Recent Advances in Lipases and Their Applications in the Food and Nutraceutical Industry
Abstract
:1. Bioactives, Nutraceuticals, and Functional Foods Update
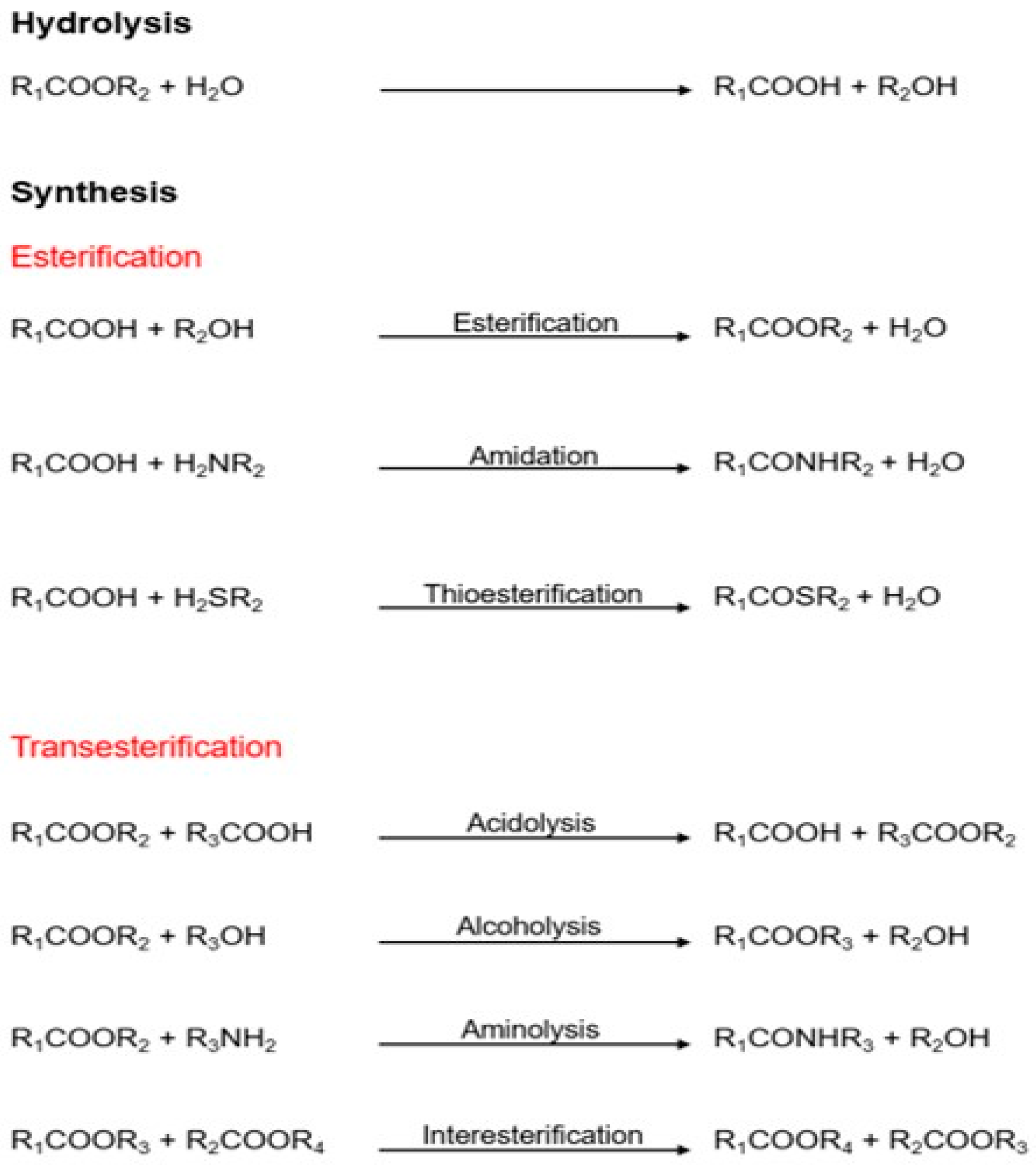
2. Lipases as Biocatalysts in the Food and Nutraceutical Industry
2.1. Lipase Characteristics
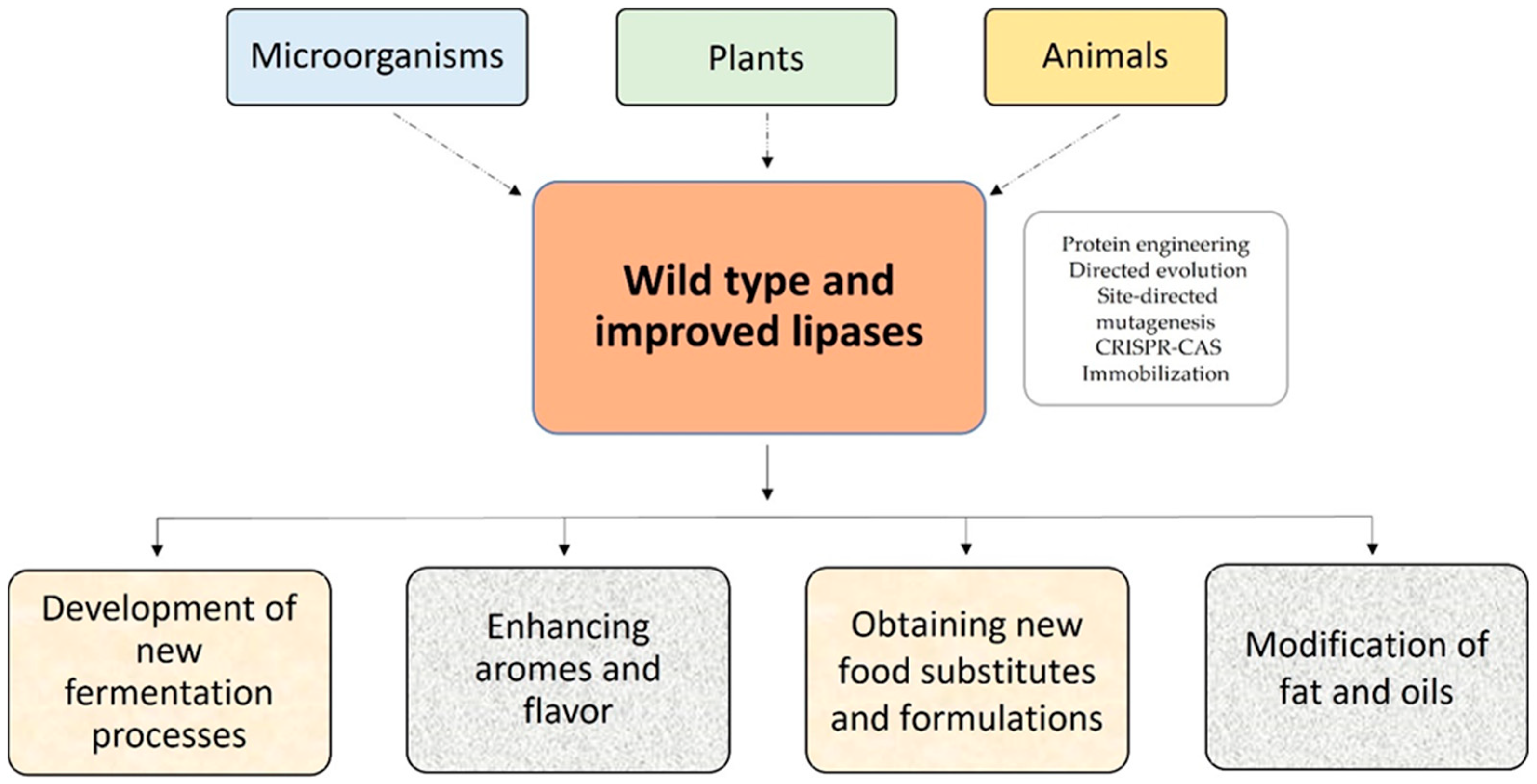
2.2. Sources and Tools to Improve Lipase-Catalyzed Reactions
3. Established Applications of Lipases in the Food and Nutraceutical Industry
3.1. Fats and Oils
3.1.1. Dairy Products
3.1.2. Structured Lipids
3.2. Vitamin Esters
3.2.1. Retinol (Vitamin A) Esters
3.2.2. Fatty Acid Esters of L-Ascorbic Acid (Vitamin C)
3.2.3. Tocopherols (Vitamin E) Esters
3.3. Bakery Products
3.4. Flavors and Fragances
3.4.1. Short-Chain Fatty Acids and Isoamyl Alcohol Esters
3.4.2. Other Flavors and Alternative Reaction Systems
4. Trends in the Use of Lipases in Food and Nutraceuticals
4.1. Phenolic Antioxidants
4.2. Prebiotics and Biosurfactants
5. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martirosyan, D.M.; Singh, J. A new definition of functional food by FFC: What makes a new definition unique? Funct. Foods Health Dis. 2015, 5, 209–223. [Google Scholar] [CrossRef]
- Trifković, K.; Benković, M. Chapter 1-Introduction to Nutraceuticals and Pharmaceuticals. In Nutraceuticals and Natural Product Pharmaceuticals; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–31. [Google Scholar]
- Enespa; Chandra, P.; Singh, D. Sources, purification, immobilization and industrial applications of microbial lipases: An overview. Crit. Rev. Food Sci. Nutr. 2022, 18, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Baião, D.; Tahmasb, H.; Martínez, J.; Ciftci, O. Biocatalytic production of isoamyl acetate from fusel oil in supercritical CO2. J. Supercrit. Fluids 2020, 164, 104917. [Google Scholar] [CrossRef]
- Sloan, E. Top 10 Functional Food Trends. 2022. Available online: https://www.ift.org/news-and-publications/food-technology-magazine/issues/2022/april/features/top-10-functional-food-trends (accessed on 6 June 2022).
- Contor, L. Functional Food Science in Europe. Nutr. Metab. Cardiovasc. Dis. NMCD 2001, 11, 20–23. [Google Scholar]
- EFSA. European Food Safety Authority. General Function Health Claims under Article 13. 2022. Available online: https://www.efsa.europa.eu/en/topics/topic/general-function-health-claims-under-article-13 (accessed on 6 June 2022).
- Helal, N.; Eassa, H.; Amer, A.; Eltokhy, M.; Edafiogho, I.; Nounou, M. Nutraceuticals’ Novel Formulations: The Good, the Bad, the Unknown and Patents Involved. Recent Pat. Drug Deliv. Formul. 2019, 13, 105–156. [Google Scholar] [CrossRef]
- Borrelli, M.; Trono, D. Recombinant Lipases and Phospholipases and Their Use as Biocatalysts for Industrial Applications. Int. J. Mol. Sci. 2015, 16, 20774–20840. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Liu, K.; Liu, H.; Jiang, Y.; Wang, R.; Wang, W.; Wang, T. A Valuable Product of Microbial Cell Factories: Microbial Lipase. Front. Microbiol. 2021, 12, 743377. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.; Selamoglu, Z. Use of Enzymes in Dairy Industry: A Review of Current Progress. Arch. Razi Inst. 2020, 75, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, T.; Cybulska, J.; Podleśny, M.; Frąc, M. Various Perspectives on Microbial Lipase Production Using Agri-Food Waste and Renewable Products. Agriculture 2021, 11, 540. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Factories 2020, 19, 169. [Google Scholar] [CrossRef]
- Bilal, M.; Fernandes, C.D.; Mehmood, T.; Nadeem, F.; Tabassam, Q.; Ferreira, L.F.R. Immobilized lipases-based nano-biocatalytic systems—A versatile platform with incredible biotechnological potential. Int. J. Biol. Macromol. 2021, 175, 108–122. [Google Scholar] [CrossRef]
- Casas, L.; Duquesne, S.; Bordes, F.; Sandoval, G.; Marty, A. Lipases: An Overview. In Lipases and Phospholipases: Methods and Protocols; Sandoval, G., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 3–30. [Google Scholar] [CrossRef]
- Sandoval, G. Lipases and Phospholipases, 2nd ed.; Springer: Berlin, Germany, 2018; Volume 1835, p. 437. [Google Scholar]
- Ali, Y.; Verger, R.; Abousalham, A. Lipases or esterases: Does it really matter? Toward a new bio-physico-chemical classification. Methods Mol. Biol. 2012, 861, 31–51. [Google Scholar] [CrossRef]
- Priyanka, P.; Tan, Y.; Kinsella, G.; Henehan, G.; Ryan, B. Solvent stable microbial lipases: Current understanding and biotechnological applications. Biotechnol. Lett. 2019, 41, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, O. Lipases associated with plant defense against pathogens. Plant Sci. 2019, 279, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Rivera, I.; Mateos, J.; Sandoval, G. Plant Lipases: Partial Purification of Carica papaya Lipase. In Lipases and Phospholipases: Methods and Protocols; Sandoval, G., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 115–122. [Google Scholar]
- Gao, M.; Yin, X.; Yang, W.; Lam, S.; Tong, X.; Liu, J.; Wang, X.; Li, Q.; Shui, G.; He, Z. GDSL lipases modulate immunity through lipid homeostasis in rice. PLoS Pathog. 2017, 13, e1006724. [Google Scholar] [CrossRef] [PubMed]
- Rivera, I.; Gutiérrez, A.; Sandoval, G. Functional Expression of Plant Lipases: The Case of CpLip1 from Carica papaya. Methods Mol. Biol. 2018, 1835, 169–178. [Google Scholar]
- Villeneuve, P. Plant lipases and their applications in oils and fats modification. Eur. J. Lipid Sci. Technol. 2003, 105, 308–317. [Google Scholar] [CrossRef]
- Raveendran, S.; Parameswaran, B.; Ummalyma, S.; Abraham, A.; Mathew, A.; Madhavan, A.; Rebello, S.; Pandey, A. Applications of Microbial Enzymes in Food Industry. Food Technol. Biotechnol. 2018, 56, 16–30. [Google Scholar] [CrossRef]
- Valero, F. Heterologous Expression Systems for Lipases: A Review. In Lipases and Phospholipases: Methods and Protocols; Sandoval, G., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 161–178. [Google Scholar]
- Hamdan, S.H.; Maiangwa, J.; Ali, M.S.M.; Normi, Y.M.; Sabri, S.; Leow, T.C. Thermostable lipases and their dynamics of improved enzymatic properties. Appl. Microbiol. Biotechnol. 2021, 105, 7069–7094. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, B.; Lv, K.; Kumissay, L.; Wu, B.; Chu, J.; He, B. Specific immobilization of lipase on functionalized 3D printing scaffolds via enhanced hydrophobic interaction for efficient resolution of racemic 1-indanol. Biochem. Biophys. Res. Commun. 2021, 546, 111–117. [Google Scholar] [CrossRef]
- Monteiro, R.; Virgen, J.; Berenguer, Á.; da Rocha, T.; dos Santos, J.; Alcántara, A.; Fernandez, R. Biotechnological relevance of the lipase A from Candida antarctica. Catal. Today 2021, 362, 141–154. [Google Scholar] [CrossRef]
- Javed, S.; Azeem, F.; Hussain, S.; Rasul, I.; Siddique, M.; Riaz, M.; Afzal, M.; Kouser, A.; Nadeem, H. Bacterial lipases: A review on purification and characterization. Prog. Biophys. Mol. Biol. 2018, 132, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Juturu, V.; Wu, J. Heterologous Protein Expression in Pichia pastoris: Latest Research Progress and Applications. ChemBioChem 2018, 19, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Hee, K.; Huang, B.X.; Spector, A.A. Phosphatidylserine in the brain: Metabolism and function. Prog. Lipid Res. 2014, 56, 1–18. [Google Scholar] [CrossRef]
- Souza, M.; Santos, K.; Freire, R.; Barreto, A.; Fechine, P.; Gonçalves, L. Production of flavor esters catalyzed by lipase B from Candida antarctica immobilized on magnetic nanoparticles. Braz. J. Chem. Eng. 2017, 34, 681–690. [Google Scholar] [CrossRef]
- Kurtovic, I.; Nalder, T.D.; Cleaver, H.; Marshall, S. Immobilisation of Candida rugosa lipase on a highly hydrophobic support: A stable immobilised lipase suitable for non-aqueous synthesis. Biotechnol. Rep. 2020, 28, e00535. [Google Scholar] [CrossRef] [PubMed]
- Trbojević, J.; Veličković, D.; Dimitrijević, A.; Bezbradica, D.; Dragačević, V.; Gavrović, M.; Milosavić, N. Design of biocompatible immobilized Candida rugosa lipase with potential application in food industry. J. Sci. Food Agric. 2016, 96, 4281–4287. [Google Scholar] [CrossRef]
- Monoj, G. Chapter 13-Trans Fat Alternatives and Challenges. In Practical Guide to Vegetable Oil Processing, 2nd ed.; Gupta, M.K., Ed.; AOCS Press: Ubana, IL, USA, 2017; pp. 341–374. [Google Scholar]
- Dayton, C. 11-Enzymatic Interesterification. In Green Vegetable Oil Processing; Farr, W.E., Proctor, A., Eds.; AOCS Press: Ubana, IL, USA, 2014; pp. 205–224. [Google Scholar]
- Gricajeva, A.; Kazlauskas, S.; Kalėdienė, L.; Bendikienė, V. Analysis of Aspergillus sp. lipase immobilization for the application in organic synthesis. Int. J. Biol. Macromol. 2018, 108, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Muniandy, M.; Lasekan, O.; Ghazali, M.H.; Rahman Mohd, B. Lipase-Catalyzed formation of pentyl nonanoate using screened immobilized from Rhizomucor meihei. Braz. J. Chem. Eng. Life Sci. 2019, 36, 1089–1097. [Google Scholar] [CrossRef]
- Zhang, X.; Ai, N.; Wang, J.; Tong, L.; Zheng, F.; Sun, B. Lipase-catalyzed modification of the flavor profiles in recombined skim milk products by enriching the volatile components. J. Dairy Sci. 2016, 99, 8665–8679. [Google Scholar] [CrossRef]
- Shaimaa, H.; Shaaban, H.; Mahmoud, H.; Abbas, K.; Farouk, A. Preparation of Ras Cheese Flavour Concentrate using Lipolyzed Cream and Skim Milk Curd. Int. J. Dairy Sci. 2017, 12, 275–281. [Google Scholar] [CrossRef]
- Tecelão, C.; Rivera, I.; Sandoval, G.; Ferreira-Dias, S. Carica papaya latex: A low-cost biocatalyst for human milk fat substitutes production. Eur. J. Lipid Sci. Technol. 2012, 114, 266–276. [Google Scholar] [CrossRef]
- Konkit, M.; Kim, W. Activities of amylase, proteinase, and lipase enzymes from Lactococcus chungangensis and its application in dairy products. J. Dairy Sci. 2016, 99, 4999–5007. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.; Mancheño, J.; de las Rivas, B.; Muñoz, R. Characterization of a halotolerant lipase from the lactic acid bacteria Lactobacillus plantarum useful in food fermentations. LWT-Food Sci. Technol. 2015, 60, 246–252. [Google Scholar] [CrossRef]
- Alvarez, Y.; Esteban, M.; Cortés, Á.; Gago, F.; Acebrón, I.; Benavente, R.; Mardo, K.; de las Rivas, B.; Muñoz, R.; Mancheño, J. Esterase LpEst1 from Lactobacillus plantarum: A Novel and Atypical Member of the αβ Hydrolase Superfamily of Enzymes. PLoS ONE 2014, 9, e92257. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Hou, M.; Hu, N.; Chen, C.; Shaw, J. Crystallographic analysis of the Staphylococcus epidermidis lipase involved in esterification in aqueous solution. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2018, 74, 351–354. [Google Scholar] [CrossRef]
- Molina, M.; Hakalin, N.; Rodríguez, L.; Alcaraz, L.; López, F.; Martínez, M.; Prieto, A. Effect of the Immobilization Strategy on the Efficiency and Recyclability of the Versatile Lipase from Ophiostoma piceae. Molecules 2019, 24, 1313. [Google Scholar] [CrossRef]
- Knob, A.; Izidoro, S.; Lacerda, L.; Rodrigues, A.; de Lima, V. A novel lipolytic yeast Meyerozyma guilliermondii: Efficient and low-cost production of acid and promising feed lipase using cheese whey. Biocatal. Agric. Biotechnol. 2020, 24, 101565. [Google Scholar] [CrossRef]
- Chaves, A.F.L.; Castro, J.M.P.; Medeiros, T.B.; Soares, F.M.B. Trends in lipase immobilization: Bibliometric review and patent analysis. Process Biochem. 2021, 110, 37–51. [Google Scholar] [CrossRef]
- Castillo, E.; Casas, L.; Sandoval, G. Medium-engineering: A useful tool for modulating lipase activity and selectivity. Biocatalysis 2016, 1, 178–188. [Google Scholar] [CrossRef]
- Itoh, T. Activation of Lipase-Catalyzed Reactions Using Ionic Liquids for Organic Synthesis. In Application of Ionic Liquids in Biotechnology; Itoh, T., Koo, Y.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 79–104. [Google Scholar]
- Sandoval, G.; Quintana, P.; Baldessari, A.; Ballesteros, A.; Plou, F. Lipase-catalyzed preparation of mono- and diesters of ferulic acid. Biocatal. Biotransformation 2015, 33, 89–97. [Google Scholar] [CrossRef]
- Xu, P.; Zheng, G.; Zong, M.; Li, N.; Lou, W. Recent progress on deep eutectic solvents in biocatalysis. Bioresour. Bioprocess. 2017, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.L.S.; Orlandelli, R.C. Immobilized microbial lipases in the food industry: A systematic literature review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1689–1703. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, Y.; Lai, X.; Nong, J.; Zhao, G.; Xiao, X. One-pot biocatalytic synthesis and antioxidant activities of highly lipophilic naringin derivatives by using bi-functional whole-cells. Food Res. Int. 2020, 136, 109291. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, N. Microbial Lipase Mediated by Health Beneficial Modification of Cholesterol and Flavors in Food Products: A Review. Recent Pat. Biotechnol. 2018, 12, 81–91. [Google Scholar] [CrossRef]
- Khanniri, E.; Bagheripoor, N.; Sohrabvandi, S.; Mortazavian, M.; Khosravi, K.; Mohammad, R. Application of Liposomes in Some Dairy Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 484–493. [Google Scholar] [CrossRef]
- Shojaei, M.; Golmakani, M.; Eskandari, M.; Toh, M.; Liu, S. Natural flavor biosynthesis by lipase in fermented milk using in situ produced ethanol. J. Food Sci. Technol. 2021, 58, 1858–1868. [Google Scholar] [CrossRef]
- Ferreira, S.; Osório, N.; Tecelão, C. 9-Bioprocess technologies for production of structured lipids as nutraceuticals. In Current Developments in Biotechnology and Bioengineering; Rai, A.K., Singh, S.P., Pandey, A., Larroche, C., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 209–237. [Google Scholar]
- Guo, Y.; Cai, Z.; Xie, Y.; Ma, A.; Zhang, H.; Rao, P.; Wang, Q. Synthesis, physicochemical properties, and health aspects of structured lipids: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 759–800. [Google Scholar] [CrossRef]
- Burdge, G.; Calder, P. Introduction to fatty acids and lipids. World Rev. Nutr. Diet. 2015, 112, 1–16. [Google Scholar] [CrossRef]
- Astrup, A.; Magkos, F.; Bier, D.; Brenna, J.; de Oliveira Otto, M.; Hill, J.; King, J.; Mente, A.; Ordovas, J.; Volek, J.; et al. Saturated Fats and Health: A Reassessment and Proposal for Food-Based Recommendations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 844–857. [Google Scholar] [CrossRef]
- Temkov, M.; Mureșan, V. Tailoring the Structure of Lipids, Oleogels and Fat Replacers by Different Approaches for Solving the Trans-Fat Issue-A Review. Foods 2021, 10, 1376. [Google Scholar] [CrossRef] [PubMed]
- Bahari, A.; Akoh, C. Synthesis of a Cocoa Butter Equivalent by Enzymatic Interesterification of Illipe Butter and Palm Midfraction. J. Am. Oil Chem. Soc. 2018, 95, 547–555. [Google Scholar] [CrossRef]
- Bahari, A.; Akoh, C. Texture, rheology and fat bloom study of ‘chocolates’ made from cocoa butter equivalent synthesized from illipe butter and palm mid-fraction. LWT 2018, 97, 349–354. [Google Scholar] [CrossRef]
- Casas, L.; Gasteazoro, F.; Duquesne, S.; Bordes, F.; Marty, A.; Sandoval, G. Lipases: An Overview. In Lipases and Phospholipases: Methods and Protocols; Sandoval, G., Ed.; Springer New York: New York, NY, USA, 2018; pp. 3–38. [Google Scholar] [CrossRef]
- Simões, T.; Valero, F.; Tecelão, C.; Ferreira, S. Production of Human Milk Fat Substitutes Catalyzed by a Heterologous Rhizopus oryzae Lipase and Commercial Lipases. J. Am. Oil Chem. Soc. 2014, 91, 411–419. [Google Scholar] [CrossRef]
- Mota, D.; Rajan, D.; Heinzl, G.; Osório, N.; Gominho, J.; Krause, L.; Soares, C.; Nampoothiri, M.; Sukumaran, R.; Ferreira, S. Production of low-calorie structured lipids from spent coffee grounds or olive pomace crude oils catalyzed by immobilized lipase in magnetic nanoparticles. Bioresour. Technol. 2020, 307, 123223. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Akoh, C.C. Recent Research Trends on the Enzymatic Synthesis of Structured Lipids. J. Food Sci. 2015, 80, C1713–C1724. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, S.; Lee, W.J.; Lai, O.M.; Tan, C.P.; Wang, Y. Production of Structured Triacylglycerol via Enzymatic Interesterification of Medium-Chain Triacylglycerol and Soybean Oil Using a Pilot-Scale Solvent-Free Packed Bed Reactor. J. Am. Oil Chem. Soc. 2020, 97, 271–280. [Google Scholar] [CrossRef]
- Tecelão, C.; Perrier, V.; Dubreucq, E.; Ferreira, S. Production of Human Milk Fat Substitutes by Interesterification of Tripalmitin with Ethyl Oleate Catalyzed by Candida parapsilosis Lipase/Acyltransferase. J. Am. Oil Chem. Soc. 2019, 96, 777–787. [Google Scholar] [CrossRef]
- Jiang, X.; Zou, X.; Chao, Z.; Xu, X. Preparation of Human Milk Fat Substitutes: A Review. Life 2022, 12, 187. [Google Scholar] [CrossRef]
- Wei, W.; Jin, Q.; Wang, X. Human milk fat substitutes: Past achievements and current trends. Prog. Lipid Res. 2019, 74, 69–86. [Google Scholar] [CrossRef]
- Hasibuan, H.A.; Sitanggang, A.B.; Andarwulan, N.; Hariyadi, P. Enzymatic Synthesis of Human Milk Fat Substitute—A Review on Technological Approaches. Food Technol. Biotechnol. 2021, 59, 475–495. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xin, F.; Alper, H. Bio-synthesis of food additives and colorants-a growing trend in future food. Biotechnol. Adv. 2021, 47, 107694. [Google Scholar] [CrossRef] [PubMed]
- Tomasz, P.; Maciej, B. The study on the use of flavonodi- prhosphatidylcholilne coating in extending the oxidative stability of flaxseed oil during storage. Food Packag. Sheld Life 2021, 28, 5. [Google Scholar] [CrossRef]
- Farooq, S.; Abdullah; Zhang, H.; Weiss, J. A comprehensive review on polarity, partitioning, and interactions of phenolic antioxidants at oil-water interface of food emulsions. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4250–4277. [Google Scholar] [CrossRef] [PubMed]
- Boutillier, S.; Fourmentin, S.; Laperche, B. Food additives and the future of health: An analysis of the ongoing controversy on titanium dioxide. Futures 2020, 122, 102598. [Google Scholar] [CrossRef]
- de Carvalho, C.; Caramujo, M. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef]
- Carocho, M.; Barreiro, M.; Morales, P.; Ferreira, I. Adding Molecules to Food, Pros and Cons: A Review on Synthetic and Natural Food Additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef]
- Revilla, I.; González, M.; Vivar, A.; Blanco, M.; Lobos, I.; Hernández, J. Antioxidant capacity of different cheeses: Affectinf factors and prediction by near infrared spectroscopy. J. Dairy 2015, 99, 5074–5082. [Google Scholar] [CrossRef]
- Gammone, M.; Riccioni, G.; Parrinello, G.; D’Orazio, N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018, 11, 46. [Google Scholar] [CrossRef]
- Pop, P.A.M.a.A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Zieniuk, B.; Wołoszynowska, M.; Białecka, E.; Fabiszewska, A. Application of freeze-dried Yarrowia lipolytica biomass in the synthesis of lipophilic antioxidants. Biotechnol. Lett. 2021, 43, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Zieniuk, B.; Białecka, E.; Wierzchowska, K.; Fabiszewska, A. Recent advances in the enzymatic synthesis of lipophilic antioxidant and antimicrobial compounds. World J. Microbiol. Biotechnol. 2021, 38, 11. [Google Scholar] [CrossRef] [PubMed]
- Oteng, A.; Kersten, S. Mechanisms of Action of trans Fatty Acids. Adv. Nutr. 2020, 11, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Reviews. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Godic, A.; Poljšak, B.; Adamic, M.; Dahmane, R. The Role of Antioxidants in Skin Cancer Prevention and Treatment. Oxidative Med. Cell. Longev. 2014, 2014, 860479. [Google Scholar] [CrossRef]
- Khalaf, A.T.; Wei, Y.; Alneamah, S.J.A.; Al-Shawi, S.G.; Kadir, S.Y.A.; Zainol, J.; Liu, X. What Is New in the Preventive and Therapeutic Role of Dairy Products as Nutraceuticals and Functional Foods? BioMed Res. Int. 2021, 2021, 8823222. [Google Scholar] [CrossRef]
- Ceriello, A.; Esposito, K.; La Sala, L.; Pujadas, G.; De Nigris, V.; Testa, R.; Bucciarelli, L.; Rondinelli, M.; Genovese, S. The protective effect of the Mediterranean diet on endothelial resistance to GLP-1 in type 2 diabetes: A preliminary report. Cardiovasc. Diabetol. 2014, 13, 140. [Google Scholar] [CrossRef]
- Tan, B.; Norhaizan, M.; Liew, W. Nutrients and Oxidative Stress: Friend or Foe? Oxidative Med. Cell Longev. 2018, 2018, 9719584. [Google Scholar] [CrossRef]
- Diamanti, E.; Papalou, O.; Kandaraki, E.; Kassi, G. Mechanisms in endocrinology: Nutrition as a mediator of oxidative stress in metabolic and reproductive disorders in women. Eur. J. Endocrinol. 2017, 176, R79–R99. [Google Scholar] [CrossRef]
- Klaunig, J. Oxidative Stress and Cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.M.; Dayem, A.A.; Cho, S.G. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef]
- Lee, J.D.; Cai, Q.; Shu, X.O.; Nechuta, S.J. The Role of Biomarkers of Oxidative Stress in Breast Cancer Risk and Prognosis: A Systematic Review of the Epidemiologic Literature. J. Women’s Health (2002) 2017, 26, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, L.; Gao, G.; Wei, G.; Zheng, Y.; Wang, C.; Gao, N.; Zhao, Y.; Deng, J.; Chen, H.; et al. Elevation of GPRC5A expression in colorectal cancer promotes tumor progression through VNN-1 induced oxidative stress. Int. J. Cancer 2017, 140, 2734–2747. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Santini, A. Nutraceuticals in Human Health. Foods 2020, 9, 370. [Google Scholar] [CrossRef]
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current Prospects of Nutraceuticals: A Review. Curr. Pharm. Biotechnol. 2020, 21, 884–896. [Google Scholar] [CrossRef]
- Aydin, S.; Dalgic, S.; Karaman, M.; Kirlangic, F.; Yildirim, H. Effects of Fulvic Acid on Different Cancer Cell Lines. Proceedings 2017, 1, 1031. [Google Scholar]
- Swat, M.; Rybicka, I.; Gliszczyńska, A. Characterization of Fulvic Acid Beverages by Mineral Profile and Antioxidant Capacity. Foods 2019, 8, 605. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Martinelli, A.; Giannini, L.; Branduardi, P. Enzymatic Modification of Cellulose To Unlock Its Exploitation in Advanced Materials. ChemBioChem 2021, 22, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Karaki, N.; Aljawish, A.; Humeau, C.; Muniglia, L.; Jasniewski, J. Enzymatic modification of polysaccharides: Mechanisms, properties, and potential applications: A review. Enzym. Microb. Technol. 2016, 90, 1–18. [Google Scholar] [CrossRef]
- de Araújo, M.; Franco, Y.; Messias, M.; Longato, G.; Pamphile, J.; Carvalho, P. Biocatalytic Synthesis of Flavonoid Esters by Lipases and Their Biological Benefits. Planta Med. 2017, 83, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Hammerling, U. Retinol as electron carrier in redox signaling, a new frontier in vitamin A research. Hepatobiliary Surg. Nutr. 2016, 5, 15–28. [Google Scholar] [CrossRef]
- O’Byrne, S.; Blaner, W. Retinol and retinyl esters: Biochemistry and physiology. J. Lipid Res. 2013, 54, 1731–1743. [Google Scholar] [CrossRef]
- Athar, M.; Zaidi, S.; Hassan, S. Intensification and optimization of biodiesel production using microwave-assisted acid-organo catalyzed transesterification process. Sci. Rep. 2020, 10, 21239. [Google Scholar] [CrossRef]
- Huang, K.; Li, Y.; Kuo, C.; Twu, Y.; Shieh, C. Highly Efficient Synthesis of an Emerging Lipophilic Antioxidant: 2-Ethylhexyl Ferulate. Molecules 2016, 21, 478. [Google Scholar] [CrossRef]
- Huang, S.-M.; Li, H.-J.; Liu, Y.-C.; Kuo, C.-H.; Shieh, C.-J. An Efficient Approach for Lipase-Catalyzed Synthesis of Retinyl Laurate Nutraceutical by Combining Ultrasound Assistance and Artificial Neural Network Optimization. Molecules 2017, 22, 1972. [Google Scholar] [CrossRef]
- Caritá, A.; Fonseca, B.; Shultz, J.; Michniak, B.; Chorilli, M.; Leonardi, G. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102117. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.; Tu, Y.; Schlegel, H. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- Taira, N.; Katsuyama, Y.; Yoshioka, M.; Muraoka, O.; Morikawa, T. Structural Requirements of Alkylglyceryl-l-Ascorbic Acid Derivatives for Melanogenesis Inhibitory Activity. Int. J. Mol. Sci. 2018, 19, 1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tufiño, C.; Bernal, C.; Ottone, C.; Romero, O.; Illanes, A.; Wilson, L. Synthesis with Immobilized Lipases and Downstream Processing of Ascorbyl Palmitate. Molecules 2019, 24, 3227. [Google Scholar] [CrossRef] [PubMed]
- Kharat, M.; Skrzynski, M.; Decker, E.; McClements, D. Enhancement of chemical stability of curcumin-enriched oil-in-water emulsions: Impact of antioxidant type and concentration. Food Chem. 2020, 320, 126653. [Google Scholar] [CrossRef]
- Khalid, N.; Kobayashi, I.; Neves, M.; Uemura, K.; Nakajima, M. Microchannel emulsification: A promising technique towards encapsulation of functional compounds. Crit. Rev. Food Sci. Nutr. 2018, 58, 2364–2385. [Google Scholar] [CrossRef]
- Díaz, Z.; Menghi, K.; Guerrero, M.; Nocelli, N.; Fanani, M. l-Ascorbic acid alkyl esters action on stratum corneum model membranes: An insight into the mechanism for enhanced skin permeation. Colloids Surf. B Biointerfaces 2020, 185, 110621. [Google Scholar] [CrossRef] [PubMed]
- Tanini, D.; Lupori, B.; Malevolti, G.; Ambrosi, M.; Nostro, P.; Capperucci, A. Direct biocatalysed synthesis of first sulfur-, selenium- and tellurium- containing l-ascorbyl hybrid derivatives with radical trapping and GPx-like properties. Chem. Commun. 2019, 55, 5705–5708. [Google Scholar] [CrossRef]
- Yadav, M.; Kavadia, M.; Vadgama, R.; Odaneth, A.; Lali, A. Production of 6-O-l-Ascorbyl Palmitate by Immobilized Candida antarctica Lipase B. Appl. Biochem. Biotechnol. 2018, 184, 1168–1186. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Luo, P.; Zeng, Z.; Wang, H.; Malafa, M.; Suh, N. Vitamin E and cancer prevention: Studies with different forms of tocopherols and tocotrienols. Mol. Carcinog. 2020, 59, 365–389. [Google Scholar] [CrossRef]
- Lee, G.; Han, S. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef]
- De la Fuente, M.; Sánchez, C.; Vallejo, C.; Díaz, E.; Arnalich, F.; Hernanz, Á. Vitamin C and vitamin C plus E improve the immune function in the elderly. Exp. Gerontol. 2020, 142, 111118. [Google Scholar] [CrossRef]
- Zhou, P.; Shao, R.; Wang, H.; Miao, J.; Wang, X. Dietary vitamin A, C, and E intake and subsequent fracture risk at various sites: A meta-analysis of prospective cohort studies. Medicine 2020, 99, e20841. [Google Scholar] [CrossRef] [PubMed]
- Busso, D.; David, A.; Penailillo, R.; Echeverría, G.; Rigotti, A.; Kovalskys, I.; Gómez, G.; Cortés Sanabria, L.Y.; Yépez García, M.C.; Pareja, R.G.; et al. Intake of Vitamin E and C in Women of Reproductive Age: Results from the Latin American Study of Nutrition and Health. Nutrients 2021, 13, 1954. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Chen, L.; Zhang, Y.; Wen, R.; Zhao, D.; Xia, C. Lipase-Catalyzed Synthesis of α-Tocopheryl Ferulate. Food Biotechnol. 2011, 25, 43–57. [Google Scholar] [CrossRef]
- Zu, G.; Zhang, L.; Wang, Y. Research on Synthesis of Polyunsaturated Fatty Acid in Fish Oil and Vitamin E. Dalian Inst. Light Ind. 1999, 18, 294–298. [Google Scholar]
- Casas, L.; Arrizon, J.; Arrieta, D.; Plou, F.; Sandoval, G. Synthesis and emulsifying properties of carbohydrate fatty acid esters produced from Agave tequilana fructans by enzymatic acylation. Food Chem. 2016, 204, 437–443. [Google Scholar] [CrossRef]
- Mène-Saffrané, L.; Pellaud, S. Current strategies for vitamin E biofortification of crops. Curr. Opin. Biotechnol. 2017, 44, 189–197. [Google Scholar] [CrossRef]
- Giménez, T.; Mula, D.; Gea, S.; Martínez, M.; Martí, N.; Valero, M.; Saura, D. Lipase catalyzed deacidification of tocopherol-rich distillates obtained from natural Vitamin E sources. Process Biochem. 2019, 77, 70–76. [Google Scholar] [CrossRef]
- Chen, D.; Li, B.; Li, B.; Zhang, X.; Wei, L.; Zheng, W. Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system. Green Process. Synth. 2019, 8, 667–676. [Google Scholar] [CrossRef]
- Zou, Z.; Dai, L.; Liu, D.; Du, W. Research Progress in Enzymatic Synthesis of Vitamin E Ester Derivatives. Catalysts 2021, 11, 739. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Dusemund, B.; Durjava, M.F.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Assessment of a feed additive consisting of all-rac-alpha-tocopheryl acetate (vitamin E) for all animal species for the renewal of its authorisation (DSM). EFSA J. 2021, 19, e06533. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for vitamin E as α-tocopherol. EFSA J. 2015, 13, 4149. [Google Scholar] [CrossRef]
- Gill, B.; Indyk, H. Separation of RRR-α-Tocopherol by Chiral Chromatography. J. AOAC Int. 2020, 103, 1288–1292. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Htar, T.; De Silva, L.; Tan, D.; Chuah, L. Chromatographic Separation of Vitamin E Enantiomers. Molecules 2017, 22, 233. [Google Scholar] [CrossRef]
- Leal, L.; Beltrán, J.; Marc, B.; Bello, J.; den Hartog, L.; Hendriks, W.; Martín, J. Supplementation of lamb diets with vitamin E and rosemary extracts on meat quality parameters. J. Sci. Food Agric. 2020, 100, 2922–2931. [Google Scholar] [CrossRef]
- Shan, L.C.; De Brún, A.; Henchion, M.; Li, C.; Murrin, C.; Wall, P.G.; Monahan, F.J. Consumer evaluations of processed meat products reformulated to be healthier—A conjoint analysis study. Meat Sci. 2017, 131, 82–89. [Google Scholar] [CrossRef]
- Melis, S.; Delcour, J. Impact of wheat endogenous lipids on the quality of fresh bread: Key terms, concepts, and underlying mechanisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3715–3754. [Google Scholar] [CrossRef]
- Dai, Y.; Tyl, C. A review on mechanistic aspects of individual versus combined uses of enzymes as clean label-friendly dough conditioners in breads. J. Food Sci. 2021, 86, 1583–1598. [Google Scholar] [CrossRef]
- Lien, G.; Bram, P.; Karolien, D.; Jan, D. Lipases and Their Functionality in the Production of Wheat-Based Food Systems. Compr. Rev. Food Sci. Food Saf. 2014, 13, 978–989. [Google Scholar] [CrossRef]
- Min, B.; Salt, L.; Wilde, P.; Kosik, O.; Hassall, K.; Przewieslik, A.; Burridge, A.; Poole, M.; Snape, J.; Wingen, L.; et al. Genetic variation in wheat grain quality is associated with differences in the galactolipid content of flour and the gas bubble properties of dough liquor. Food Chem. X 2020, 6, 100093. [Google Scholar] [CrossRef]
- Lien, R.G.; Bram, P.; Hanne, G.M.; Jan, A.D. A lipase based approach to understand the role of wheat endogenous lipids in bread crumb firmness evolution during storage. LWT-Food Sci. Technol. 2015, 64, 874–880. [Google Scholar] [CrossRef]
- Gomes, A.; Meneses, A.; Araújo, P.; Oliveira, D. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci. Technol. 2017, 69, 95–105. [Google Scholar] [CrossRef]
- López, J.; Benaiges, M.; Valero, F. Rhizopus oryzae Lipase, a Promising Industrial Enzyme: Biochemical Characteristics, Production and Biocatalytic Applications. Catalysts 2020, 10, 1277. [Google Scholar] [CrossRef]
- Nitesh, C.; Himanshu, V.; Roshan, D. Flavors and Fragrance Market by Type (Flavors and Fragrance), Nature (Natural and Synthetic), and Application (Food & Beverages, Cosmetics & Personal Care, Home Care and Fabric Care): Global Opportunity Analysis and Industry Forecast, 2021–2027. Available online: https://www.researchandmarkets.com/reports/5341604/flavors-and-fragrance-market-by-type-nature-and (accessed on 11 August 2021).
- Heinsman, N.; Franssen, M.; van der Padt, A.; Boom, R.; van’t Riet, K. Lipase-mediated Resolution of Branched Chain Fatty Acids. Biocatal. Biotransformation 2002, 20, 297–309. [Google Scholar] [CrossRef]
- Leffingwell, J. Chirality & Odour Perception. Available online: www.leffingwell.com/chirality/chirality.htm (accessed on 23 April 2022).
- Akacha, N.B.; Gargouri, M. Microbial and enzymatic technologies used for the production of natural aroma compounds: Synthesis, recovery modeling, and bioprocesses. Food Bioprod. Process. 2015, 94, 675–706. [Google Scholar] [CrossRef]
- Bruno Nicolau, P.; Adones, S.; Lorena, F.; Gláucia Maria, P.; Gustavo, M.; Juliano Lemos, B. Recent advances in the microbial and enzymatic production of aroma compounds. Curr. Opin. Food Sci. 2021, 37, 98–106. [Google Scholar] [CrossRef]
- Bansode, S.; Rathod, V. Enzymatic sythesis of Isoamyl butyrate under microwave irradiation. Chem. Eng. Process.-Process Intensif. 2018, 129, 71–76. [Google Scholar] [CrossRef]
- Bansode, S.; Hardikar, M.; Rathod, V. Evaluation of reaction parameters and kinetic modelling for Novozym 435 catalysed synthesis of isoamyl butyrate. J. Chem. Technol. Biotechnol. 2017, 92, 1306–1314. [Google Scholar] [CrossRef]
- Ferreira, S.; Sandoval, G.; Plou, F.; Valero, F. The potential use of lipases in the production of fatty acid derivatives for the food and nutraceutical industries. Electron. J. Biotechnol. 2013, 16, 1–38. [Google Scholar] [CrossRef]
- Mahdieh, Z.; Mohammad, G.; Mehrdad, N. Lipase synthesis of isoamyl acetate using different acyl donors: Comparison of novel esterification techniques. LWT 2019, 101, 214–219. [Google Scholar] [CrossRef]
- Tohid, T.; Atefeh, A.; Amin, T.; Somayeh, M.; Alieh, A.; Hamid, F.; Sara, T.; Mohammad, F. Lipase@zeolitic imidazolate framework ZIF-90: A highly stable and recyclable biocatalyst for the synthesis of fruity banana flavour. Int. J. Biol. Macromol. 2021, 166, 1301–1311. [Google Scholar] [CrossRef]
- Hanen, G.; Karra, M.; Bezzine, S.; Nabil, M.; Youssef, G. Production of isoamyl acetate with immobilized Staphylococcus simulans lipase in a solvent-free system. Enzym. Microb. Technol. 2006, 38, 788–794. [Google Scholar] [CrossRef]
- Macedo, G.; Pastore, G.; Rodrigues, M. Optimising the synthesis of isoamyl butyrate using Rhizopus sp. lipase with a central composite rotatable design. Process Biochem. 2004, 39, 687–693. [Google Scholar] [CrossRef]
- Hari, K.; Prapulla, S.; Karanth, N. Enzymatic synthesis of isoamyl butyrate using immobilized Rhizomucor miehei lipase in non-aqueous media. J. Ind. Microbiol. Biotechnol. 2000, 25, 147–154. [Google Scholar] [CrossRef]
- Bansode, S.; Rathod, V. Ultrasound assisted lipase catalysed synthesis of isoamyl butyrate. Process Biochem. 2014, 49, 1297–1303. [Google Scholar] [CrossRef]
- Ozyilmaz, G.; Yağız, E. Isoamylacetate production by entrapped and covalently bound Candida rugosa and porcine pancreatic lipases. Food Chem. 2012, 135, 2326–2332. [Google Scholar] [CrossRef]
- Cvjetko, M.; Jasna, V.; Polona, Ž. Isoamyl acetate synthesis in imidazolium-based ionic liquids using packed bed enzyme microreactor. Process Biochem. 2012, 47, 1344–1350. [Google Scholar] [CrossRef]
- Azudin, N.Y.; Sangaran, S.; Abd Shukor, S.R. Non-enzymatic synthesis route for production of isoamyl acetate in a solvent-free system using miniaturized intensified reactor. J. Environ. Chem. Eng. 2020, 8, 103186. [Google Scholar] [CrossRef]
- Muhammad, H.; Daniele, C.; Lasse, R. Two-stage catalytic hydrotreatment of highly nitrogenous biocrude from continuous hydrothermal liquefaction: A rational design of the stabilization stage. Biomass Bioenergy 2020, 139, 105658. [Google Scholar] [CrossRef]
- Thi, T.; Suwadee, K.; Surachai, K.; Prasert, R.; Guoqing, G.; Narong, C.; Chanatip, S. Selective production of green solvent (isoamyl acetate) from fusel oil using a sulfonic acid-functionalized KIT-6 catalyst. Mol. Catal. 2020, 484, 110724. [Google Scholar] [CrossRef]
- Anschau, A.; Aragão, V.; Porciuncula, B.; Kalil, S.; Burkert, C. Enzymatic Synthesis Optimization of Isoamyl Butyrate. J. Braz. Chem. 2011, 22, 2148–2156. [Google Scholar] [CrossRef]
- Romero, M.; Calvo, L.; Alba, C.; Daneshfar, A.; Ghaziaskar, H. Enzymatic synthesis of isoamyl acetate with immobilized Candida antarctica lipase in n-hexane. Enzym. Microb. Technol. 2005, 37, 42–48. [Google Scholar] [CrossRef]
- Kirdi, R.; Ben Akacha, N.; Messaoudi, Y.; Gargouri, M. Enhanced synthesis of isoamyl acetate using liquid-gas biphasic system by the transesterification reaction of isoamyl alcohol obtained from fusel oil. Biotechnol. Bioprocess Eng. 2017, 22, 413–422. [Google Scholar] [CrossRef]
- López, J.; Benaiges, M.; Sebastian, X.; Bueno, J.; Valero, F. Producing Natural Flavours from Isoamyl Alcohol and Fusel Oil by Using Immobilised Rhizopus oryzae Lipase. Catalysts 2022, 12, 639. [Google Scholar] [CrossRef]
- Gamayurova, V.; Shnaider, K.; Jamai, M. Enzymatic synthesis of butyrates of fusel oil. Catal. Ind. 2017, 9, 85–90. [Google Scholar] [CrossRef]
- Vincenti, S.; Mariani, M.; Alberti, J.-C.; Jacopini, S.; Brunini-Bronzini de Caraffa, V.; Berti, L.; Maury, J. Biocatalytic Synthesis of Natural Green Leaf Volatiles Using the Lipoxygenase Metabolic Pathway. Catalysts 2019, 9, 873. [Google Scholar] [CrossRef]
- Jin, Z.; Ntwali, J.; Han, S.; Zheng, S.; Lin, Y. Production of flavor esters catalyzed by CALB-displaying Pichia pastoris whole-cells in a batch reactor. J. Biotechnol. 2012, 159, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Meilleur, C.; Hupé, J.-F.; Juteau, P.; Shareck, F. Isolation and characterization of a new alkali-thermostable lipase cloned from a metagenomic library. J. Ind. Microbiol. Biotechnol. 2009, 36, 853–861. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Guillén, M.; Benaiges, M.; Valero, F. Biosynthesis of ethyl butyrate by immobilized recombinant Rhizopus oryzae lipase expressed in Pichia pastoris. Biochem. Eng. J. 2012, 65, 1–9. [Google Scholar] [CrossRef]
- Guillén, M.; Benaiges, M.; Valero, F. Improved ethyl butyrate synthesis catalyzed by an immobilized recombinant Rhizopus oryzae lipase: A comprehensive statistical study by production, reaction rate and yield analysis. J. Mol. Catal. B Enzym. 2016, 133, S371–S376. [Google Scholar] [CrossRef]
- Grosso, C.; Ferreira, S.; Pires, P. Modelling and optimization of ethyl butyrate production catalysed by Rhizopus oryzae lipase. J. Food Eng. 2013, 115, 475–480. [Google Scholar] [CrossRef]
- Verma, D.K.; Al-Sahlany, S.T.G.; Niamah, A.K.; Thakur, M.; Shah, N.; Singh, S.; Baranwal, D.; Patel, A.R.; Uta-ma, G.L.; Aguilar, C.N. Recent trends in microbial flavour Compounds: A review on Chemistry, synthesis mechanism and their application in food. Saudi J. Biol. Sci. 2022, 29, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, C.; Johannsen, J.; Waluga, T.; Fieg, G.; Liese, A.; Bubenheim, P. A Multi-Enzyme Cascade for the Production of High-Value Aromatic Compounds. Catalysts 2020, 10, 1216. [Google Scholar] [CrossRef]
- Esparan, V.; Krings, U.; Struch, M.; Berger, R. A three-enzyme-system to degrade curcumin to natural vanillin. Molecules 2015, 20, 6640–6653. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, H.; Negi, P. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef] [PubMed]
- Gómez, A.; Escobedo, Z.; Welti, J. Phenolic Compounds in Mesoamerican Fruits-Characterization, Health Potential and Processing with Innovative Technologies. Int. J. Mol. Sci. 2020, 21, 8357. [Google Scholar] [CrossRef]
- Van, P. Phenolic Compounds of Cereals and Their Antioxidant Capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Zhang, H.; Wang, X.; Fan, J.; Zhang, X. Phenolic compounds and bioactivity evaluation of aqueous and methanol extracts of Allium mongolicum Regel. Food Sci. Nutr. 2019, 7, 779–787. [Google Scholar] [CrossRef]
- Hussain, S.; Rehman, A.; Luckett, D.; Blanchard, C.; Obied, H.; Strappe, P. Phenolic Compounds with Antioxidant Properties from Canola Meal Extracts Inhibit Adipogenesis. Int. J. Mol. Sci. 2019, 21, 1. [Google Scholar] [CrossRef]
- Wu, D.; Nie, X.; Shen, D.; Li, H.; Zhao, L.; Zhang, Q.; Lin, D.; Qin, W. Phenolic Compounds, Antioxidant Activities, and Inhibitory Effects on Digestive Enzymes of Different Cultivars of Okra (Abelmoschus esculentus). Molecules 2020, 25, 1276. [Google Scholar] [CrossRef]
- Nagarajan, S.; Nagarajan, R.; Kumar, J.; Salemme, A.; Togna, A.; Saso, L.; Bruno, F. Antioxidant Activity of Synthetic Polymers of Phenolic Compounds. Polymers 2020, 12, 1646. [Google Scholar] [CrossRef] [PubMed]
- González, J.; Míguez, N.; Padilla, D.; Leemans, L.; Poveda, A.; Jiménez, J.; Ballesteros, A.; Sandoval, G.; Plou, F. Optimization of Regioselective α-Glucosylation of Hesperetin Catalyzed by Cyclodextrin Glucanotransferase. Molecules 2018, 23, 2885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.M.; Xu, T.T.; Peng, Q.X.; Chen, Y.S.; Zhou, H.; Lu, Y.Y.; Yan, R.A. Enzymatic acylation of rutin with benzoic acid ester and lipophilic, antiradical, and antiproliferative properties of the acylated derivatives. J. Food Sci. 2021, 86, 1714–1725. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Z.; Wang, Y.; Fu, L. Dietary protein-phenolic interactions: Characterization, biochemical-physiological consequences, and potential food applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 3589–3615. [Google Scholar] [CrossRef]
- Wenfei, T.; Gengjun, C.; Michael, T.; Yonghui, L. Changes in phenolic profiles and antioxidant activities during the whole wheat bread-making process. Food Chem. 2021, 345, 128851. [Google Scholar] [CrossRef]
- Panche, A.; Diwan, A.; Chandra, S. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Zieniuk, B.; Groborz, K.; Wołoszynowska, M.; Ratusz, K.; Białecka, E.; Fabiszewska, A. Enzymatic Synthesis of Lipophilic Esters of Phenolic Compounds, Evaluation of Their Antioxidant Activity and Effect on the Oxidative Stability of Selected Oils. Biomolecules 2021, 11, 314. [Google Scholar] [CrossRef]
- Yang, W.; Kortesniemi, M.; Yang, B.; Zheng, J. Enzymatic Acylation of Anthocyanins Isolated from Alpine Bearberry (Arctostaphylos alpina) and Lipophilic Properties, Thermostability, and Antioxidant Capacity of the Derivatives. J. Agric. Food Chem. 2018, 66, 2909–2916. [Google Scholar] [CrossRef]
- Cumming, H.; Marshall, S.N. Lipase-catalysed synthesis of mono- and di-acyl esters of glyceryl caffeate in propylene carbonate and their antioxidant properties in tuna oil. J. Biotechnol. 2021, 325, 217–225. [Google Scholar] [CrossRef]
- Soares, V.; Marini, M.; de Paula, L.; Gabry, P.; Amaral, A.; Malafaia, C.; Leal, I. Umbelliferone esters with antibacterial activity produced by lipase-mediated biocatalytic pathway. Biotechnol. Lett. 2021, 43, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Bian, L.; Zhu, Y.; Zhang, R.; Shao, S.; Wu, Y.; Chen, Y.; Dang, Y.; Ding, Y.; Sun, H. Multifunctional alkyl ferulate esters as potential food additives: Antibacterial activity and mode of action against Listeria monocytogenes and its application on American sturgeon caviar preservation. Food Control 2019, 96, 390–402. [Google Scholar] [CrossRef]
- Torres, P.; Poveda, A.; Jimenez-Barbero, J.; Ballesteros, A.; Plou, F.J. Regioselective lipase-catalyzed synthesis of 3-o-acyl derivatives of resveratrol and study of their antioxidant properties. J. Agric. Food Chem. 2010, 58, 807–813. [Google Scholar] [CrossRef]
- Torres, P.; Reyes, D.; Ballesteros, A.; Plou, F. Lipase-Catalyzed Modification of Phenolic Antioxidants. In Lipases and Phospholipases: Methods and Protocols; Sandoval, G., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 435–443. [Google Scholar]
- Torres, P.; Plou, F.; Ballesteros, A. Modificación de antioxidantes fenólicos mediante procesos enzimáticos. In Obtención Enzimática de Compuestos Bioactivos a Partir de Recursos Naturales Iberoamericanos; Plou, F.J., Sandoval, G., Eds.; CSIC: Madrid, Spain, 2012; pp. 151–163. [Google Scholar]
- Xu, L.; Yang, T.; Wang, J.; Huang, F.; Zheng, M. Immobilized Lipase Based on Hollow Mesoporous Silicon Spheres for Efficient Enzymatic Synthesis of Resveratrol Ester Derivatives. J. Agric. Food Chem. 2021, 69, 9067–9075. [Google Scholar] [CrossRef] [PubMed]
- Davani, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Shi, Y.; Wu, Y.; Bian, L.; Zhu, Y.; Huang, X.; Pan, Y.; Zeng, L.; Zhang, R. Lipase-Catalyzed Synthesis of Sucrose Monolaurate and Its Antibacterial Property and Mode of Action against Four Pathogenic Bacteria. Molecules 2018, 23, 1118. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Cruces, M.A.; Bernabé, M.; Ballesteros, A.; Plou, F.J. Lipase-catalyzed regioselective acylation of sucrose in two-solvent mixtures. Biotechnol. Bioeng. 1999, 65, 10–16. [Google Scholar] [CrossRef]
- Sandoval, G.; Arrizon, J.; Gonalez-Avila, M.; Padilla-Camberos, E.; Matinez-Velazquez, M.; Villanueva-Rodriguez, S.; Casas-Godoy, L. Bioconjugate Molecules with Biological and Techno-Functional Activity, Method for the Production Thereof and Use Thereof. Patent Number MX 358789, 18 December 2013. [Google Scholar]
- Neta, N.S.; Teixeira, J.A.; Rodrigues, L.R. Sugar ester surfactants: Enzymatic synthesis and applications in food industry. Crit. Rev. Food Sci. Nutr. 2015, 55, 595–610. [Google Scholar] [CrossRef]
- van Rantwijk, F.; Madeira, L.; Sheldon, R. Biocatalytic transformations in ionic liquids. Trends Biotechnol. 2003, 21, 131–138. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, Y.; Lu, X.; Ren, Y.; Wang, Q.; Zhu, C.; Yu, D.; Wang, H. Lipase-catalyzed esterification of ferulic acid with lauryl alcohol in ionic liquids and antibacterial properties in vitro against three food-related bacteria. Food Chem. 2017, 220, 249–256. [Google Scholar] [CrossRef]



| Source/Commercial Name | Type | Application/Products | Reference |
|---|---|---|---|
| Candida antarctica lipase B (CALB)/Novozym 435/Lipozyme 435 | Recombinant | Flavor esters | [32] |
| Candida rugosa | Wild type | Glycerides, production flavor compounds | [33,34] |
| Termomyces lanuginosus/Lipozyme TL IM | Engineered | Food formulation, Interesterification of fats and oils | [35,36] |
| Aspergillus sp. | Wild type | Flavor and fragance | [37] |
| Aspergillus oryzae | Wild type | Interesterification of fats and oils | [36] |
| Geotrichum candidum | Wild type | Oil with increased unsaturation | [36] |
| Rhizomucor miehei/Lipozyme RM IM | Recombinant | Enhancing fruit fragrance | [38] |
| Modification of the amount and composition of volatile components in bovine milk | [39] | ||
| Ras Cheese Flavor Concentrate (RCFC) | [40] | ||
| Rhizopus oryzae | Wild type | Human Milk Fat Substitutes | [41] |
| Lactococcus chungangensis | Wild type | Flavoring in milk, cream cheese, yogurt and butter. | [42] |
| Lactobacillus plantarum | Wild type | Fermented food and cheese | [43,44] |
| Staphylococcus epidermidis | Wild type | Flavor-compound production | [45] |
| Ophiostoma piceae | Wild type | Flavor-compound production | [46] |
| Meyerozyma guilliermondii | Wild type | Feed industry | [47] |
| Vitamin Derivative | Acyl Donor | Solvent | Biocatalyst | Reaction Conditions | Conversion (%) | Reference |
|---|---|---|---|---|---|---|
| L-ascorbyl palmitate | Palmitic acid | tert-butyl alcohol | Indigenously immobilized lipase PyCal (CALB) | AA:PA molar ratio 1:5; 20 mL tert-butyl alcohol, 0.6 g of biocatalyst, 60 °C (batch) | 50 | [119] |
| L-ascorbyl palmitate | Palmitic acid | tert-butyl alcohol | Novozym 435 | AA:PA molar ratio 1:5; 20 mL tert-butyl alcohol, 0.6 g of biocatalyst, 60 °C (batch) | 50 | |
| L-ascorbyl palmitate | Palmitic acid | 2-Methyl-2-butanol (2M2B) | Novozym 435 | AA:PA molar ratio 1:8; 12 g/L biocatalyst; 55 °C | 81 | [114] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Reyes, A.L.; Valero Barranco, F.; Sandoval, G. Recent Advances in Lipases and Their Applications in the Food and Nutraceutical Industry. Catalysts 2022, 12, 960. https://doi.org/10.3390/catal12090960
Reyes-Reyes AL, Valero Barranco F, Sandoval G. Recent Advances in Lipases and Their Applications in the Food and Nutraceutical Industry. Catalysts. 2022; 12(9):960. https://doi.org/10.3390/catal12090960
Chicago/Turabian StyleReyes-Reyes, Ana Laura, Francisco Valero Barranco, and Georgina Sandoval. 2022. "Recent Advances in Lipases and Their Applications in the Food and Nutraceutical Industry" Catalysts 12, no. 9: 960. https://doi.org/10.3390/catal12090960
APA StyleReyes-Reyes, A. L., Valero Barranco, F., & Sandoval, G. (2022). Recent Advances in Lipases and Their Applications in the Food and Nutraceutical Industry. Catalysts, 12(9), 960. https://doi.org/10.3390/catal12090960








