Characterization of a New Marine Leucine Dehydrogenase from Pseudomonas balearica and Its Application for L-tert-Leucine Production
Abstract
:1. Introduction
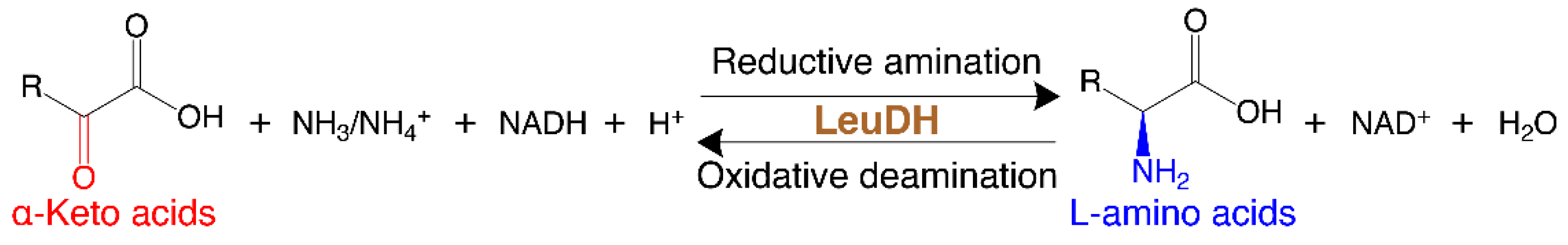
2. Results and Discussion
2.1. Sequence Analysis of PbLeuDH
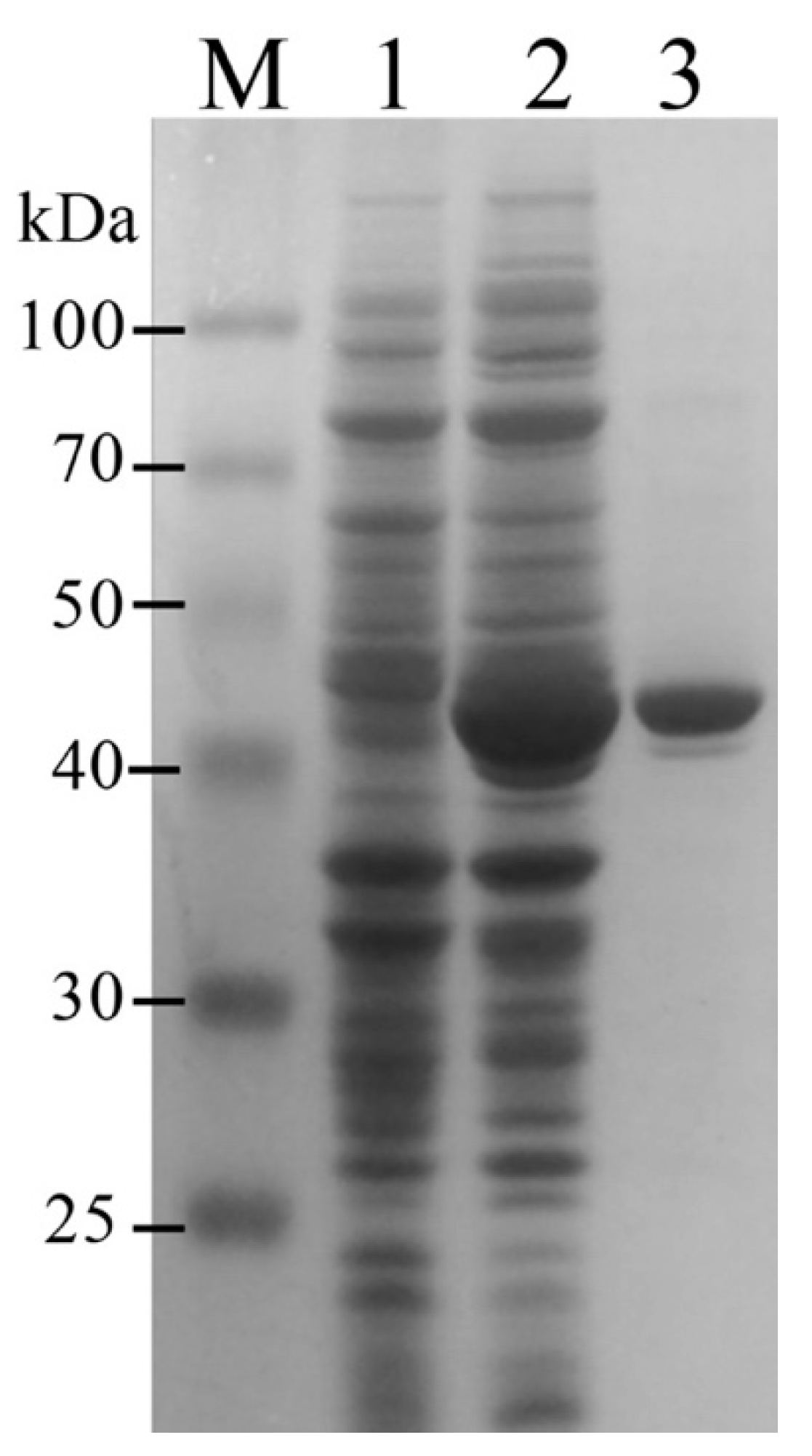
2.2. Expression and Purification of Recombinant PbLeuDH
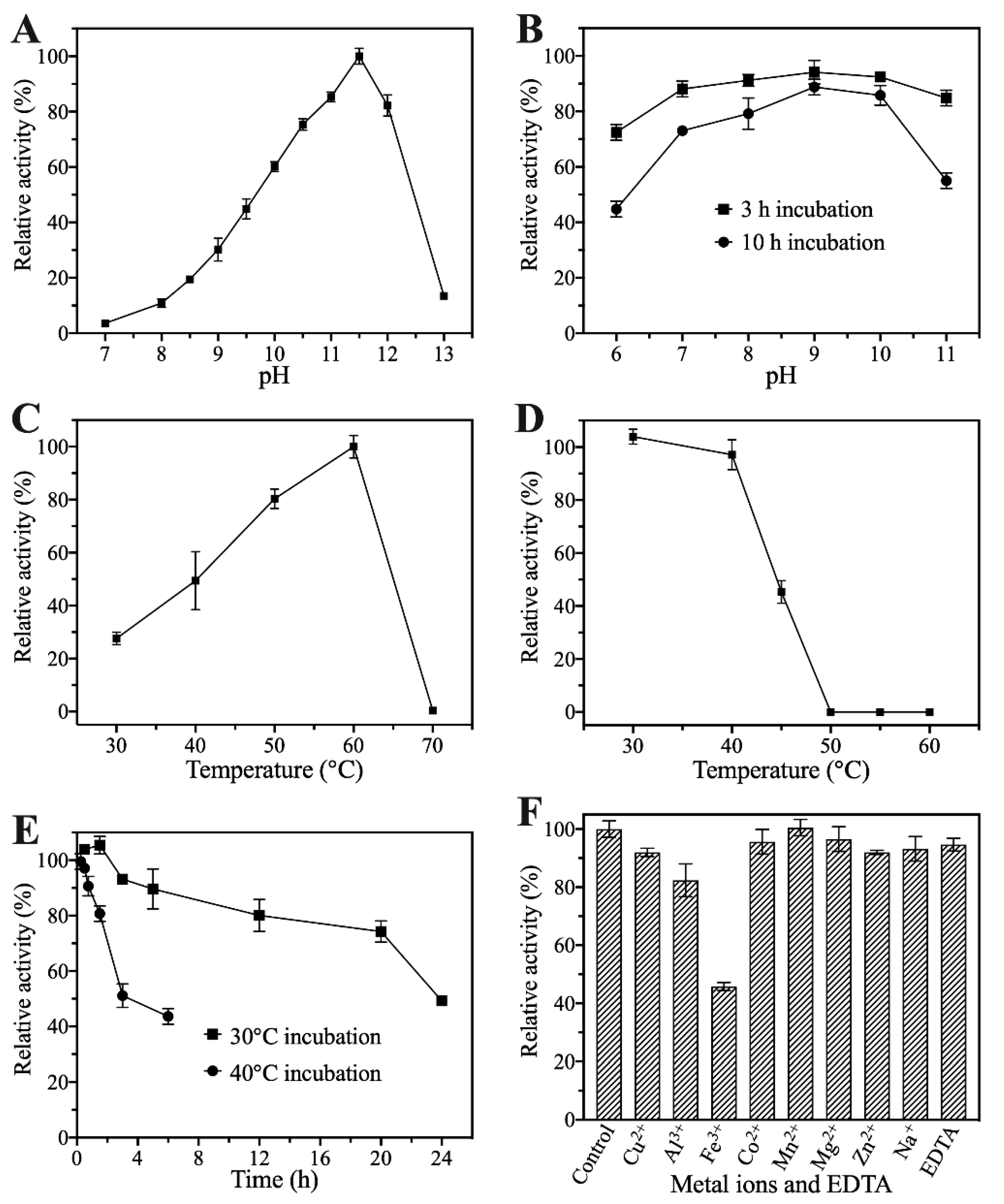
2.3. Biochemical Characterization of PbLeuDH toward L-Leucine
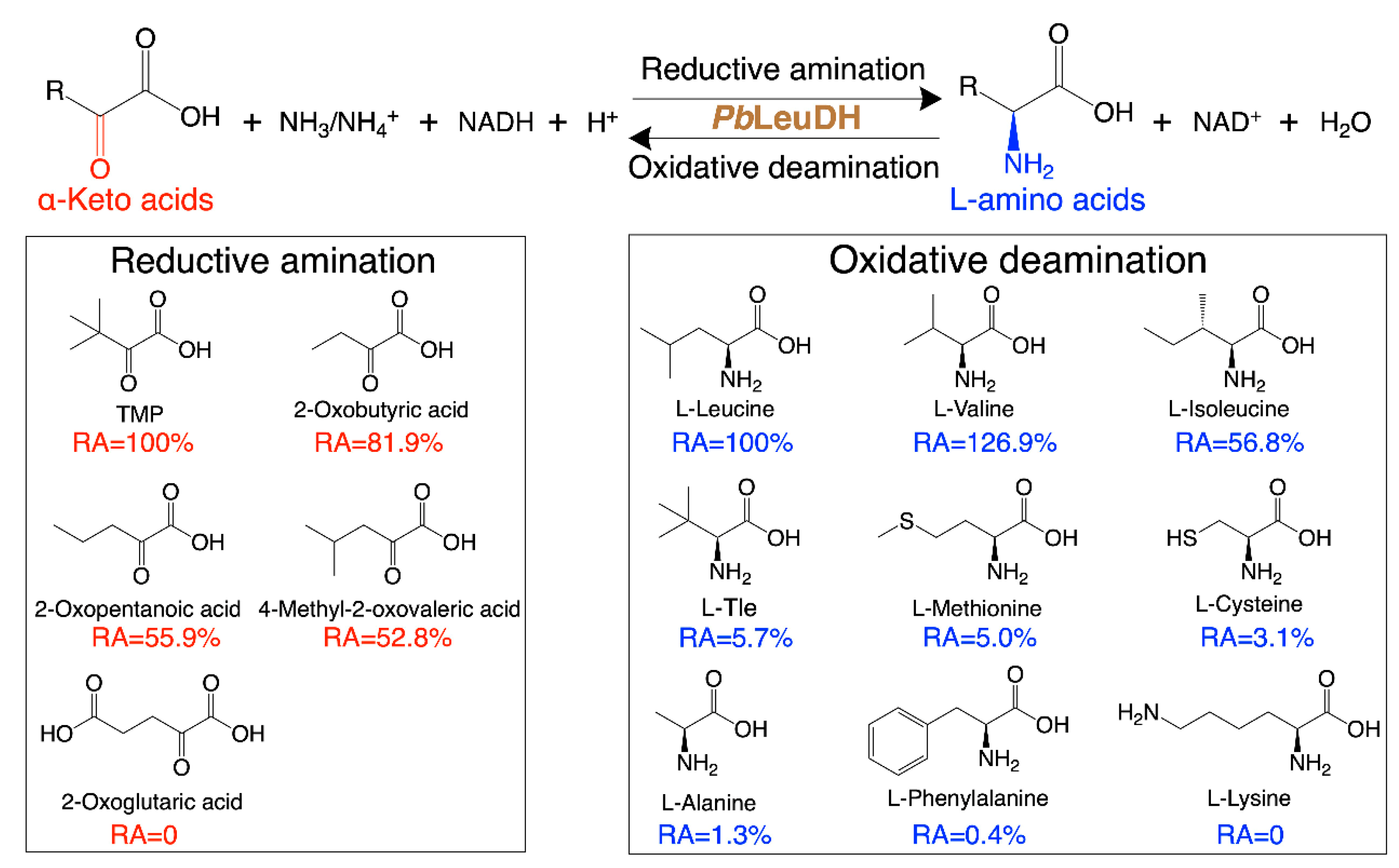
2.4. Substrate Specificity and Kinetic Parameters of PbLeuDH
2.5. Construction of Whole-Cell Biocatalyst Coexpressing PbLeuDH and BmGDH
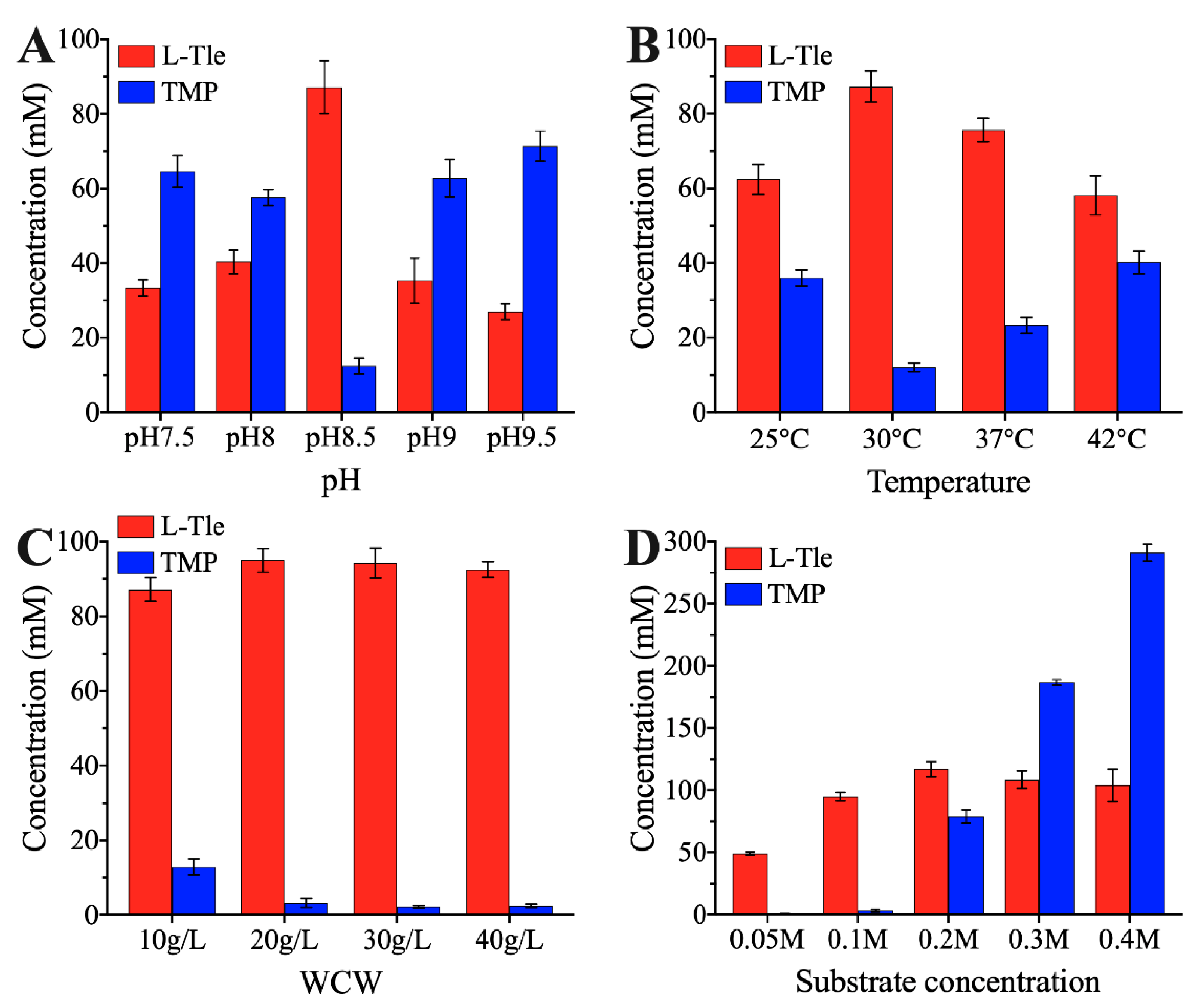
2.6. Effects of pH, Temperature, WCW, and Substrate Concentration on L-Tle Production by E. coli (pET28a-Pbleudh-Bmgdh)
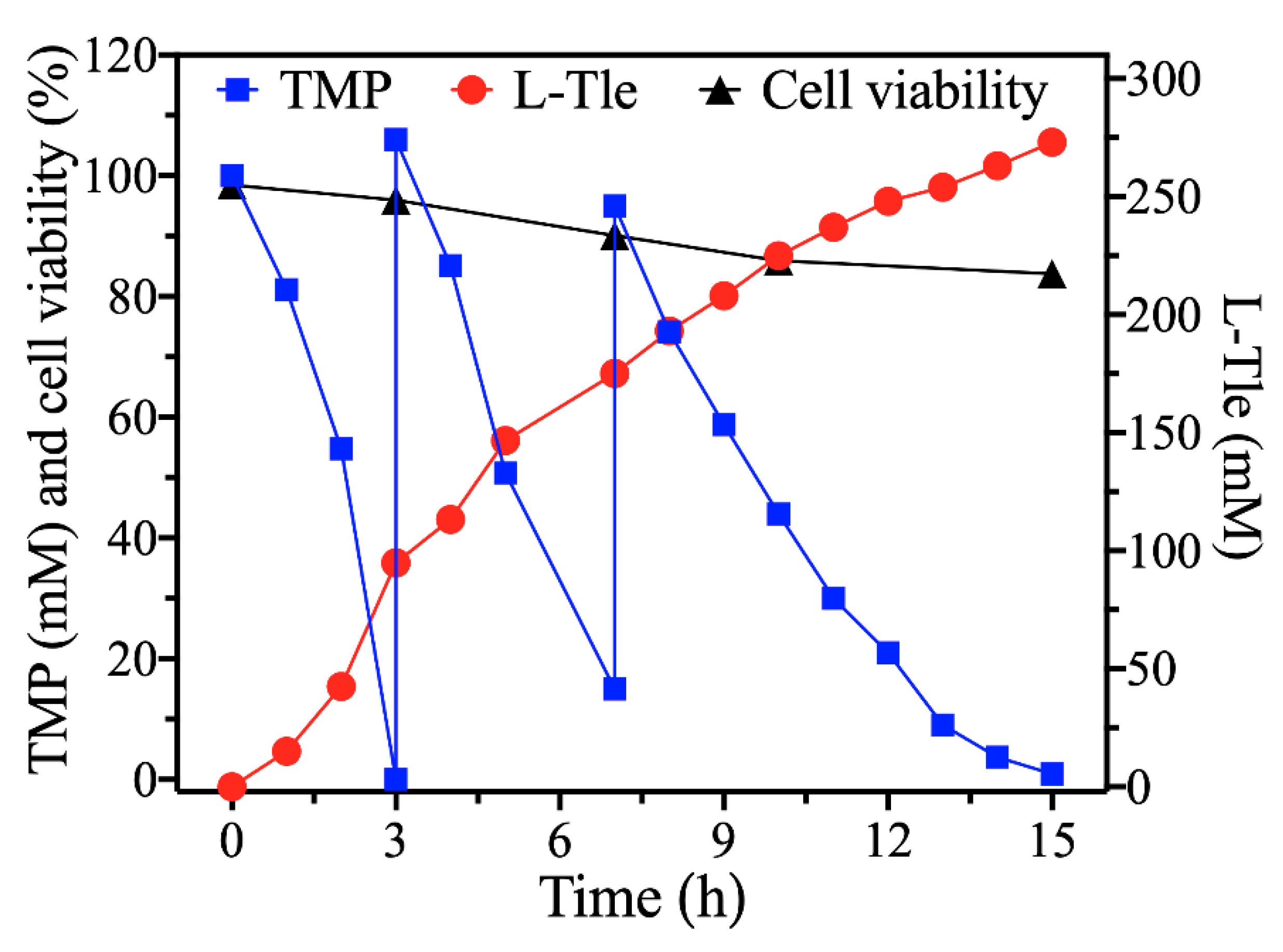
2.7. Fed-Batch Synthesis of L-Tle from TMP by E. coli (pET28a-Pbleudh-Bmgdh)
3. Materials and Methods
3.1. Strains, Plasmids, Primers, and Chemicals
3.2. Cloning, Expression, and Purification of PbLeuDH
3.3. Enzyme Activity Assay
3.4. Biochemical Characterization of PbLeuDH toward L-Leucine
3.5. Substrate Specificity and Kinetic Parameters of PbLeuDH
3.6. Construction of Whole-Cell Biocatalyst Coexpressing PbLeuDH and BmGDH
3.7. Whole-Cell Biocatalyst Preparation
3.8. L-Tle Production from TMP by Whole-Cell Biocatalysis
3.9. Analytical Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Narancic, T.; Almahboub, S.A.; O’Connor, K.E. Unnatural amino acids: Production and biotechnological potential. World J. Microbiol. Biotechnol. 2019, 35, 67. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Chen, J.; Xu, M.; Yang, T.; Zheng, J.; Zhang, X.; Rao, Z. Rational engineering of Bacillus cereus leucine dehydrogenase towards α-keto acid reduction for improving unnatural amino acid production. Biotechnol. J. 2019, 14, 1800253. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.-F.; Zeng, Y.-J.; Xu, P.; Guo, Z.-W.; Ou, X.-Y.; Peng, F.; Yang, J.-G.; Zong, M.-H.; Lou, W.-Y. Characterization of a novel methylaspartate ammonia lyase from E. coli O157:H7 for efficient asymmetric synthesis of unnatural amino acids. ACS Sustain. Chem. Eng. 2020, 8, 329–334. [Google Scholar] [CrossRef]
- Jia, Y.-Y.; Xie, Y.-L.; Yang, L.-L.; Shi, H.-L.; Lu, Y.-F.; Zhang, S.-P.; Tang, C.-D.; Yao, L.-G.; Kan, Y.-C. Expression of novel L-leucine dehydrogenase and high-level production of L-tert-leucine catalyzed by engineered Escherichia coli. Front. Bioeng. Biotechnol. 2021, 9, 655522. [Google Scholar]
- Zhou, F.; Mu, X.; Nie, Y.; Xu, Y. Enhanced catalytic efficiency and coenzyme affinity of leucine dehydrogenase by comprehensive screening strategy for L-tert-leucine synthesis. Appl. Microbiol. Biotechnol. 2021, 105, 3625–3634. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, Z.; Jin, J.-M.; Tang, S.-Y. Directed evolution of leucine dehydrogenase for improved efficiency of L-tert-leucine synthesis. Appl. Microbiol. Biotechnol. 2016, 100, 5805–5813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Wang, S.; Fang, B. Engineering bi-functional enzyme complex of formate dehydrogenase and leucine dehydrogenase by peptide linker mediated fusion for accelerating cofactor regeneration. Eng. Life Sci. 2017, 17, 989–996. [Google Scholar] [CrossRef]
- You, Z.-N.; Zhou, K.; Han, Y.; Yang, B.-Y.; Chen, Q.; Pan, J.; Qian, X.-L.; Li, C.-X.; Xu, J.-H. Design of a self-sufficient hydride-shuttling cascade for concurrent bioproduction of 7,12-dioxolithocholate and L-tert-leucine. Green Chem. 2021, 23, 4125–4133. [Google Scholar] [CrossRef]
- Jiang, W.; Fang, B. Construction of a tunable multi-enzyme-coordinate expression system for biosynthesis of chiral drug intermediates. Sci. Rep. 2016, 6, 30462. [Google Scholar] [CrossRef]
- Meng, X.; Yang, L.; Liu, Y.; Wang, H.; Shen, Y.; Wei, D. Identification and rational engineering of a high substrate-tolerant leucine dehydrogenase effective for the synthesis of L-tert-leucine. ChemCatChem 2021, 13, 3340–3349. [Google Scholar] [CrossRef]
- Hummel, W.; Kuzu, M.; Geueke, B. An efficient and selective enzymatic oxidation system for the synthesis of enantiomerically pure D-tert-leucine. Org. Lett. 2003, 5, 3649–3650. [Google Scholar] [CrossRef]
- Ouyang, S.; Li, X.; Sun, X.; Ouyang, J.; Yong, Q. A thermostable leucine dehydrogenase from Bacillus coagulans NL01: Expression, purification and characterization. Process Biochem. 2020, 90, 89–96. [Google Scholar] [CrossRef]
- Zhu, W.; Li, Y.; Jia, H.; Wei, P.; Zhou, H.; Jiang, M. Expression, purification and characterization of a thermostable leucine dehydrogenase from the halophilic thermophile Laceyella sacchari. Biotechnol. Lett. 2016, 38, 855–861. [Google Scholar] [CrossRef]
- Li, H.; Zhu, D.; Hyatt, B.A.; Malik, F.M.; Biehl, E.R.; Hua, L. Cloning, protein sequence clarification, and substrate specificity of a leucine dehydrogenase from Bacillus sphaericus ATCC4525. Appl. Biochem. Biotechnol. 2009, 158, 343–351. [Google Scholar] [CrossRef]
- Luo, W.; Zhu, J.; Zhao, Y.; Zhang, H.; Yang, X.; Liu, Y.; Rao, Z.; Yu, X. Cloning and expression of a novel leucine dehydrogenase: Characterization and L-tert-leucine production. Front. Bioeng. Biotechnol. 2020, 8, 186. [Google Scholar] [CrossRef]
- Li, J.; Pan, J.; Zhang, J.; Xu, J.-H. Stereoselective synthesis of L-tert-leucine by a newly cloned leucine dehydrogenase from Exiguobacterium sibiricum. J. Mol. Catal. B Enzym. 2014, 105, 11–17. [Google Scholar] [CrossRef]
- Liu, W.; Ma, H.; Luo, J.; Shen, W.; Xu, X.; Li, S.; Hu, Y.; Huang, H. Efficient synthesis of L-tert-leucine through reductive amination using leucine dehydrogenase and formate dehydrogenase coexpressed in recombinant E. coli. Biochem. Eng. J. 2014, 91, 204–209. [Google Scholar] [CrossRef]
- Zhao, Y.; Wakamatsu, T.; Doi, K.; Sakuraba, H.; Ohshima, T. A psychrophilic leucine dehydrogenase from Sporosarcina psychrophila: Purification, characterization, gene sequencing and crystal structure analysis. J. Mol. Catal. B Enzym. 2012, 83, 65–72. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, W.; Gao, Z.; Zhou, H.; Cao, F.; Jiang, M.; Li, Y.; Jia, H.; Wei, P. Biosynthetic L-tert-leucine using Escherichia coli co-expressing a novel NADH-dependent leucine dehydrogenase and a formate dehydrogenase. Electron. J. Biotechnol. 2020, 47, 83–88. [Google Scholar] [CrossRef]
- Zhang, C.; Kim, S.-K. Research and application of marine microbial enzymes: Status and prospects. Mar. Drugs 2010, 8, 1920–1934. [Google Scholar] [CrossRef]
- Jiang, W.; Sun, D.; Lu, J.; Wang, Y.; Wang, S.; Zhang, Y.; Fang, B. A cold-adapted leucine dehydrogenase from marine bacterium Alcanivorax dieselolei: Characterization and L-tert-leucine production. Eng. Life Sci. 2016, 16, 283–289. [Google Scholar] [CrossRef]
- Chen, R.; Liao, Y.-T.; Gao, T.-T.; Zhang, Y.-M.; Lu, L.-H.; Wang, C.-H. Novel salt-tolerant leucine dehydrogenase from marine Pseudoalteromonas rubra DSM 6872. Mol. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Liu, J.; Kong, W.; Bai, J.; Li, Y.; Dong, L.; Zhou, L.; Liu, Y.; Gao, J.; Bradshaw Allen, R.T.; Turner, N.J.; et al. Amine dehydrogenases: Current status and potential value for chiral amine synthesis. Chem Catal. 2022, 2, 1288–1314. [Google Scholar] [CrossRef]
- Mahdizadehdehosta, R.; Kianmehr, A.; Khalili, A. Isolation and characterization of leucine dehydrogenase from a thermophilic Citrobacter freundii JK-91 strain isolated from Jask Port. Iran. J. Microbiol. 2013, 5, 278–284. [Google Scholar]
- He, Y.; Chen, F.; Sun, M.; Gao, H.; Guo, Z.; Lin, H.; Chen, J.; Jin, W.; Yang, Y.; Zhang, L.; et al. Efficient (3S)-acetoin and (2S,3S)-2,3-butanediol production from meso-2,3-butanediol using whole-cell biocatalysis. Molecules 2018, 23, 691. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, X.; He, Y.; Yang, T.; Gao, H.; Li, G.; Chen, F.; Sun, M.; Lee, J.-K.; Zhang, L. Efficient (3R)-acetoin production from meso-2,3-butanediol using a new whole-cell biocatalyst with co-expression of meso-2,3-butanediol dehydrogenase, NADH oxidase, and Vitreoscilla hemoglobin. J. Microbiol. Biotechnol. 2017, 27, 92–100. [Google Scholar] [CrossRef]
- Lin, H.; Xu, J.; Sun, W.; Hu, W.; Gao, H.; Hu, K.; Qiu, J.; Huang, B.; Zhang, L. Efficient 1-hydroxy-2-butanone production from 1,2-butanediol by whole cells of engineered E. coli. Catalysts 2021, 11, 1184. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, Y.; Wang, Y.; Fu, Y.; Zhang, A.; Qiu, R.; Yang, S.; Fang, B. Construction and characterization of a novel glucose dehydrogenase-leucine dehydrogenase fusion enzyme for the biosynthesis of L-tert-leucine. Microb. Cell Fact. 2021, 20, 3. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.-Y.; Zong, M.-H.; Li, N. Sacrificial substrate-free whole-cell biocatalysis for the synthesis of 2,5-furandicarboxylic acid by engineered Escherichia coli. ACS Sustain. Chem. Eng. 2020, 8, 4341–4345. [Google Scholar] [CrossRef]


| Enzyme | Total Activity (U) | Total Protein (mg) | Specific Activity (U mg−1) | Purification Yield (%) | Purification Factor |
|---|---|---|---|---|---|
| Crude extract | 106.72 | 14.38 ± 0.52 | 7.42 ± 0.15 | 100.00 | 1.00 |
| Purified PbLeuDH | 71.82 | 2.71 ± 0.06 | 26.50 ± 0.27 | 67.30 | 3.57 |
| Substrate | Vmax (U mg−1) | Km (mM) | kcat (s−1) | kcat/Km(s−1 mM−1) |
|---|---|---|---|---|
| TMP | 91.78 ± 6.41 | 4.92 ± 0.31 | 120.49 ± 6.04 | 24.49 |
| L-leucine | 23.68 ± 2.37 | 0.40 ± 0.03 | 14.97 ± 1.27 | 37.43 |
| Strains or Plasmids | Characteristic | Source |
|---|---|---|
| Strains | ||
| P. balearica | Wild type | Lab stock |
| B. megaterium ATCC 14581 | Wild type | Lab stock |
| E. coli DH5α | General cloning host | Tiangen Biotech |
| E. coli BL21(DE3) | General expression host | Tiangen Biotech |
| E. coli (pET28a-Pbleudh) | E. coli BL21(DE3) with plasmid pET28a-Pbleudh | This study |
| E. coli (pET28a-Pbleudh-Bmgdh) | E. coli BL21(DE3) with plasmid pET28a-Pbleudh-Bmgdh | This study |
| Plasmids | ||
| pET28a | Kmr; expression vector | Laboratory stock |
| pET28a-Pbleudh | Kmr; Pbleudh in pET28a | This study |
| pET28a-Pbleudh-Bmgdh | Kmr; Pbleudh and Bmgdh in pET28a | This study |
| Primers | Sequences | Source |
|---|---|---|
| P1 | CAGCAAATGGGTCGCGGATCCATGGCAGGGCGCCAGACCA | BamHI |
| P2 | CTCGAGTGCGGCCGCAAGCTTTCAGGCGTGCAAGCGCAGG | HindIII |
| P3 | CAGCAAATGGGTCGCGGATCCATGGCAGGGCGCCAGACCA | BamHI |
| P4 | TATATCTCCTTTTTAACTCAGGCGTGCAAGCGCAG | RBS |
| P5 | GTTAAAAAGGAGATATAATGTATAAAGATTTAGAAGG | RBS |
| P6 | CTCGAGTGCGGCCGCAAGCTTTTATCCGCGTCCTGCTTGG | HindIII |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Chen, D.; Xiong, Q.; Liang, M.; Li, P.; Gong, Z.; Qiu, J.; Zhang, L. Characterization of a New Marine Leucine Dehydrogenase from Pseudomonas balearica and Its Application for L-tert-Leucine Production. Catalysts 2022, 12, 971. https://doi.org/10.3390/catal12090971
Guo Z, Chen D, Xiong Q, Liang M, Li P, Gong Z, Qiu J, Zhang L. Characterization of a New Marine Leucine Dehydrogenase from Pseudomonas balearica and Its Application for L-tert-Leucine Production. Catalysts. 2022; 12(9):971. https://doi.org/10.3390/catal12090971
Chicago/Turabian StyleGuo, Zewang, Denghui Chen, Qi Xiong, Miao Liang, Pengfei Li, Zehui Gong, Junzhi Qiu, and Liaoyuan Zhang. 2022. "Characterization of a New Marine Leucine Dehydrogenase from Pseudomonas balearica and Its Application for L-tert-Leucine Production" Catalysts 12, no. 9: 971. https://doi.org/10.3390/catal12090971
APA StyleGuo, Z., Chen, D., Xiong, Q., Liang, M., Li, P., Gong, Z., Qiu, J., & Zhang, L. (2022). Characterization of a New Marine Leucine Dehydrogenase from Pseudomonas balearica and Its Application for L-tert-Leucine Production. Catalysts, 12(9), 971. https://doi.org/10.3390/catal12090971






