Effect of Morphology-Dependent Oxygen Vacancies of CeO2 on the Catalytic Oxidation of Toluene
Abstract
:1. Introduction
2. Result and Discussion
2.1. Bulk Structure Analysis
2.2. SEM and TEM Analysis
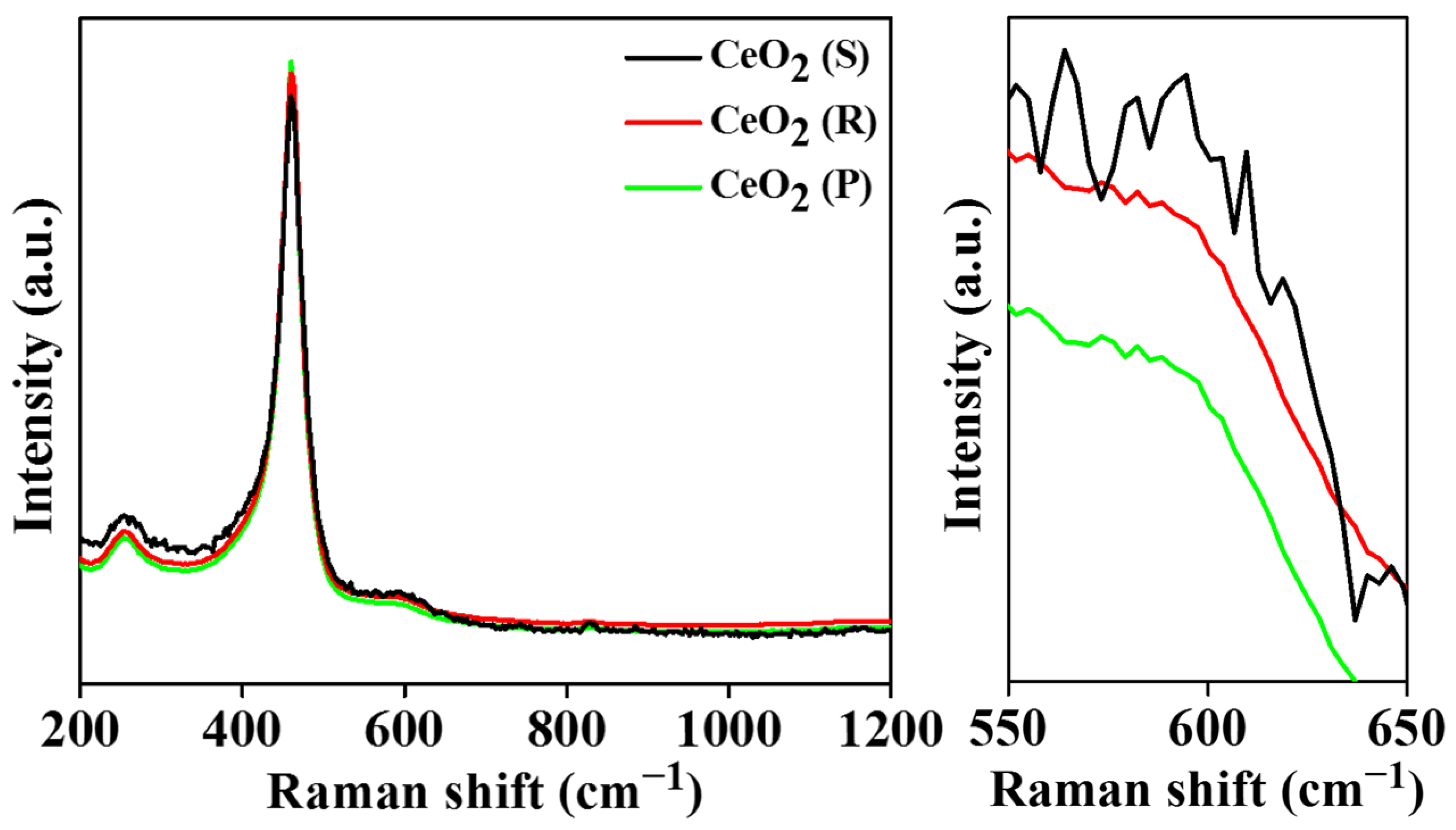
2.3. H2-TPR and O2-TPD Analysis
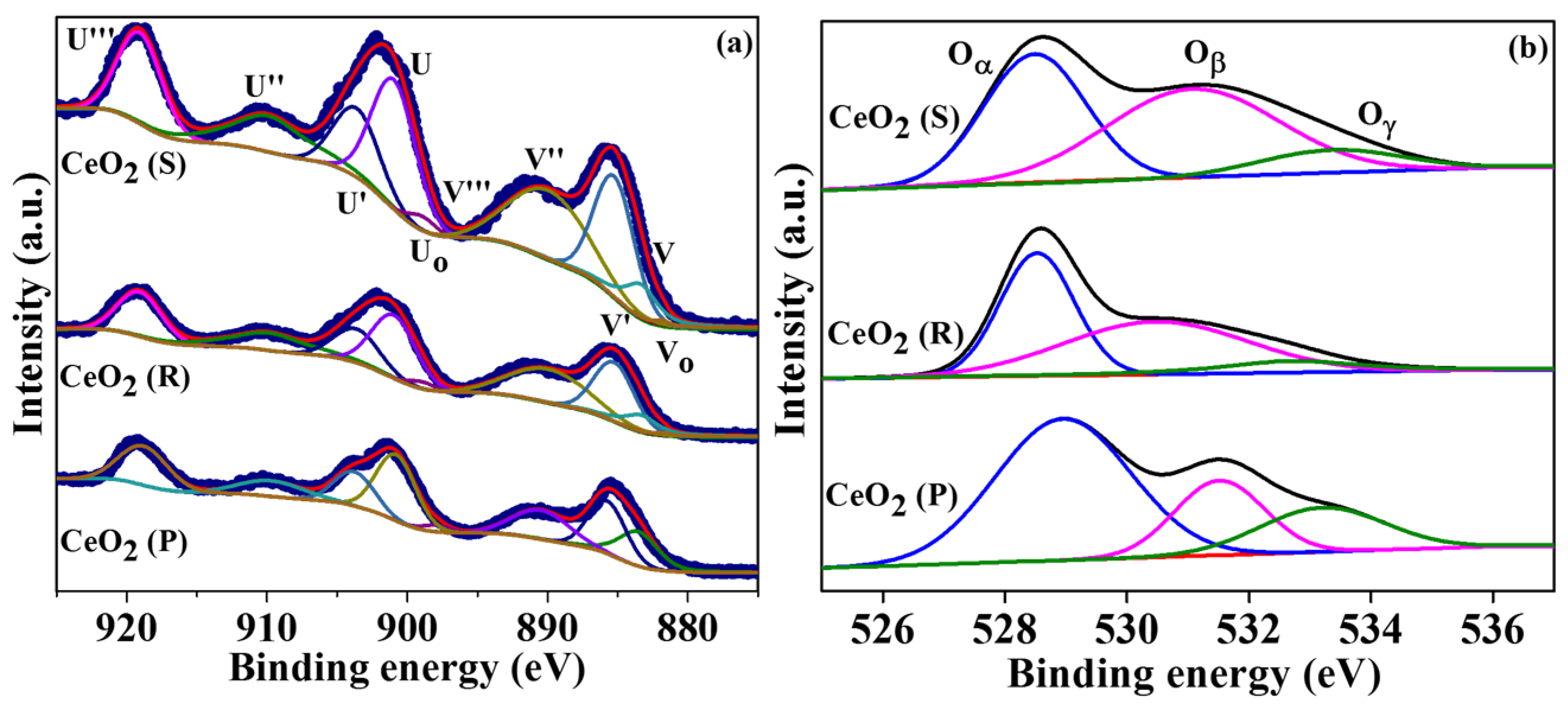
2.4. XPS Analysis
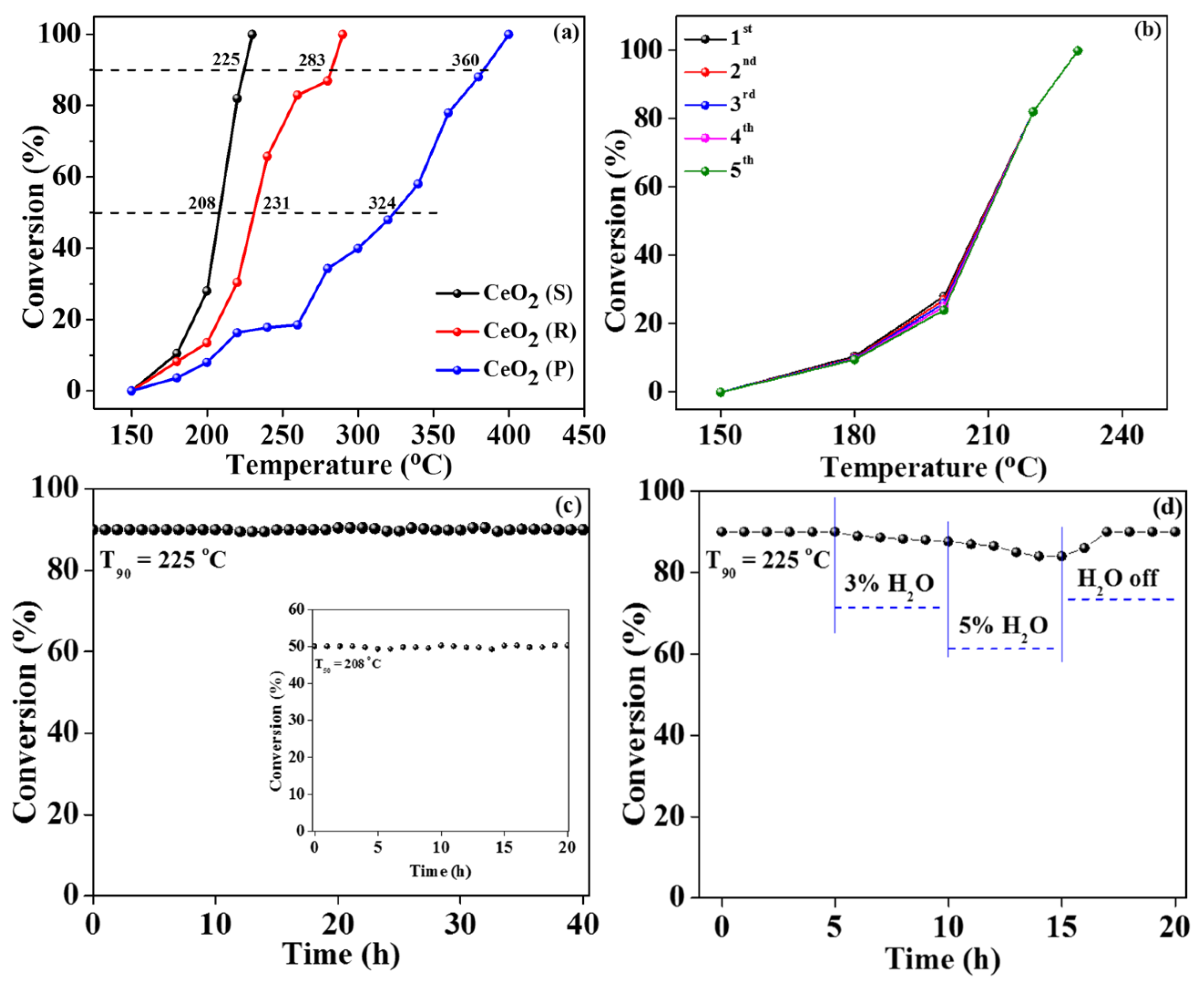
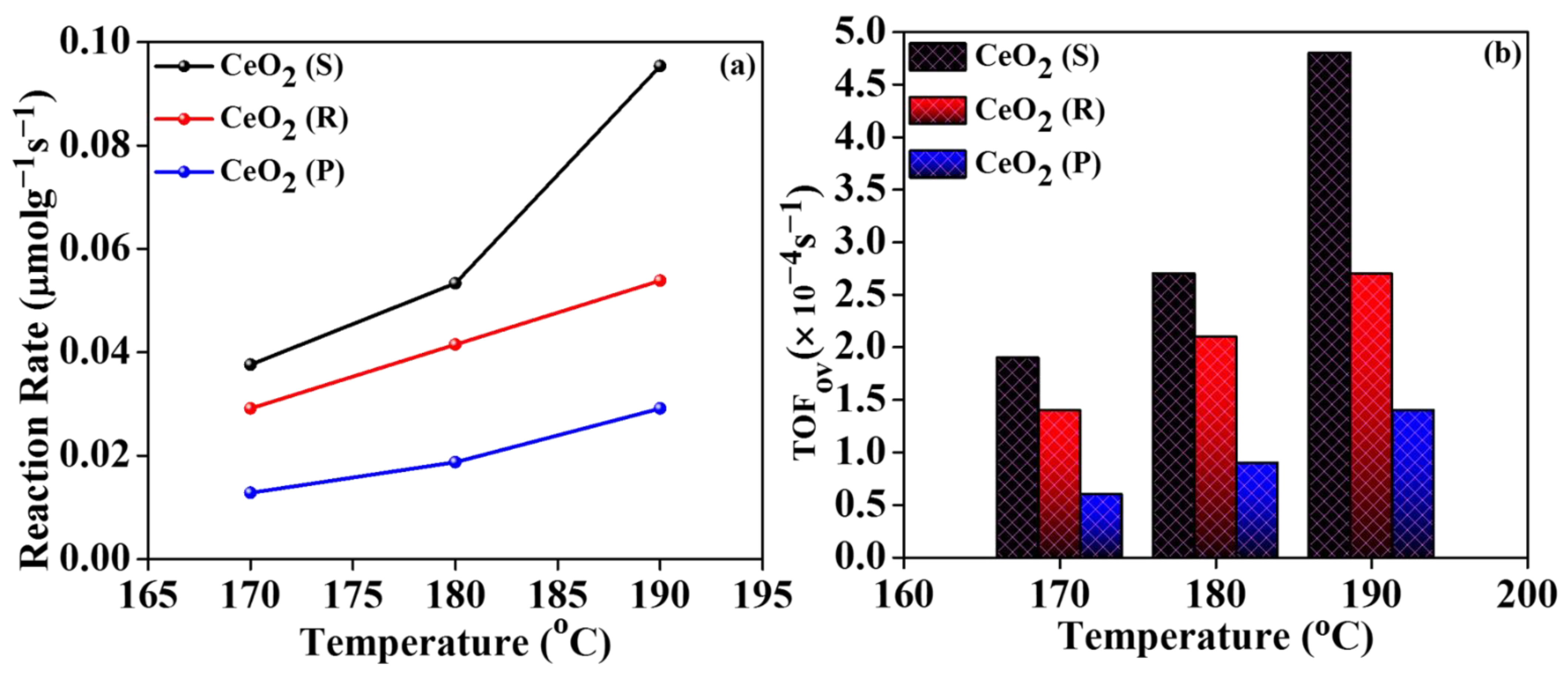
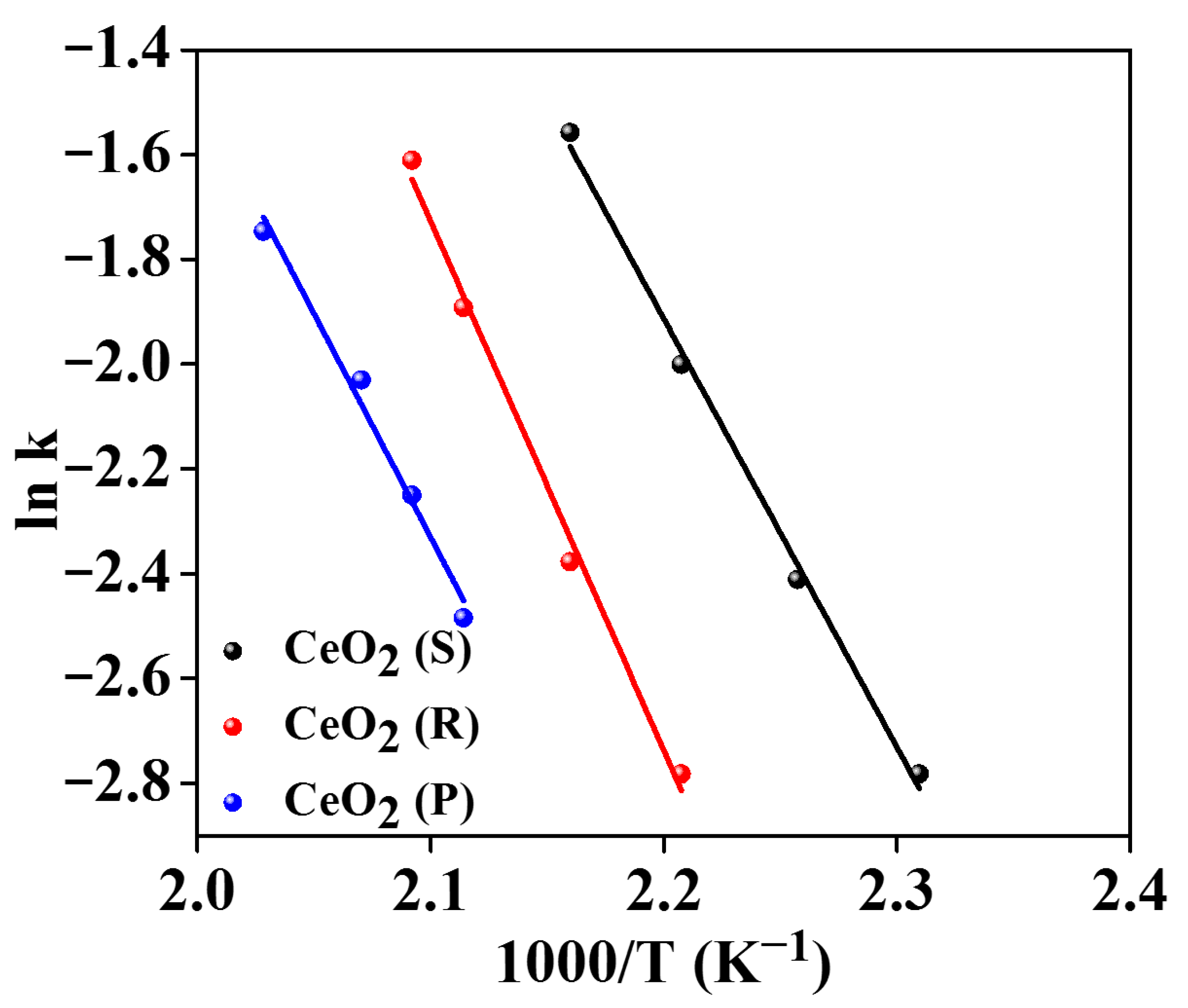
2.5. Catalytic Oxidation Activity
2.6. Influences of Different Morphologies CeO2 Catalysts on Oxidation Activity
3. Experimental
3.1. Chemicals
3.2. Preparation of Catalysts
3.3. Characterization
3.4. Catalytic Oxidation Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, K.; Li, J.; Tong, S.; Wang, W.; Huang, R.J.; Ge, M. Characteristics of wintertime VOCs in suburban and urban beijing: Concentrations, emission ratios, and festival effects. Atmos. Chem. Phys. 2019, 19, 8021–8036. [Google Scholar] [CrossRef]
- Lyu, X.P.; Chen, N.; Guo, H.; Zhang, W.H.; Wang, N.; Wang, Y.; Liu, M. Ambient volatile organic compounds and their effect on ozone production in wuhan, central china. Sci. Total Environ. 2016, 541, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, C.; Fan, L.; Lian, Y.; Niu, X.; Zhu, Y. Enhanced catalytic oxidation of toluene over manganese oxide modified by lanthanum with a coral-like hierarchical structure nanosphere. ACS Appl. Mater. Interfaces 2021, 13, 10089–10100. [Google Scholar] [CrossRef]
- Ismail, A.; Li, M.; Zahid, M.; Fan, L.; Zhang, C.; Li, Z.; Zhu, Y. Effect of strong interaction between Co and Ce oxides in CoxCe1−xO2−δ oxides on its catalytic oxidation of toluene. Mol. Catal. 2021, 502, 111356. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Schick, L.; Sanchis, R.; Gonzalez-Alfaro, V.; Agouram, S.; Lopez, J.M.; Torrente-Murciano, L.; García, T.; Solsona, B. Size-activity relationship of iridium particles supported on silica for the total oxidation of volatile organic compounds (VOCs). Chem. Eng. J. 2019, 366, 100–111. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, M.; Ren, Q.; Mo, S.; Peng, R.; Yan, D.; Fu, M.; Chen, L.; Wu, J.; Ye, D. Design of 3-dimensionally self-assembled CeO2 hierarchical nanosphere as high efficiency catalysts for toluene oxidation. Chem. Eng. J. 2019, 369, 18–25. [Google Scholar] [CrossRef]
- Yan, D.; Mo, S.; Sun, Y.; Ren, Q.; Feng, Z.; Chen, P.; Wu, J.; Fu, M.; Ye, D. Morphology-activity correlation of electrospun CeO2 for toluene catalytic combustion. Chemosphere 2020, 247, 125860. [Google Scholar] [CrossRef]
- He, C.; Yu, Y.; Yue, L.; Qiao, N.; Li, J.; Shen, Q.; Yu, W.; Chen, J.; Hao, Z. Low-temperature removal of toluene and propanal over highly active mesoporous CuCeOx catalysts synthesized via a simple self-precipitation protocol. Appl. Catal. B Environ. 2014, 147, 156–166. [Google Scholar] [CrossRef]
- Saqer, S.M.; Kondarides, D.I.; Verykios, X.E. Catalytic oxidation of toluene over binary mixtures of copper, manganese and cerium oxides supported on γ-Al2O3. Appl. Catal. B Environ. 2011, 103, 275–286. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and catalytic applications of CeO2-based materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Sun, X.; Li, S.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Shape effect of Pt/CeO2 catalysts on the catalytic oxidation of toluene. Chem. Eng. J. 2016, 306, 1234–1246. [Google Scholar] [CrossRef]
- Feng, Z.; Ren, Q.; Peng, R.; Mo, S.; Zhang, M.; Fu, M.; Chen, L.; Ye, D. Effect of CeO2 morphologies on toluene catalytic combustion. Catal. Today 2019, 332, 177–182. [Google Scholar] [CrossRef]
- Hu, F.; Chen, J.; Peng, Y.; Song, H.; Li, K.; Li, J. Novel nanowire self-assembled hierarchical CeO2 microspheres for low temperature toluene catalytic combustion. Chem. Eng. J. 2018, 331, 425–434. [Google Scholar] [CrossRef]
- Liao, Y.; He, L.; Zhao, M.; Ye, D. Ultrasonic-assisted hydrothermal synthesis of ceria nanorods and their catalytic properties for toluene oxidation. J. Environ. Chem. Eng. 2017, 5, 5054–5060. [Google Scholar] [CrossRef]
- Meher, S.K.; Rao, G.R. Polymer-assisted hydrothermal synthesis of highly reducible shuttle-shaped CeO2: Microstructural effect on promoting Pt/C for methanol electrooxidation. ACS Catal. 2012, 2, 2795–2809. [Google Scholar] [CrossRef]
- Li, H.; Meng, F.; Gong, J.; Fan, Z.; Qin, R. Structural, morphological and optical properties of shuttle-like CeO2 synthesized by a facile hydrothermal method. J. Alloy. Compd. 2017, 722, 489–498. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Yu, E.; Cai, S.; Jia, H.; Chen, J.; Liang, P. In situ pyrolysis of Ce-MOF to prepare CeO2 catalyst with obviously improved catalytic performance for toluene combustion. Chem. Eng. J. 2018, 344, 469–479. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, J.; Zhang, Q.; Liu, Z.; Fu, M.; Wu, J.; Hu, Y.; Ye, D. Enhanced oxygen vacancies to improve ethyl acetate oxidation over MnOx-CeO2 catalyst derived from MOF template. Chem. Eng. J. 2019, 371, 78–87. [Google Scholar] [CrossRef]
- Hojo, H.; Mizoguchi, T.; Ohta, H.; Findlay, S.D.; Shibata, N.; Yamamoto, T.; Ikuhara, Y. Atomic structure of a CeO2 grain boundary: The role of oxygen vacancies. Nano Lett. 2010, 10, 4668–4672. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Z.; Wang, D.; Chen, D.; Chen, F.; Li, X. Morphology and crystal-plane effects of Fe/W-CeO2 for selective catalytic reduction of NO with NH3. Catalysts 2019, 9, 288. [Google Scholar] [CrossRef]
- Yao, X.; Xiong, Y.; Zou, W.; Zhang, L.; Wu, S.; Dong, X.; Gao, F.; Deng, Y.; Tang, C.; Chen, Z.; et al. Correlation between the physicochemical properties and catalytic performances of CexSn1−xO2 mixed oxides for NO reduction by CO. Appl. Catal. B Environ. 2014, 144, 152–165. [Google Scholar] [CrossRef]
- Torrente-Murciano, L.; Gilbank, A.; Puertolas, B.; Garcia, T.; Solsona, B.; Chadwick, D. Shape-dependency activity of nanostructured CeO2 in the total oxidation of polycyclic aromatic hydrocarbons. Appl. Catal. B Environ. 2013, 132–133, 116–122. [Google Scholar] [CrossRef]
- Ali, S.; Chen, L.; Yuan, F.; Li, R.; Zhang, T.; Bakhtiar, S.u.H.; Leng, X.; Niu, X.; Zhu, Y. Synergistic effect between copper and cerium on the performance of Cux-Ce0.5−x-Zr0.5 (x = 0.1–0.5) oxides catalysts for selective catalytic reduction of NO with ammonia. Appl. Catal. B Environ. 2017, 210, 223–234. [Google Scholar] [CrossRef]
- Lykaki, M.; Pachatouridou, E.; Carabineiro, S.A.C.; Iliopoulou, E.; Andriopoulou, C.; Kallithrakas-Kontos, N.; Boghosian, S.; Konsolakis, M. Ceria nanoparticles shape effects on the structural defects and surface chemistry: Implications in CO oxidation by Cu/CeO2 catalysts. Appl. Catal. B Environ. 2018, 230, 18–28. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Q.; Dai, Q.; Wang, X. Fe doped CeO2 nanosheets for catalytic oxidation of 1,2-dichloroethane: Effect of preparation method. Chem. Eng. J. 2017, 307, 1037–1046. [Google Scholar] [CrossRef]
- Tang, X.; Xu, Y.; Shen, W. Promoting effect of copper on the catalytic activity of MnOx-CeO2 mixed oxide for complete oxidation of benzene. Chem. Eng. J. 2008, 144, 175–180. [Google Scholar] [CrossRef]
- Fan, L.; Li, M.; Zhang, C.; Ismail, A.; Hu, B.; Zhu, Y. Effect of Cu/Co ratio in CuaCo1−aOx (a = 0.1, 0.2, 0.4, 0.6) flower structure on its surface properties and catalytic performance for toluene oxidation. J. Colloid Interface Sci. 2021, 599, 404–415. [Google Scholar] [CrossRef]
- Liu, W.; Liu, R.; Zhang, H.; Jin, Q.; Song, Z.; Zhang, X. Fabrication of Co3O4 nanospheres and their catalytic performances for toluene oxidation: The distinct effects of morphology and oxygen species. Appl. Catal. A Gen. 2020, 597, 117539. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Zhu, S.; Fu, S.; Dong, X.; Ida, S.; Zhang, L.; Guo, L. Layered δ-MnO2 as an active catalyst for toluene catalytic combustion. Appl. Catal. A Gen. 2020, 602, 117715. [Google Scholar] [CrossRef]
- Bo, Z.; Zhu, J.; Yang, S.; Yang, H.; Yan, J.; Cen, K. Enhanced plasma-catalytic decomposition of toluene over Co-Ce binary metal oxide catalysts with high energy efficiency. RSC Adv. 2019, 9, 7447–7456. [Google Scholar] [CrossRef] [PubMed]
- Bo, Z.; Yang, S.; Kong, J.; Zhu, J.; Wang, Y.; Yang, H.; Li, X.; Yan, J.; Cen, K.; Tu, X. Solar-enhanced plasma-catalytic oxidation of toluene over a bifunctional graphene fin foam decorated with nanofin-like MnO2. ACS Catal. 2020, 10, 4420–4432. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Samples | Crystallite Size (nm) a | BET Surface Area (m2∙g−1) b | Pore Size (nm) b | Pore Volume (cm3∙g−1) b | A600/A461 c | Ce3+/(Ce) d (%) | Oβ/(O) e (%) |
|---|---|---|---|---|---|---|---|
| CeO2 (S) | 9 | 116 | 3.85 | 0.54 | 0.62 | 39.6 | 55.4 |
| CeO2 (R) | 11 | 79 | 3.72 | 0.43 | 0.23 | 31.4 | 48.3 |
| CeO2 (P) | 12 | 60 | 3.98 | 0.09 | 0.16 | 23.8 | 45.2 |
| Samples | T50 (°C) | T90 (°C) | Reaction Rate (×10−2) (μmol∙g−1s−1) (190 °C) | TOFov (×10−4 s−1) (190 °C) | Ea (kJ∙mol−1) |
|---|---|---|---|---|---|
| CeO2 (S) | 208 | 225 | 14.37 | 4.8 | 67.4 |
| CeO2 (R) | 231 | 283 | 7.36 | 2.7 | 85.8 |
| CeO2 (P) | 324 | 360 | 4.26 | 1.4 | 103.2 |
| Catalyst | Morphology | Toluene Concentration (ppm) | WHSV mL·g−1·h−1 | T90% | Ref. |
|---|---|---|---|---|---|
| CeO2 | Nanotubes | 100 | 60,000 | 255 | 8 |
| CeO2 | Wire in nanotubes | 100 | 60,000 | 345 | 8 |
| CeO2 | Nanocubes | 1000 | 48,000 | 296 | 13 |
| CeO2 | Non-hierarchical Microspheres | 1000 | 60,000 | 240 | 14 |
| CeO2 | Nanorods | 1000 | 20,000 | 300 | 15 |
| Co3O4 | Hydrangea-like microsphere | 500 | 60,000 | 248 | 29 |
| Co3O4 | Pompon microsphere | 500 | 60,000 | 298 | 29 |
| Co3O4 | Spiky microspheres | 500 | 60,000 | 269 | 29 |
| CeO2 | Shuttle | 1000 | 40,000 | 225 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, A.; Zahid, M.; Hu, B.; Khan, A.; Ali, N.; Zhu, Y. Effect of Morphology-Dependent Oxygen Vacancies of CeO2 on the Catalytic Oxidation of Toluene. Catalysts 2022, 12, 1034. https://doi.org/10.3390/catal12091034
Ismail A, Zahid M, Hu B, Khan A, Ali N, Zhu Y. Effect of Morphology-Dependent Oxygen Vacancies of CeO2 on the Catalytic Oxidation of Toluene. Catalysts. 2022; 12(9):1034. https://doi.org/10.3390/catal12091034
Chicago/Turabian StyleIsmail, Ahmed, Muhammad Zahid, Boren Hu, Adnan Khan, Nauman Ali, and Yujun Zhu. 2022. "Effect of Morphology-Dependent Oxygen Vacancies of CeO2 on the Catalytic Oxidation of Toluene" Catalysts 12, no. 9: 1034. https://doi.org/10.3390/catal12091034





