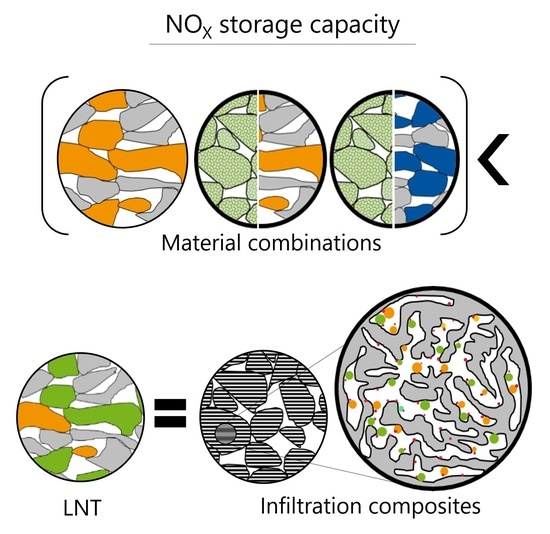
Contributions of Washcoat Components in Different Configurations to the NOX and Oxygen Storage Performance of LNT Catalysts
Abstract
:1. Introduction
2. Results
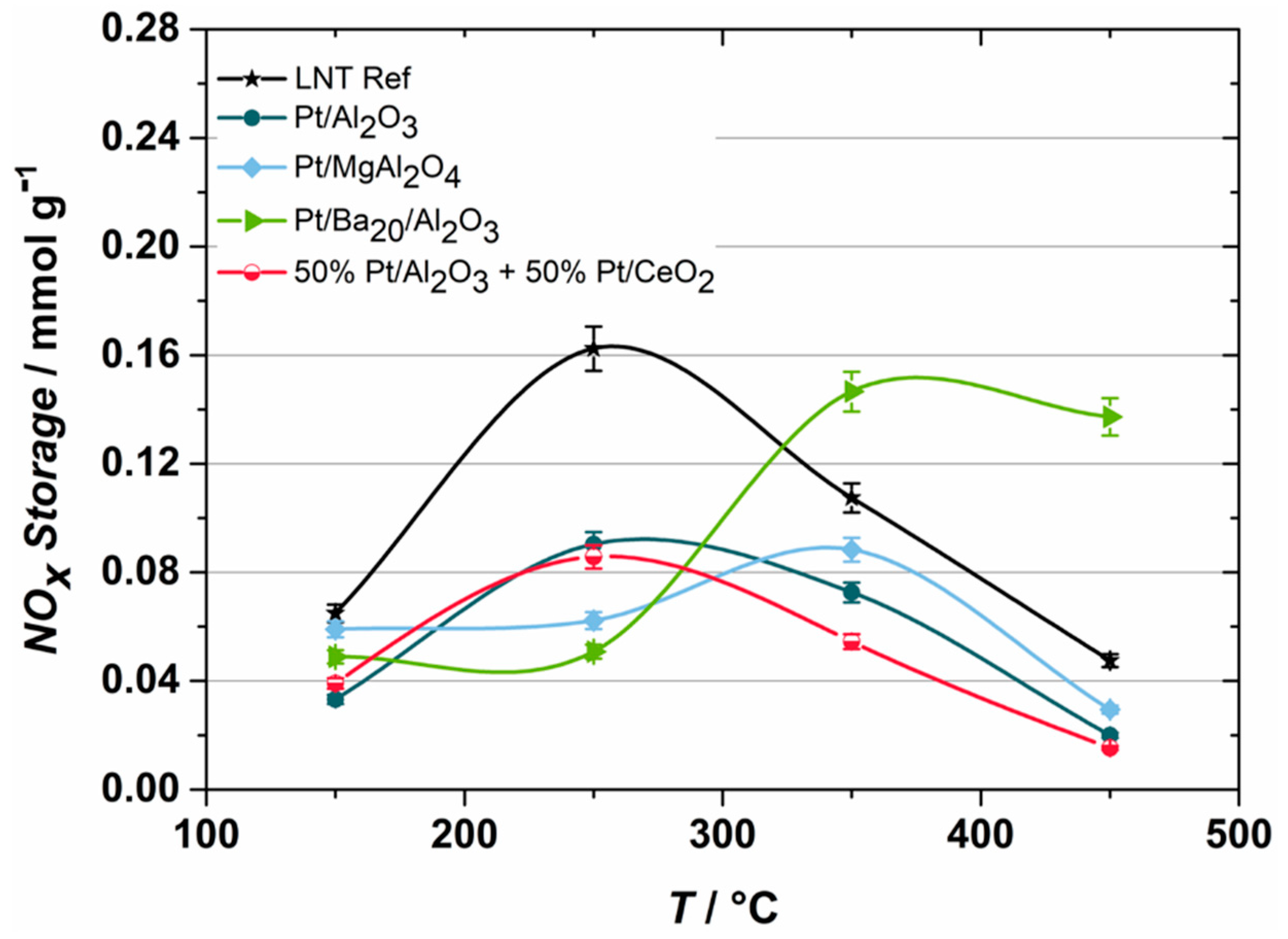
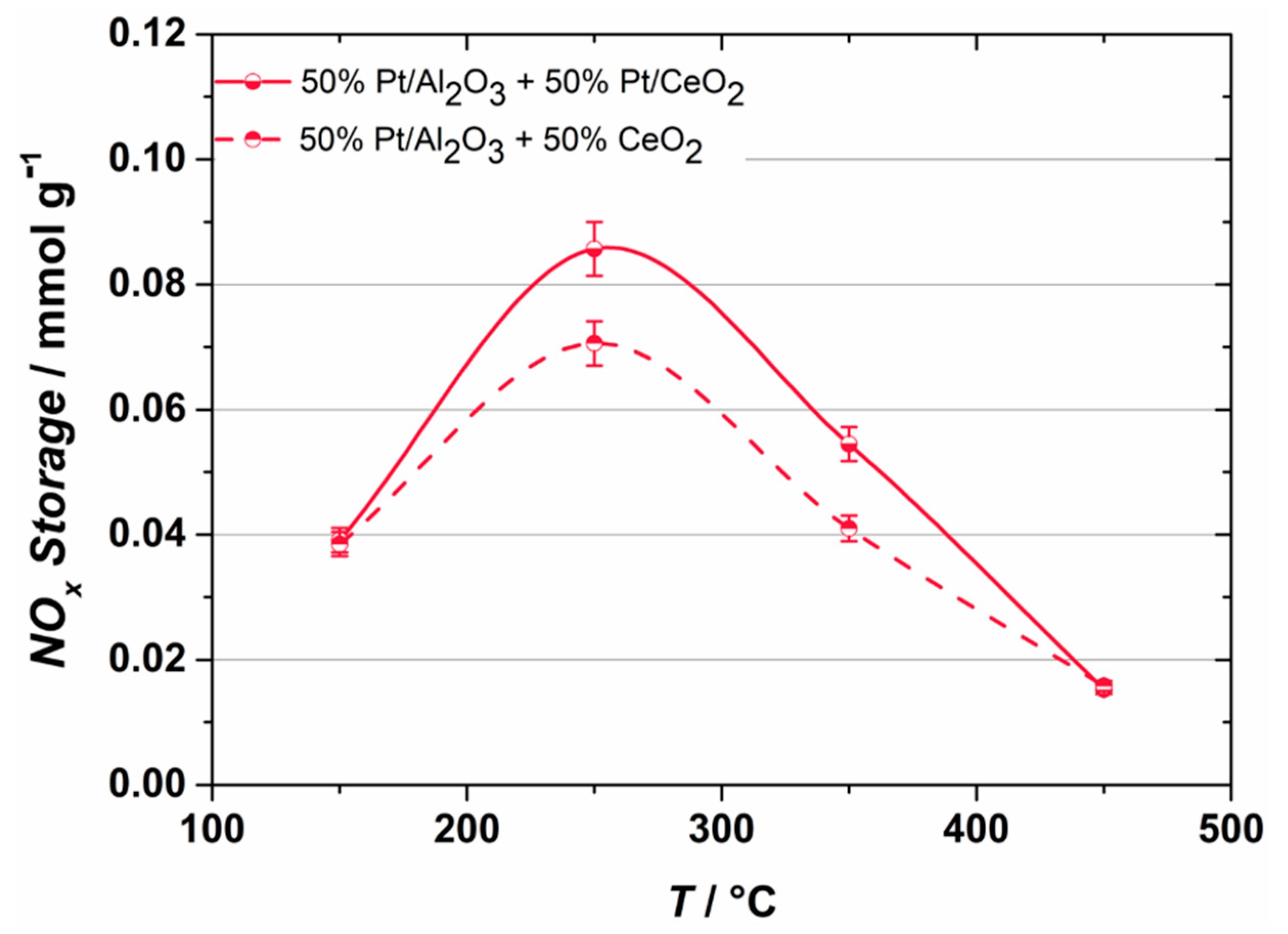
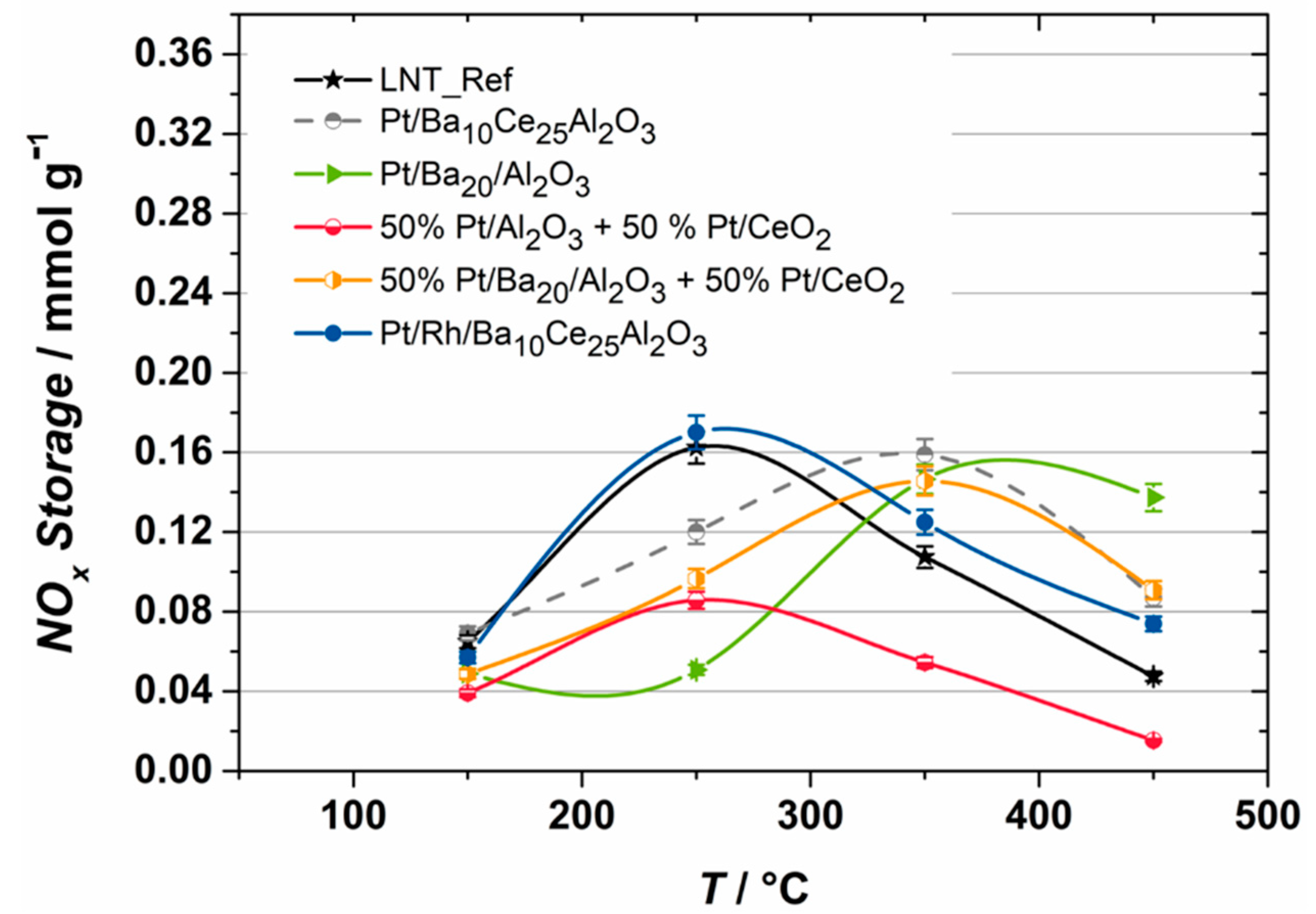
2.1. NOX Storage Performance
2.2. Oxygen Storage Capacity
3. Discussion
4. Materials and Methods
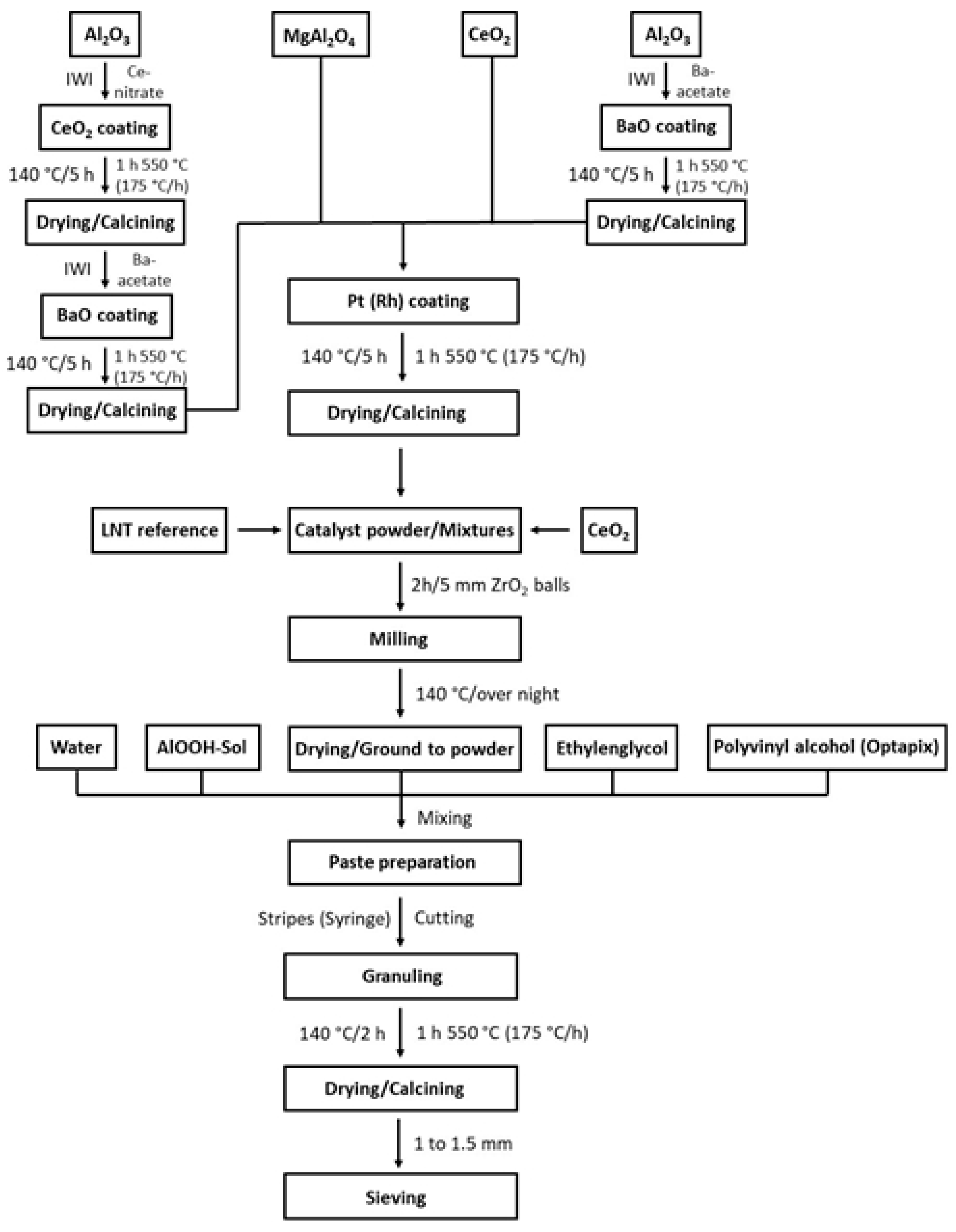
4.1. Catalyst Preparation
4.1.1. Granule Formation
4.1.2. Grain Size Distribution
4.2. Reference Catalyst
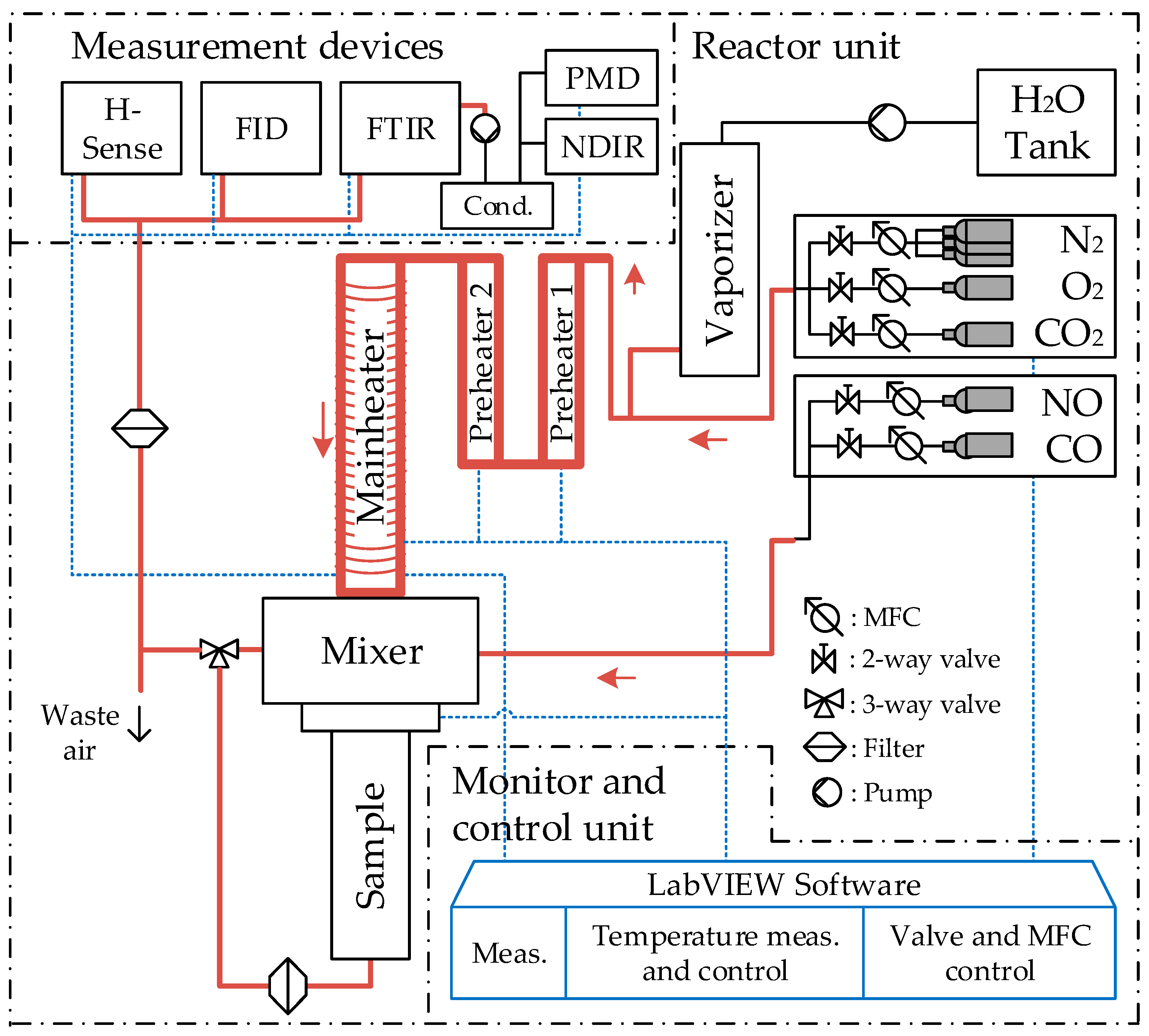
4.3. Experimental Setup
4.4. NOX Storage Measurements
4.5. Oxygen Storage Capacity Measurements
5. Conclusions
- Due to synergistic effects, combination materials, in particular physical mixtures between Mg spinel and barium oxide or ceria and barium oxide, have been developed that can store more NOX at higher temperatures than the commercially manufactured reference. As a result, modeling the combined performance by simply interpolating the NSC of the individual materials is not possible.
- The investigations with different material groups showed that a mixing process at the chemical level by impregnation of PGM, BaO, and ceria within the pore system on an alumina support is required to achieve a high NOX adsorption capability at low temperatures by reducing the diffusion pathways for complex reactions of all participants.
- The variation of PGM within these infiltration components shows that only the addition of Rh could improve the NSC under lean conditions at compared to the reference LNT.
- The oxygen storage capacity of the infiltration composites can be reduced by decreasing the amount of ceria without performance loss during NOX storage, which could improve the NOX regeneration efficiency.
- Due to the comparable NOX storage performance with the reference LNT, the investigated Pt/RhBa10Ce25Al2O3 catalyst could be used as a single compound in commercial LNTs. However, further investigations and optimizations must still be executed in terms of regeneration behavior and HC oxidation activity (e.g., addition of Pd) to verify the suitability.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, L. NOx control technologies for Euro 6 diesel passenger cars: Market penetration and experimental performance assessment. ICCT 2015. [Google Scholar] [CrossRef]
- Johnson, T.V. Review of Vehicular Emissions Trends. SAE Int. J. Engines 2015, 8, 1152–1167. [Google Scholar] [CrossRef]
- Nova, I.; Tronconi, E. Urea-SCR Technology for deNOx after Treatment of Diesel Exhausts; Springer: New York, NY, USA, 2014; ISBN 978-1-4899-8071-7. [Google Scholar]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The State of the Art in Selective Catalytic Reduction of NO x by Ammonia Using Metal-Exchanged Zeolite Catalysts. Catal. Rev. 2008, 50, 492–531. [Google Scholar] [CrossRef]
- Miyoshi, N.; Matsumoto, S.; Katoh, K.; Tanaka, T.; Harada, J.; Takahashi, N.; Yokota, K.; Sugiura, M.; Kasahara, K. Development of New Concept Three-Way Catalyst for Automotive Lean-Burn Engines. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 1995. [Google Scholar]
- Liu, G.; Gao, P.-X. A review of NOx storage/reduction catalysts: Mechanism, materials and degradation studies. Catal. Sci. Technol. 2011, 1, 552. [Google Scholar] [CrossRef]
- Roy, S.; Baiker, A. NOx storage-reduction catalysis: From mechanism and materials properties to storage-reduction performance. Chem. Rev. 2009, 109, 4054–4091. [Google Scholar] [CrossRef]
- Schönebaum, S.; Dornseiffer, J.; Mauermann, P.; Wolkenar, B.; Sterlepper, S.; Wessel, E.; Iskandar, R.; Mayer, J.; Weirich, T.E.; Pischinger, S.; et al. Composition/Performance Evaluation of Lean NOx Trap Catalysts for Coupling with SCR Technology. ChemCatChem 2021, 50, 2274. [Google Scholar] [CrossRef]
- Dupré, J.; Bazin, P.; Marie, O.; Daturi, M.; Jeandel, X.; Meunier, F. Effects of temperature and rich-phase composition on the performance of a commercial NOx-Storage-Reduction material. Appl. Catal. B Environ. 2016, 181, 534–541. [Google Scholar] [CrossRef]
- Epling, W.S.; Campbell, L.E.; Yezerets, A.; Currier, N.W.; Parks, J.E. Overview of the Fundamental Reactions and Degradation Mechanisms of NOx Storage/Reduction Catalysts. Catal. Rev. 2004, 46, 163–245. [Google Scholar] [CrossRef]
- Abdulhamid, H.; Fridell, E.; Skoglundh, M. The reduction phase in NOx storage catalysis: Effect of type of precious metal and reducing agent. Appl. Catal. B Environ. 2006, 62, 319–328. [Google Scholar] [CrossRef]
- Amberntsson, A.; Fridell, E.; Skoglundh, M. Influence of platinum and rhodium composition on the NOx storage and sulphur tolerance of a barium based NOx storage catalyst. Appl. Catal. B Environ. 2003, 46, 429–439. [Google Scholar] [CrossRef]
- Breen, J.P.; Burch, R.; Fontaine-Gautrelet, C.; Hardacre, C.; Rioche, C. Insight into the key aspects of the regeneration process in the NOx storage reduction (NSR) reaction probed using fast transient kinetics coupled with isotopically labelled 15NO over Pt and Rh-containing Ba/Al2O3 catalysts. Appl. Catal. B Environ. 2008, 81, 150–159. [Google Scholar] [CrossRef]
- Olsson, L.; Westerberg, B.; Persson, H.; Fridell, E.; Skoglundh, M.; Andersson, B. A Kinetic Study of Oxygen Adsorption/Desorption and NO Oxidation over Pt/Al2O3 Catalysts. J. Phys. Chem. B 1999, 103, 10433–10439. [Google Scholar] [CrossRef]
- Olsson, L.; Fridell, E. The Influence of Pt Oxide Formation and Pt Dispersion on the Reactions NO2⇔NO+1/2 O2 over Pt/Al2O3 and Pt/BaO/Al2O3. J. Catal. 2002, 210, 340–353. [Google Scholar] [CrossRef]
- Bhatia, D.; McCabe, R.W.; Harold, M.P.; Balakotaiah, V. Experimental and kinetic study of NO oxidation on model Pt catalysts. J. Catal. 2009, 266, 106–119. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalysis by Ceria and Related Materials; Imperial College Press: London, UK, 2005; ISBN 1860942997. [Google Scholar]
- Bunluesin, T.; Gorte, R.J.; Graham, G.W. Studies of the water-gas-shift reaction on ceria-supported Pt, Pd, and Rh: Implications for oxygen-storage properties. Appl. Catal. B Environ. 1998, 15, 107–114. [Google Scholar] [CrossRef]
- Phatak, A.A.; Koryabkina, N.; Rai, S.; Ratts, J.L.; Ruettinger, W.; Farrauto, R.J.; Blau, G.E.; Delgass, W.N.; Ribeiro, F.H. Kinetics of the water–gas shift reaction on Pt catalysts supported on alumina and ceria. Catal. Today 2007, 123, 224–234. [Google Scholar] [CrossRef]
- Pereda-Ayo, B.; De La Torre, U.; González-Marcos, M.P.; González-Velasco, J.R. Influence of ceria loading on the NOx storage and reduction performance of model Pt–Ba/Al2O3 NSR catalyst. Catal. Today 2015, 241, 133–142. [Google Scholar] [CrossRef]
- Ji, Y.; Toops, T.J.; Crocker, M. Effect of Ceria on the Storage and Regeneration Behavior of a Model Lean NOx Trap Catalyst. Catal. Lett. 2007, 119, 257–264. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; He, H. Oxygen vacancies on nanosized ceria govern the NOx storage capacity of NSR catalysts. Catal. Sci. Technol. 2016, 6, 3950–3962. [Google Scholar] [CrossRef]
- Constantinou, C.; Li, W.; Qi, G.; Epling, W.S. NOX storage and reduction over a perovskite-based lean NOX trap catalyst. Appl. Catal. B Environ. 2013, 134–135, 66–74. [Google Scholar] [CrossRef]
- López-Suárez, F.E.; Illán-Gómez, M.J.; Bueno-López, A.; Anderson, J.A. NOx Storage reduction on a SrTiCuO3 perovskite catalyst studied by operando DRIFTS. Appl. Catal. B Environ. 2011, 104, 261–267. [Google Scholar] [CrossRef]
- Ander Onrubia-Calvo, J.; Pereda-Ayo, B.; De-La-Torre, U.; Ramón González-Velasco, J. Perovskite-Based Formulations as Rival Platinum Catalysts for NO x Removal in Diesel Exhaust Aftertreatment. In Perovskite Materials, Devices and Integration; He, T., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Wen, W.; Wang, X.; Jin, S.; Wang, R. LaCoO3 perovskite in Pt/LaCoO3 /K/Al2O3 for the improvement of NOx storage and reduction performances. RSC Adv. 2016, 6, 74046–74052. [Google Scholar] [CrossRef]
- Fornasari, G.; Trifirò, F.; Vaccari, A.; Prinetto, F.; Ghiotti, G.; Centi, G. Novel low temperature NO storage-reduction catalysts for diesel light-duty engine emissions based on hydrotalcite compounds. Catal. Today 2002, 75, 421–429. [Google Scholar] [CrossRef]
- Li, Q.; Meng, M.; Xian, H.; Tsubaki, N.; Li, X.; Xie, Y.; Hu, T.; Zhang, J. Hydrotalcite-derived MnxMg3-xAlO catalysts used for soot combustion, NOx storage and simultaneous soot-NOx removal. Environ. Sci. Technol. 2010, 44, 4747–4752. [Google Scholar] [CrossRef]
- Yu, J.J.; Jiang, Z.; Zhu, L.; Hao, Z.P.; Xu, Z.P. Adsorption/desorption studies of NOx on well-mixed oxides derived from Co-Mg/Al Hydrotalcite-like compounds. J. Phys. Chem. B 2006, 110, 4291–4300. [Google Scholar] [CrossRef]
- Silletti, B.A.; Adams, R.T.; Sigmon, S.M.; Nikolopoulos, A.; Spivey, J.J.; Lamb, H.H. A novel Pd/MgAlOx catalyst for NOx storage-reduction. Catal. Today 2006, 114, 64–71. [Google Scholar] [CrossRef]
- Fornasari, G.; Glöckler, R.; Livi, M.; Vaccari, A. Role of the Mg/Al atomic ratio in hydrotalcite-based catalysts for NOx storage/reduction. Appl. Clay Sci. 2005, 29, 258–266. [Google Scholar] [CrossRef]
- Mahzoul, H.; Limousy, L.; Brilhac, J.F.; Gilot, P. Experimental study of SO2 adsorption on barium-based NOx adsorbers. J. Anal. Appl. Pyrol. 2000, 2000, 179–193. [Google Scholar] [CrossRef]
- Westerberg, B.; Fridell, E. A transient FTIR study of species formed during NOx storage in the Pt/BaO/Al2O3 system. J. Mol. Catal. A Chem. 2000, 2001, 249–263. [Google Scholar]
- Forzatti, P.; Castoldi, L.; Nova, I.; Lietti, L.; Tronconi, E. NOx removal catalysis under lean conditions. Catal. Today 2006, 117, 316–320. [Google Scholar] [CrossRef]
- Nova, I.; Costaldi, L.; Lietti, L.; Tronconi, E.; Forzatti, P.; Prinetto, F.; Ghiotti, G. NOx adsorption study over Pt–Ba/alumina catalysts: FT-IR and pulse experiments. J. Catal. 2004, 222, 377–388. [Google Scholar] [CrossRef]
- Dupré, J.; Bazin, P.; Marie, O.; Daturi, M.; Jeandel, X.; Meunier, F. Understanding the storage function of a commercial NOx-storage-reduction material using operando IR under realistic conditions. Appl. Catal. B Environ. 2014, 160–161, 335–343. [Google Scholar] [CrossRef]
- Shi, C.; Ji, Y.; Graham, U.M.; Jacobs, G.; Crocker, M.; Zhang, Z.; Wang, Y.; Toops, T.J. NOx storage and reduction properties of model ceria-based lean NOx trap catalysts. Appl. Catal. B Environ. 2012, 119–120, 183–196. [Google Scholar] [CrossRef]
- Sedlmair, C.; Seshan, K.; Jentys, A.; Lercher, J.A. Elementary steps of NOx adsorption and surface reaction on a commercial storage–reduction catalyst. J. Catal. 2003, 214, 308–316. [Google Scholar] [CrossRef]
- Laible, T.; Pischinger, S.; Holderbaum, B. Internal and External Measures for Catalyst Light-Off Support. In SAE Technical Paper Series. Proceedings of the 12th International Conference on Engines & Vehicles, Capri, Italy, 13 September 2015; SAE International: Warrendale, PA, USA, 2015. [Google Scholar]
- Jabłońska, M.; Wolkenar, B.; Beale, A.M.; Pischinger, S.; Palkovits, R. Comparison of Cu-Mg-Al-Ox and Cu/Al2O3 in selective catalytic oxidation of ammonia (NH3-SCO). Catal. Commun. 2018, 110, 5–9. [Google Scholar] [CrossRef]
- Wolkenar, B.; Schönebaum, S.; Mauermann, P.; Dittmann, P.; Pischinger, S.; Simon, U. Storage and Oxidation of Oxygen-Free and Oxygenated Hydrocarbons on a Pt–Pd Series Production Oxidation Catalyst. Top. Catal. 2019, 62, 376–385. [Google Scholar] [CrossRef]
- Özyalcin, C.; Mauermann, P.; Dirkes, S.; Thiele, P.; Sterlepper, S.; Pischinger, S. Investigation of Filtration Phenomena of Air Pollutants on Cathode Air Filters for PEM Fuel Cells. Catalysts 2021, 11, 1339. [Google Scholar] [CrossRef]


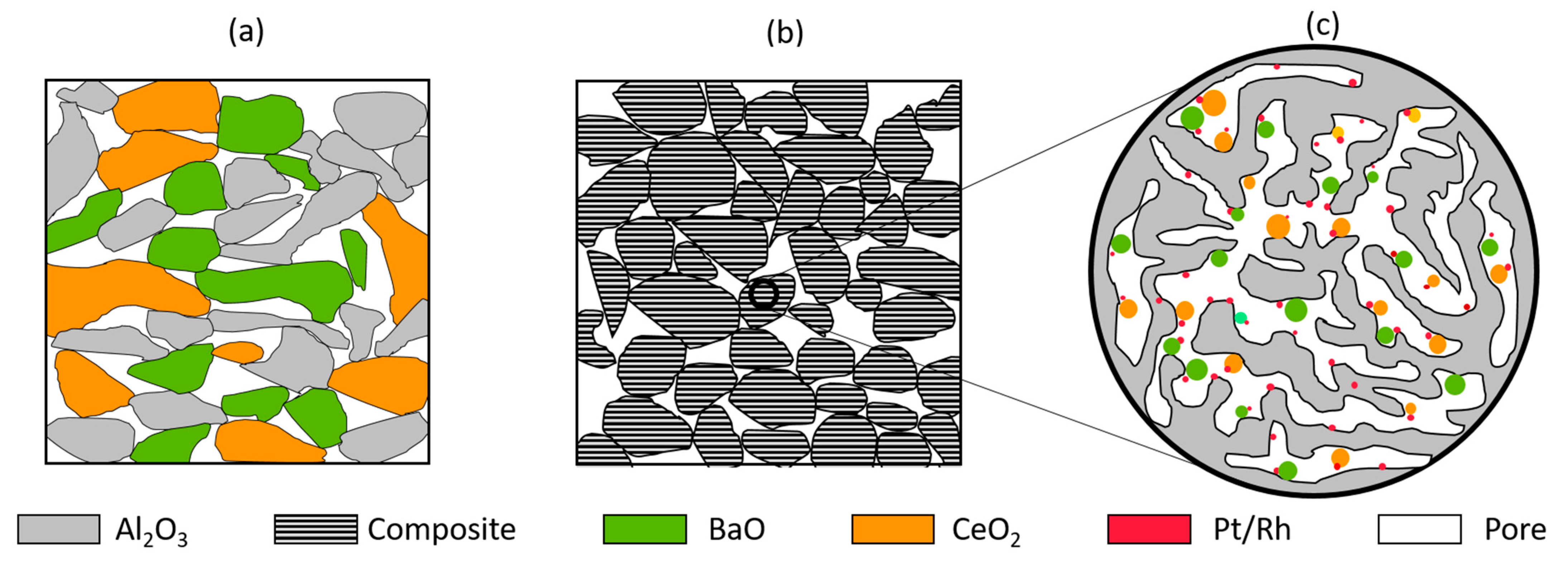

| Component | Pt | Pd | Rh | MgAl2O4 | CeO2 | BaO | Al2O3 |
|---|---|---|---|---|---|---|---|
| Specific Loading/(g/lCAT) | 2.52 | 0.8 | 0.09 | 22.2 | 132 | 10 | 80 |
| Mass Fraction/% | 1.01 | 0.32 | 0.04 | 8.87 | 52.71 | 5.11 | 31.95 |
| Samples | Material |
|---|---|
| Reference LNT | From serial production |
| Pt/Al2O3 | Commercial alumina |
| Pt/MgAl2O4 | Commercial Mg spinel |
| 50% Pt/Al2O3 + 50% CeO2 | Non-activated commercial ceria |
| 50% Pt/Al2O3 + 50% Pt/CeO2 | Activated commercial ceria |
| Pt/Ba20/Al2O3 | Activated barium (Infiltration composite) |
| 50% Pt/Ba20/Al2O3 + 50% Pt/CeO2 | Mixture of ceria and barium |
| 50% Pt/Ba20/Al2O3 + 50% Pt/MgAl2O4 | Mixture of Mg spinel and barium |
| Pt/Ba10Ce25/Al2O3 | Infiltration composite |
| Pt/Rh/Ba10Ce25/Al2O3 | Infiltration composite |
| Parameter | Value |
|---|---|
| Temperature/°C | 150, 250, 350, 450 |
| SV/1/h | 90,000 |
| NO/ppm | 500 |
| O2/% | 9 |
| CO2/% | 6 |
| H2O/% | 6 |
| N2/% | balance |
| Parameter | Lean | Rich |
| Temperature/°C | 200, 300, 400, 450 | 200, 300, 400, 450 |
| SV/1/h | 90,000 | 90,000 |
| CO/% | 0 | 2 |
| O2/% | 1 | 0 |
| N2/% | balance | balance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özyalcin, C.; Mauermann, P.; Dornseiffer, J.; Sterlepper, S.; Günther, M.; Pischinger, S. Contributions of Washcoat Components in Different Configurations to the NOX and Oxygen Storage Performance of LNT Catalysts. Catalysts 2022, 12, 953. https://doi.org/10.3390/catal12090953
Özyalcin C, Mauermann P, Dornseiffer J, Sterlepper S, Günther M, Pischinger S. Contributions of Washcoat Components in Different Configurations to the NOX and Oxygen Storage Performance of LNT Catalysts. Catalysts. 2022; 12(9):953. https://doi.org/10.3390/catal12090953
Chicago/Turabian StyleÖzyalcin, Can, Peter Mauermann, Jürgen Dornseiffer, Stefan Sterlepper, Marco Günther, and Stefan Pischinger. 2022. "Contributions of Washcoat Components in Different Configurations to the NOX and Oxygen Storage Performance of LNT Catalysts" Catalysts 12, no. 9: 953. https://doi.org/10.3390/catal12090953
APA StyleÖzyalcin, C., Mauermann, P., Dornseiffer, J., Sterlepper, S., Günther, M., & Pischinger, S. (2022). Contributions of Washcoat Components in Different Configurations to the NOX and Oxygen Storage Performance of LNT Catalysts. Catalysts, 12(9), 953. https://doi.org/10.3390/catal12090953