Chitosan Capped Copper Oxide Nanocomposite: Efficient, Recyclable, Heterogeneous Base Catalyst for Synthesis of Nitroolefins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus and Instrumentations
2.2. Preparation of Chitosan-Based CuO Nanocomposite Films
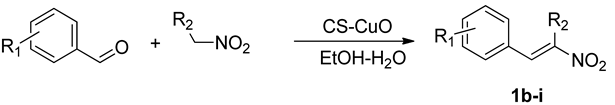
2.3. Synthesis of Nitroolefin
3. Results and Discussion
3.1. Preparation of Chitosan-CuO Nanocomposite
3.1.1. FTIR Characterization
3.1.2. X-ray Diffraction (XRD)
3.1.3. SEM and Morphological Changes
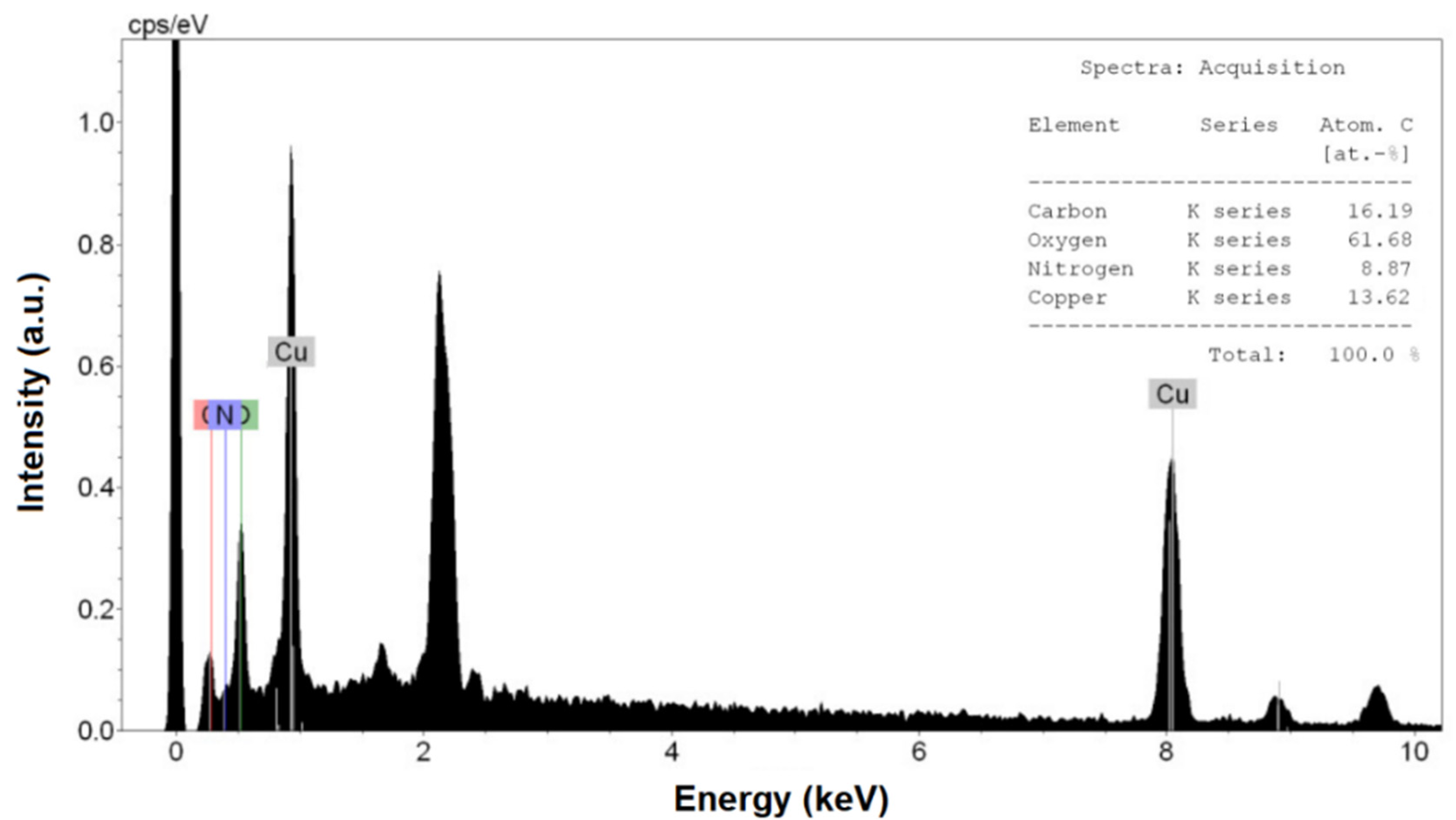
3.1.4. Energy-Dispersive X-ray Spectroscopy (EDS) and Estimation of Copper
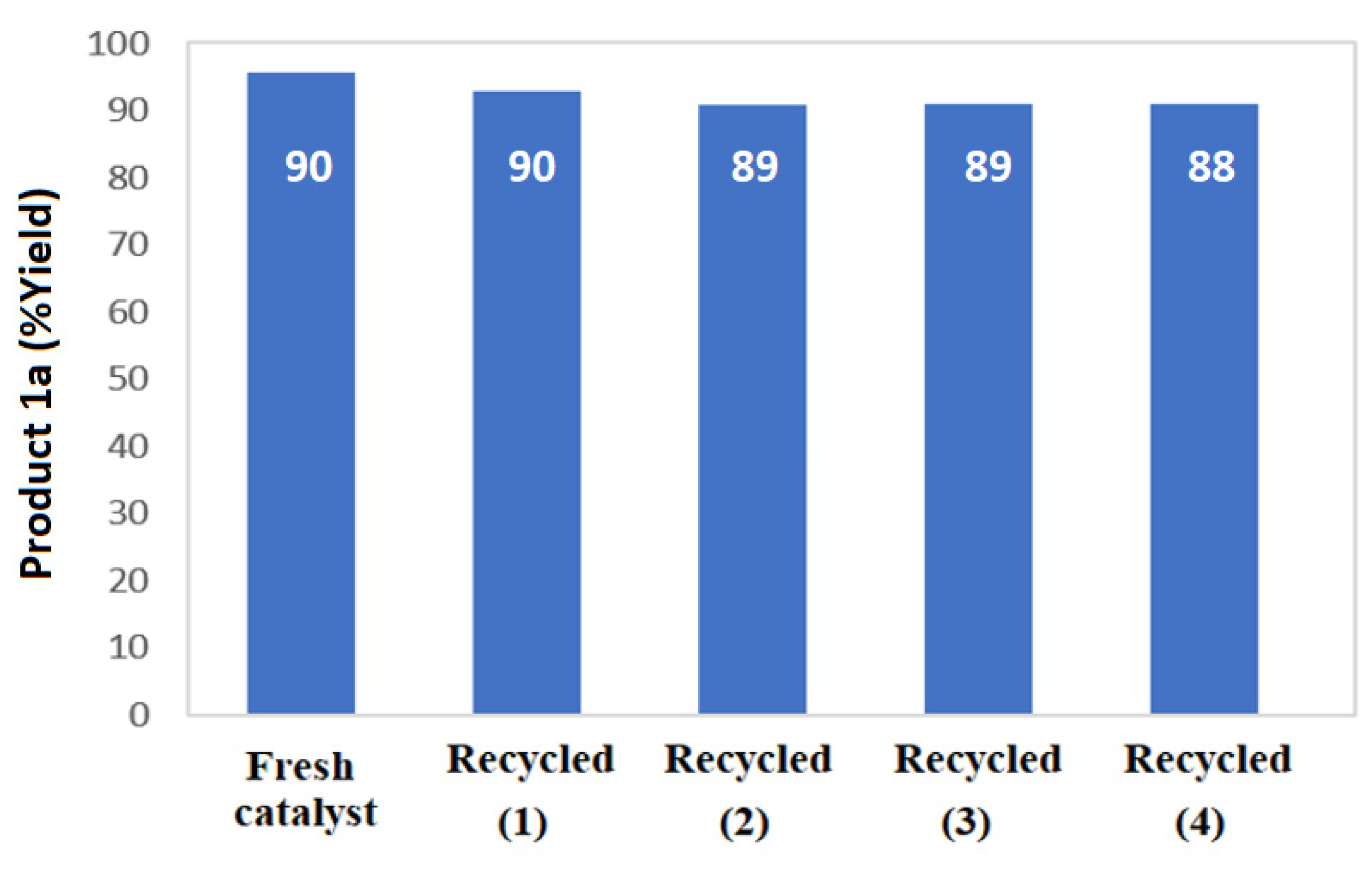
3.2. CS-CuO Nanocomposite Film as Basic Heterogeneous Catalyst in Synthesis of Nitroolefin
3.2.1. Optimization of the Reaction Conditions
Nature of Solvent
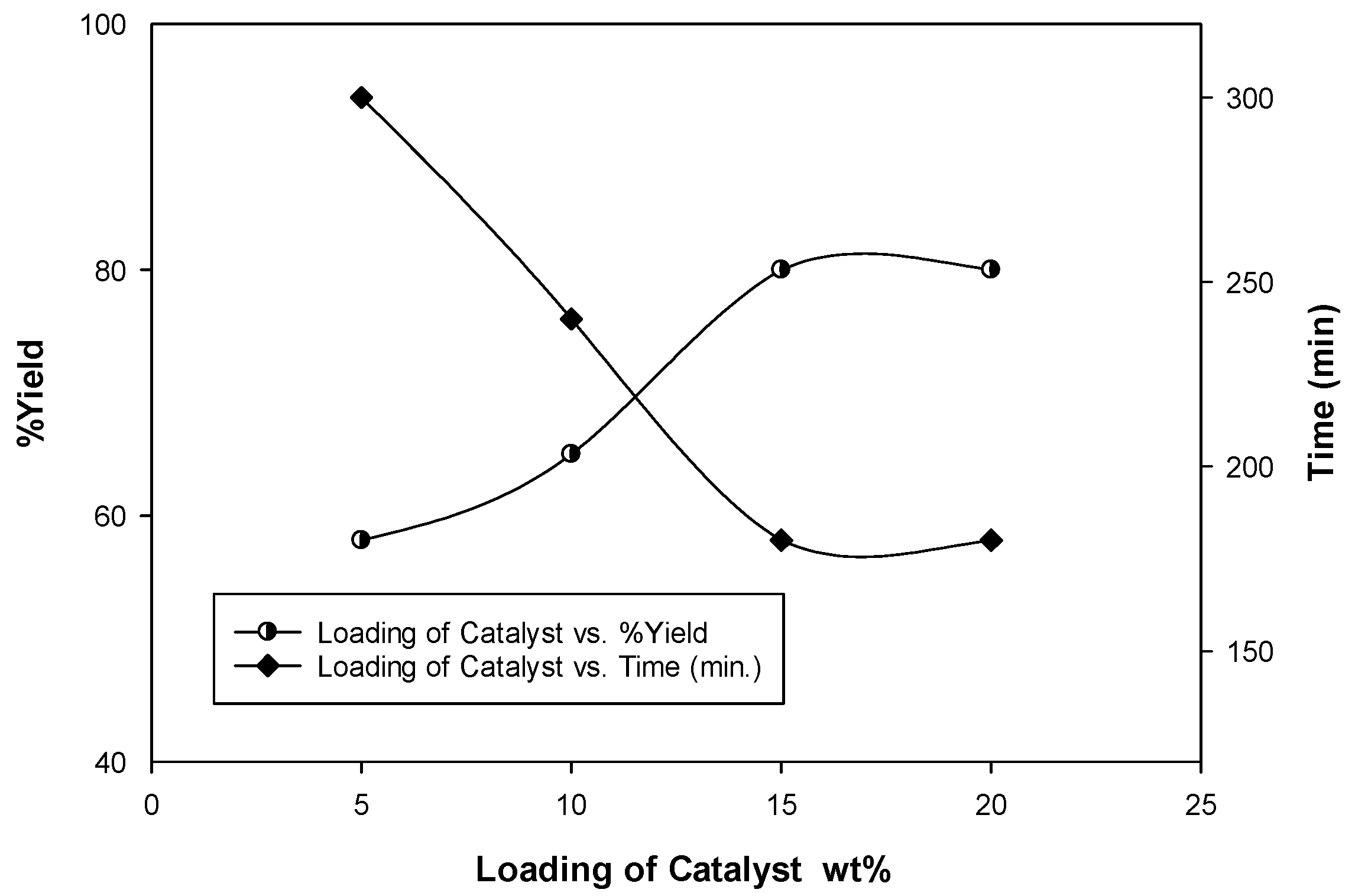
Amount of Catalyst (Catalyst Loading)
3.2.2. Scope of the Reaction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bell, A.T. The Impact of Nanoscience on Heterogeneous Catalysis. Science 2003, 299, 1688–1691. [Google Scholar] [PubMed]
- Narayanan, R. Synthesis of green nanocatalysts and industrially important green reactions. Green Chem. Lett. Rev. 2012, 5, 707–725. [Google Scholar] [CrossRef]
- Somwanshi, S.B.; Somvanshi, S.B.; Kharat, P.B. Nanocatalyst: A Brief Review on Synthesis to Applications. J. Phys. Conf. Ser. 2020, 1644, 012046. [Google Scholar] [CrossRef]
- Bhanage, B.M.; Arai, M. Catalyst product separation techniques in heck reaction. Catal. Rev. 2001, 43, 315–344. [Google Scholar] [CrossRef]
- Hemalatha, K.; Madhumitha, G.; Kajbafvala, A.; Anupama, N.; Sompalle, R.; Mohana, R.S. Function of Nanocatalyst in Chemistry of Organic Compounds Revolution: An Overview. J. Nanomater. 2013, 2013, 4. [Google Scholar]
- Jammi, S.; Sakthivel, S.; Rout, L.; Mukherjee, T.; Mandal, S.; Mitra, R.; Saha, P.; Punniyamurthy, T. CuO Nanoparticles Catalyzed C-N, C-O, and C-S Cross-Coupling Reactions: Scope and Mechanism. J. Org. Chem. 2009, 74, 1974–1976. [Google Scholar]
- Kidwai, M.; Bhardwaj, S.; Poddar, R. C-Arylation reactions catalyzed by CuO-nanoparticles under ligand free conditions. Beil. J. Org. Chem. 2010, 6, 35–41. [Google Scholar]
- Ahmady, A.Z.; Keshavarz, M.; Kardani, M.; Mohtasham, N. CuO Nanoparticles as an Efficient Catalyst for the Synthesis of Flavanones. Orient. J. Chem. 2015, 31, 1841–1846. [Google Scholar]
- Satish, G.; Reddy, K.H.V.; Ramesh, K.; Karnakar, K.; Nageswar, Y.V.D. Synthesis of 2-N-substituted benzothiazoles via domino condensation-hetero cyclization process, mediated by copper oxide nanoparticles under ligand-free conditions. Tetrahedron Lett. 2012, 53, 2518–2521. [Google Scholar]
- Gaddam, S.; Kasireddy, H.R.; Konkala, K.; Katla, R.; Durga, N.Y.V. Synthesis of N-substituted-2-aminobenzothiazoles using nano copper oxide as a recyclable catalyst under ligand-free conditions, in reusable PEG-400 medium. Chin. Chem. Lett. 2014, 25, 732–736. [Google Scholar]
- Shaterian, H.R.; Moradi, F.; Mohammadnia, M. Nano copper(II) oxide catalyzed four-component synthesis of functionalized benzo[a]pyrano [2,3-c]phenazine derivatives. Comptes Rendus Chim. 2012, 15, 1055–1059. [Google Scholar] [CrossRef]
- Rawat, M.; Rawat, D.S. Copper oxide nanoparticle catalysed synthesis of imidazo[1,2-a] pyrimidine derivatives, their optical properties and selective fluorescent sensor towards zinc ion. Tetrahedron Lett. 2018, 59, 2341–2346. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, R.; Xu, B.; Li, Y. Synthesis, characterization and catalytic properties of CuO nanocrystals with various shapes. Nanotechnology 2006, 17, 3939–3943. [Google Scholar] [CrossRef]
- Varzi, Z.; Esmaeili, M.S.; Taheri-Ledari, R.; Maleki, A. Facile synthesis of imidazoles by an efficient and eco-friendly heterogeneous catalytic system constructed of FeO and CuO nanoparticles, and guarana as a natural basis. Inorg. Chem. Commun. 2021, 125, 108465–108470. [Google Scholar] [CrossRef]
- Khalil, K.D.; Riyadh, S.M.; Gomha, S.M.; Ali, I. Synthesis, characterization and application of copper oxide chitosan nanocomposite for green regioselective synthesis of [1,2,3]triazoles. Int. J. Biol. Macromol. 2019, 130, 928–937. [Google Scholar] [CrossRef]
- Aljuhani, A.; Riyadh, S.M.; Khalil, K.D. Chitosan/CuO nanocomposite films mediated regioselective synthesis of 1,3,4-trisubstituted pyrazoles under microwave irradiation. J. Saudi Chem. Soc. 2021, 25, 101276. [Google Scholar] [CrossRef]
- Khalil, K.D.; Ibrahim, E.I.; Al-Sagheer, F.A. A novel, efficient, and recyclable biocatalyst for Michael addition reactions and its iron(III) complex as promoter for alkyl oxidation reactions. Catal. Sci. Technol. 2016, 6, 1410–1416. [Google Scholar]
- Madkour, M.; Khalil, K.D.; Al-Sagheer, F.A. Heterogeneous Hybrid Nanocomposite Based on Chitosan/Magnesia Hybrid Films: Ecofriendly and Recyclable Solid Catalysts for Organic Reactions. Polymers 2021, 13, 3583. [Google Scholar] [CrossRef]
- Riyadh, S.M.; Khalil, K.D.; Bashal, A.H. Structural Properties and Catalytic Activity of Binary Poly (vinyl alcohol)/Al2O3 Nanocomposite Film for Synthesis of Thiazoles. Catalysts 2020, 10, 100. [Google Scholar] [CrossRef]
- Mizwari, Z.M.; Oladipo, A.A.; Yilmaz, E. Chitosan/metal oxide nanocomposites: Synthesis, characterization, and antibacterial activity. Int. J. Poly. Mater. Poly. Biomater. 2020, 69, 1–9. [Google Scholar] [CrossRef]
- Sunil, D. Recennt Advances on Chitosan-Metal Oxide Nanoparticles and their Biological Application. Mater. Sci. Forum 2013, 754, 99–108. [Google Scholar] [CrossRef]
- Fu, C.-C.; Hung, T.-C.; Su, C.-H.; Suryani, D.; Wu, W.-T.; Dai, W.-C.; Yeh, Y.-T. Immobilization of calcium oxide onto chitosan beads as a heterogeneous catalyst for biodiesel production. Polym. Int. 2011, 60, 957–962. [Google Scholar] [CrossRef]
- Guibal, E. Heterogeneous catalysis on chitosan-based materials: A review. Prog. Polym. Sci. 2005, 30, 71–109. [Google Scholar] [CrossRef]
- Mostafa, M.A.; Ismail, M.M.; Morsy, J.M.; Hassanin, H.M.; Abdelrazek, M.M. Synthesis, characterization, anticancer, and antioxidant activities of chitosan Schiff bases bearing quinolinone or pyranoquinolinone and their silver nanoparticles derivatives. Polym. Bull. 2022, 1–25. [Google Scholar] [CrossRef]
- Julianto, T.S.; Mumpuni, R.A. Chitosan and N-Alkyl chitosan as a heterogeneous based catalyst in the transesterification reaction of used cooking oil. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012004. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, G.; Rawat, M. A Brief Review on Synthesis and Characterization of Copper Oxide Nanoparticles and its Applications. J. Bioelectron. Nanotechnol. 2016, 1, 9. [Google Scholar]
- El-Atawy, M.A.; Ferretti, F.; Ragaini, F. A Synthetic Methodology for Pyrroles from Nitrodienes. Eur. J. Org. Chem. 2018, 34, 4818–4825. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Ferretti, F.; Ragaini, F. Palladium-Catalyzed Intramolecular Cyclization of Nitroalkenes: Synthesis of Thienopyrroles. Eur. J. Org. Chem. 2017, 14, 1902–1910. [Google Scholar] [CrossRef]
- Munoz, L.; Rodriguez, A.M.; Rosell, G.; Bosch, M.P.; Guerrero, A. Enzymatic enantiomeric resolution of phenylethylamines structurally related to amphetamine. Org. Biomol. Chem. 2011, 9, 8171–8177. [Google Scholar] [CrossRef]
- Goodman, M.M.; Kabalka, G.W.; Marks, R.C.; Knapp, F.F.; Lee, J.; Liang, Y. Synthesis and evaluation of radioiodinated 2-(2(RS)-aminopropyl)-5-iodothiophenes as brain imaging agents. J. Med. Chem. 1992, 35, 280–285. [Google Scholar] [CrossRef]
- Cardenas, G.; Miranda, S.P. FTIR and TGA studies of chitosan composite films. J. Chilean Chem. Soc. 2004, 49, 291–295. [Google Scholar] [CrossRef]
- Vaseem, M.; Umar, A.; Kim, S.H.; Hahn, Y.-B. Low-Temperature Synthesis of Flower-Shaped CuO Nanostructures by Solution Process: Formation Mechanism and Structural Properties. J. Phys. Chem. 2008, 112, 5729–5735. [Google Scholar] [CrossRef]
- Sudha, V.; Murugadoss, G.; Thangamuthu, R. Structural and morphological tuning of Cu-based metal oxide nanoparticles by a facile chemical method and highly electrochemical sensing of sulphite. Sci. Rep. 2021, 11, 3413. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liao, L.; Li, J.; Pan, C. From Copper Nanocrystalline to CuO Nanoneedle Array: Synthesis, Growth Mechanism, and Properties. J. Phys. Chem. 2007, 111, 5050–5056. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures; John Wiley and Sons Inc.: New York, NY, USA, 1954; p. 633. [Google Scholar] [CrossRef]





| Entry | Catalyst (mol%) | Solvent | Time (h) | Temperature (°C) | Yield (%) |
|---|---|---|---|---|---|
| 1 | 10 | THF | 3 | 120 | 8 |
| 2 | 10 | Toluene | 3 | 120 | 13 |
| 3 | 10 | DMF | 3 | 120 | 20 |
| 4 | 10 | CH3CN | 3 | 120 | 25 |
| 5 | 10 | EtOH | 3 | 120 | 14 |
| 6 | 10 | EtOH-H2O (1:1) | 3 | 120 | 58 |
| 7 | 10 | EtOH-H2O (1:3) | 3 | 120 | 65 |
| 8 a | 10 | EtOH-H2O (1:5) | 3 | 120 | 80 |
 | ||||
| Entry | R1 | R2 | Product | Yield (%) |
| 1 | 4-Cl | CH3 | 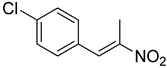 | 65 |
| 2 | 4-F | CH3 | 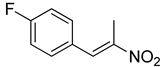 | 72 |
| 3 | 4-NO2 | CH3-CH2 | 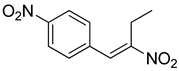 | 68 |
| 4 | 3-Cl | CH3 | 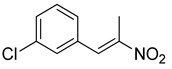 | 74 |
| 5 | 3-Br | CH3 | 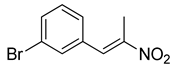 | 73 |
| 6 | 4-OCH3 | CH3 | 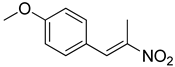 | 60 |
| 7 | 4-CH3 | CH3 | 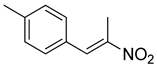 | 66 |
| 8 | 4-NMe2 | CH3 |  | 49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Atawy, M.A.; Khalil, K.D.; Bashal, A.H. Chitosan Capped Copper Oxide Nanocomposite: Efficient, Recyclable, Heterogeneous Base Catalyst for Synthesis of Nitroolefins. Catalysts 2022, 12, 964. https://doi.org/10.3390/catal12090964
El-Atawy MA, Khalil KD, Bashal AH. Chitosan Capped Copper Oxide Nanocomposite: Efficient, Recyclable, Heterogeneous Base Catalyst for Synthesis of Nitroolefins. Catalysts. 2022; 12(9):964. https://doi.org/10.3390/catal12090964
Chicago/Turabian StyleEl-Atawy, Mohamed A., Khaled D. Khalil, and Ali H. Bashal. 2022. "Chitosan Capped Copper Oxide Nanocomposite: Efficient, Recyclable, Heterogeneous Base Catalyst for Synthesis of Nitroolefins" Catalysts 12, no. 9: 964. https://doi.org/10.3390/catal12090964








