. (2022). Development and Investigation of Photoactive WO3 Nanowire-Based Hybrid Membranes. Catalysts, 12(9), 1029. https://doi.org/10.3390/catal12091029








