Modified Magnesium Alkyls for Ziegler–Natta Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Viscosity of Magnesium Alkyls
2.2. Effect of Heterocumulens on Viscosity
2.3. DFT Calculations
2.4. Ziegler–Natta Catalyst Synthesis and Polymerization Experiments
3. Materials and Methods
3.1. Magnesium Alkyl Modification
3.2. DFT-Calculations
3.3. Zieger–Natta Catalyst Preparation
3.4. Ethylen Polymerization Experiments
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics Europe: Plastics—The Facts 2020. Available online: https://plasticseurope.org/de/wp-content/uploads/sites/3/2021/11/Plastics_the_facts-WEB-2020_versionJun21_final.pdf (accessed on 27 June 2022).
- Burdett, I.D.; Eisinger, R.S. Ethylene Polymerization Processes and Manufacture of Polyethylene. In Handbook of Industrial Polyethylene and Technology; Spaldinger, M.A., Chatterjee, A.M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 61–103. [Google Scholar] [CrossRef]
- Jeremic, D. Polyethylene. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2014; pp. 1–34. [Google Scholar] [CrossRef]
- Galli, P.; Vecellio, G. Technology: Driving force behind innovation and growth of polyolefins. Prog. Polym. Sci. 2001, 26, 1287–1336. [Google Scholar] [CrossRef]
- Olabisi, O. Polyolefins. In Handbook of Thermoplastics, 2nd ed.; Olabisi, O., Adewale, K., Eds.; CRC Press, Taylor & Francsis Group: New York, NY, USA, 2015; pp. 1–52. [Google Scholar] [CrossRef]
- Gahleitner, M.; Resconi, L.; Doshev, P. Heterogenous Ziegler-Natta, metallocene, and post-metallocene catalysis: Successes and challenges in industrial applications. MRS Bull. 2013, 38, 229–233. [Google Scholar] [CrossRef]
- Malpass, D.B. Ziegler-Natta Catalysts. In Introduction to Industrial Polyethylene, 1st ed.; John Wiley & Son, Inc.: Hoboken, NJ, USA; Scrivener Publishing LCC: Salem, MA, USA, 2010; Chapter 3; pp. 33–44. [Google Scholar]
- Malpass, D.B.; Band, E.I. Ziegler-Natta Catalysts. In Introduction to Industrial Polypropylene, 1st ed.; John Wiley & Son, Inc.: Hoboken, NJ, USA; Scrivener Publishing LCC: Salem, MA, USA, 2012; Chapter 3; pp. 59–73. [Google Scholar]
- Gahleitner, M.; Paulik, C. Polypropylene. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH Co., KGaA: Weinheim, Germany, 2014; Chapter 5; pp. 13–18. [Google Scholar]
- Malpass, D.B.; Band, E.I. Ziegler-Natta Catalysts. In Introduction to Industrial Polypropylene, 1st ed.; John Wiley & Son, Inc.: Hoboken, NJ, USA; Scrivener Publishing LCC: Salem, MA, USA, 2012; Chapter 4; pp. 75–105. [Google Scholar]
- Gahleitner, M.; Paulik, C. Polypropylene. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH Co., KGaA: Weinheim, Germany, 2014; Chapter 3; pp. 6–10. [Google Scholar]
- Albizzati, E.; Cecchin, G.; Chadwick, J.C.; Collina, G.; Giannini, U.; Morini, G.; Noristi, L. Catalyst in Polymerization. In Polypropylene Handbook, 2nd ed.; Pasquini, N., Ed.; Hanser Publishers: Munich, Germany, 2005; Chapter 2; pp. 15–106. [Google Scholar]
- Severen, R. Recent Developments in Supported Polyolefin Catalysis: A Review in Multimoldal Polymers with Supported Catalysts; Albunida, A., Prades, F., Jeremic, D., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; Chapter 1; pp. 1–57. [Google Scholar]
- Soares, J.B.P.; McKenna, T.F.L. Polyolefin Reaction Engineering; Wiley-VCH: Weihnheim, Germany, 2012; pp. 53–86. ISBN 978-3-527-31710-3. [Google Scholar]
- Pokasmermsong, P.; Praserthdam, P. Comparision of activity of Ziegler-Natta catalysts. Eng. J. 2009, 13, 57–64. [Google Scholar] [CrossRef]
- Garoff, T.; Leinonen, T.; Ala-Huikku, S. Catalyst Component Comprising Magnesium, Titanium, a Halogen and an Electron Donor, Its Preparation and Use. U.S. Patent 6,420,499 B1, 16 July 2002. [Google Scholar]
- Forte, M.C.; Coutinho, F.M.B. Highly active magnesium chloride supported Ziegler-Natta catalysts with controlled morphology. Eur. Poly. J. 1996, 32, 223–231. [Google Scholar] [CrossRef]
- Klaue, A.; Kruck, M.; Friederichs, N.; Bertola, F.; Wu, H.; Morbidelli, M. Insight into the process of an industrial Ziegler-Natta catalyst. Ind. Eng. Chem. Res. 2019, 58, 886–896. [Google Scholar] [CrossRef]
- Piovano, A.; Wada, T.; Amodio, A.; Takasao, G.; Ikeda, T.; Zhu, D.; Terano, M.; Chammingkwan, P.; Groppo, E.; Taniike, T. Formation of Highly Active Ziegler-Natta Catalysts Clarified by a Multifaceted Characterization Approach. ACS Catal. 2021, 11, 13782–13796. [Google Scholar] [CrossRef]
- Wada, T.; Funako, T.; Chammingkwan, P.; Thakur, A.; Matta, A.; Terano, M.; Taniike, T. Structure-performance relationship of Mg(OEt)2-based Ziegler-Natta catalysts. J. Catal. 2020, 389, 525–532. [Google Scholar] [CrossRef]
- Potapov, A.G. Titanium-magnesium Ziegler-Natta catalysts: New insight on the active sites precursor. Mol. Catal. 2017, 432, 155–159. [Google Scholar] [CrossRef]
- Kolthammer, B.W.S.M. Ziegler-Natta Catalysis. In Handbook of Grignard Reagents; Silvermann, G.S., Rakita, P.E., Eds.; Mercel Dekker, Inc.: New York, NY, USA, 1996; pp. 677–683. [Google Scholar]
- Aigner, P.; Averina, E.; Garoff, T.; Paulik, C. Effects of Alterations to Ziegler-Natta Catalysts on Kinetics and Comonomer (1-Butene) Incorporation. Macromol. React. Eng. 2017, 11, 1700009. [Google Scholar] [CrossRef]
- Garoff, T.; Waldvogel, O.; Personen, K. Method for the Preparation of Olefin Polymerization Catalysts Support and an Olefin Polymerization Catalyst. U.S. Patent 7,432,220 B2, 7 October 2008. [Google Scholar]
- Rönkkö, H.L.; Knuuttila, H.; Denifl, P.; Leinonen, T.; Venäläinen, T. Structural studies on a solid self-supported Ziegler-Natta type catalyst for propylene polymerization. J. Mol. Catal. A Chem. 2007, 278, 127–134. [Google Scholar] [CrossRef]
- Leinonen, T.; Denifl, P. Prepartation of Olefin Polymerization Catalyst Component. U.S. Patent EP 1,273,595 A1, 8 January 2003. [Google Scholar]
- De Vries, S.M. Process for the Preparation of a Dialkyl Magnesium Compound. U.S. Patent 3,737,393, 5 June 1973. [Google Scholar]
- Sanchez, R.; Fannin, L.W.; Malpass, D.B. Hydrocarbon Soluble Magnesium Compositions of High Magnesium Content. U.S. Patent 4,207,207, 10 June 1980. [Google Scholar]
- Fannin, L.W.; Malpass, D.B. Hydrogen Soluble Straight-Chain Di-(Lower Alkyl) Magnesium Compositions. U.S. Patent 4,127,507, 28 November 1978. [Google Scholar]
- Sakurai, H.; Katayama, Y.; Ikegami, T.; Tsuyama, S. Hydrocarbon-Soluble Diorganomagnesium Compounds, Hydrocarbon Solutions Containing the Same and Processes for Preparation Thereof. U.S. Patent 4,342,708, 3 August 1982. [Google Scholar]
- Saulys, D.A.; Hill, E.A. Beryllium & magnesium: Organometallic chemistry. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Wiley: Hoboken, NJ, USA, 2011; pp. 1–27. [Google Scholar] [CrossRef]
- Kamienski, C.W.; Eastham, J.F. Diorganomagnesium Reagents and Methods of Preparing Same. U.S. Patent 3,646,231A, 29 February 1972. [Google Scholar]
- Kobetz, P.; Pinkerton, R.C. Manufacture of Magnesium Organo-Compounds. U.S. Patent 3,028,319, 3 April 1962. [Google Scholar]
- Kamienski, C.W.; McElroy, B.J.; Bach, R.O. Stable Diorganomagnesium Compounds. U.S. Patent 4,069,267, 17 January 1978. [Google Scholar]
- Screttas, C.G.; Micha-Screttas, M. Preparation of solvated and/or unsolvated simple and mixed diarylmagnesiums. J. Organomet. Chem. 1985, 292, 325–333. [Google Scholar] [CrossRef]
- Weiss, E. Die Kristallstrukturen des dimethylmagnesiums. J. Organomet. Chem. 1964, 2, 314–321. [Google Scholar] [CrossRef]
- Hoff, R.; Mathers, R.T. Commercially Available Metal Alkyls and Their Use in Polyolefin Catalysts. In Transition Metal Polymerization Catalysts, 1st ed.; Malpass, D.B., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 1–28. ISBN 13:978-0470137987. [Google Scholar]
- Stuhl, C.; Anwander, R. Dimethylmagnesium revisted. Dalton Trans. 2018, 47, 12546–12552. [Google Scholar] [CrossRef]
- Holloway, C.E.; Melnik, M. Mangesium compounds: Classification and analysis of crystallographic and structural data. J. Organomet. Chem. 1994, 465, 1–63. [Google Scholar] [CrossRef]
- Jones, P.D.; Malpass, D.B.; Smith, G.M. Viscosity Reduction of Organomagnesium Solutions. U.S. Patent 5,910,270, 8 June 1999. [Google Scholar]
- Webb, D.W.; Malpass, D.B. Organomagnesium solutions of low viscosity. U.S. Patent 4,547,477, 15 October 1985. [Google Scholar]
- Fannin, L.W.; Malpass, D.B.; Sanchez, R. Organomagnesium Solutions of Low Viscosity. U.S. Patent 4,299,781, 10 November 1981. [Google Scholar]
- Anga, S.; Bhattacharjee, J.; Harinath, A.; Panda, T.K. Synthesis, structure and reactivity study of magnesium amidinato complexes derived from carbodiimides and N,N′-bis(2,6-diisopropylphenyl)-1,4-diaza-butadiene ligands. Dalton Trans. 2015, 44, 955–965. [Google Scholar] [CrossRef]
- Chlupaty, T.; Bilek, M.; Merna, J.; Brus, J.; Ruzickova, Z.; Strassner, T.; Ruzicka, A. The addition of Grignard reagents to carbodiimides. The synthesis, structure and potential utilization of magnesium amidinates. Dalton Trans. 2019, 48, 5335–5342. [Google Scholar] [CrossRef]
- Kamienski, C.W.; Dover, B.T. Low Viscosity Hydrocarbon Solution of Dialkylmagnesium Compounds. U.S. Patent US5145600A, 8 September 1992. [Google Scholar]
- Morrison, R.C.; Rathman, T.L. Preparation of Organometallic and Organobimetallic Compounds. U.S. Patent 4,976,886, 11 December 1990. [Google Scholar]
- Becker, H.G.O.; Domschke, G.; Fanghänel, E.; Fischer, M.; Gewald, K.; Mayer, R.; Pavel, D.; Schmidt, H.; Schwetlick, K. Organikum, 17th ed.; VEB Deutscher Verlag der Wissenschaften: Berlin, Germany, 1988; pp. 481–497. [Google Scholar]
- Spence, M.W.; Plehiers, P.M. A brief overview of properties and reactions of diisocyanates. Toxicol. Ind. Health 2022. [Google Scholar] [CrossRef]
- Ribeiro, R.; Ruivo, R.; Nsiri, H.; Norsic, S.; D’Agosto, F.; Perrin, L.; Boisson, C. Deciphering the mechanism of coordinative chain transfer polymerization of ethylene using neodymocene catalysts and dialkylmagnesium. ACS Catal. 2016, 6, 851–860. [Google Scholar] [CrossRef]
- Redzic, E.; Garoff, T.; Mardare, C.C.; List, M.; Hesser, G.; Mayrhofer, L.; Hassel, A.W.; Paulik, C. Heterogeneous Ziegler-Natta catalysts with various size of MgCl2, crystallites: Synthesis and characterization. Iran Polym. J. 2016, 25, 321–337. [Google Scholar] [CrossRef]
- Göpperl, L.; Pernusch, D.; Schwarz, J.; Paulik, C. Impact of polymerization process parameters on improved comonomer imcorporation behavior in Ziegler-Natta catalyses. Macromol. React. Eng. 2022, 16, 2100042. [Google Scholar] [CrossRef]
- Piovano, A.; Signorile, M.; Braglia, L.; Torelli, P.; Martini, A.; Wada, T.; Takasao, G.; Taniike, T.; Groppo, E. Electronic properties of Ti sites in Ziegler-Natta catalysts. ACS Catal. 2021, 11, 9949–9961. [Google Scholar] [CrossRef]
- Kashiwa, N.; Yoshitake, J. The influence of the valence state of titanium in MgCl2-supported titanium catalysts on olefin polymerization. Makromol. Chem. 1984, 185, 1133–1138. [Google Scholar] [CrossRef]
- Chien, J.C.M.; Weber, S.; Hu, Y. Magnesium chloride supported catalysts for polymerization. XIX. Titanium oxidation states, catalyst deactivation, and active site structure. J. Polym. Sci. Part A Polym. Chem. 1989, 27, 1499–1514. [Google Scholar] [CrossRef]
- Fregonese, D.; Mortara, S.; Bresadola, S. Ziegler-Natta MgCl2-supported catalysts: Relationship between titanium oxidation states distribution and activity in olefin polymerization. J. Mol. Catal. A Chem. 2001, 172, 89–95. [Google Scholar] [CrossRef]
- Peacock, A.J. Handbook of Polyethylene: Structure, Properties, and Applications, 1st ed.; Marcel Dekker: New York, NY, USA, 2000; pp. 54–56. [Google Scholar]
- Chumachenko, N.N.; Zakharov, V.A.; Bukatov, G.D.; Sergeev, S.A. A study of the formation process of titanium-magnesium catalysts for propylene polymerization. Appl. Catal. A Gen. 2014, 469, 512–516. [Google Scholar] [CrossRef]
- Kaminsky, W.; Sinn, H. The influence of crystal water on the performance of a Ziegler-Natta catalyst in propylene polymerization. In Transition Metals and Organometallics as Catalysts for Olefin Polymerization, 1st ed.; Garoff, T., Iiskola, E., Sormunen, P., Eds.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 197–208. [Google Scholar]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108-1–224108-18. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Tao, J.; Perdew, J.P.; Staroverov, V.N.; Scuseria, G.E. Climbing the Density Functional Ladder: Nonempirical Meta-Generalized Gradient Approximation Designed for Molecules and Solids. Phys. Rev. Lett. 2003, 91, 146401. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Grimme, S.; Goerigk, L. Efficient and Accurate Double-Hybrid-Meta-GGA Density Functionals—Evaluation with the Extended GMTKN30 Database for General Main Group Thermochemistry, Kinetics, and Noncovalent Interactions. J. Chem. Theory Comput. 2011, 7, 291–309. [Google Scholar] [CrossRef]
- Pernusch, D.C.; Spiegel, G.; Paulik, C.; Hofer, W. Influence of Poisons Originating from Chemically Recycled Plastic Waste on the Performance of Ziegler-Natta Catalysts. Macromol. React. Eng. 2022, 16, 2100020. [Google Scholar] [CrossRef]
- Ruff, M.; Paulik, C. Controlling Polyolefin Properties by In-Reactor Blending, 1-Polymerization Process, Precise Kinetics, and Molecular Properties of UHMW-PE Polymers. Macromol. React. Eng. 2012, 6, 302–317. [Google Scholar] [CrossRef]
- Holtrichter-Rößmann, T.; Liedtke, C.; Schwarz, J.F. Novel Organo-Magnesium Compounds and Their Use. WO 2021/233930 A1, 18 May 2021. [Google Scholar]
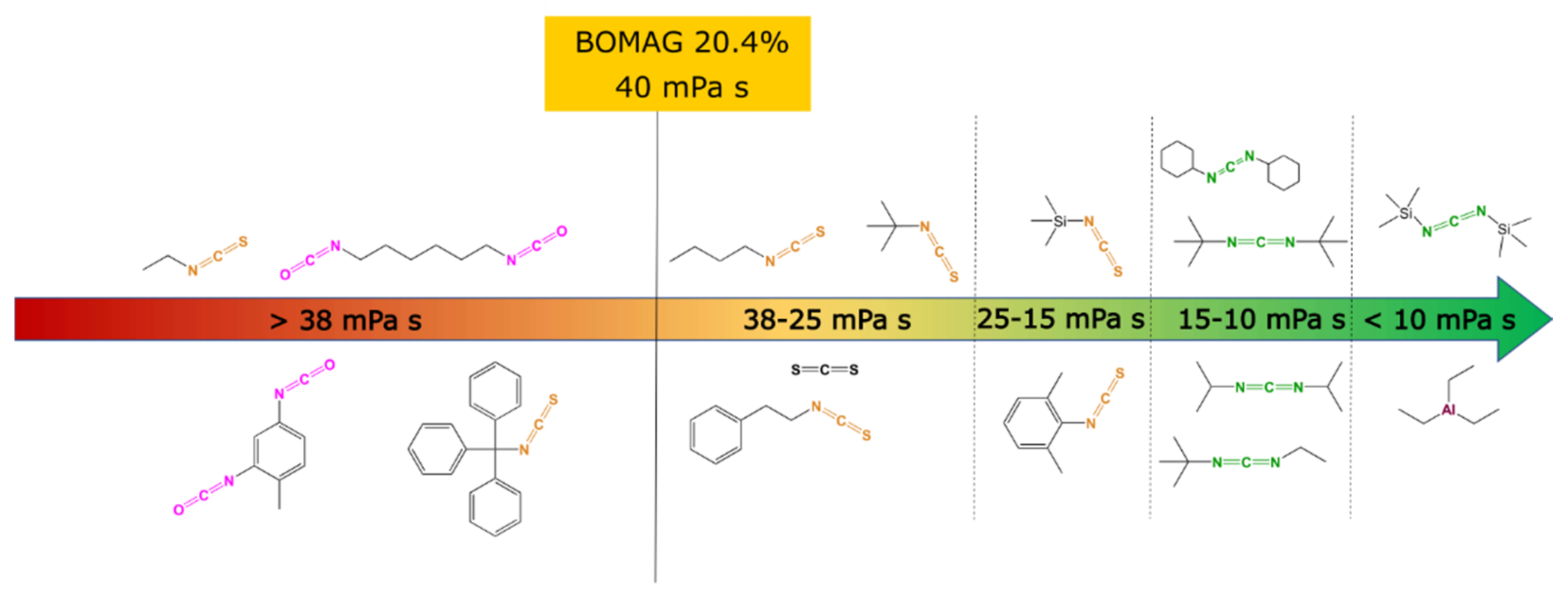

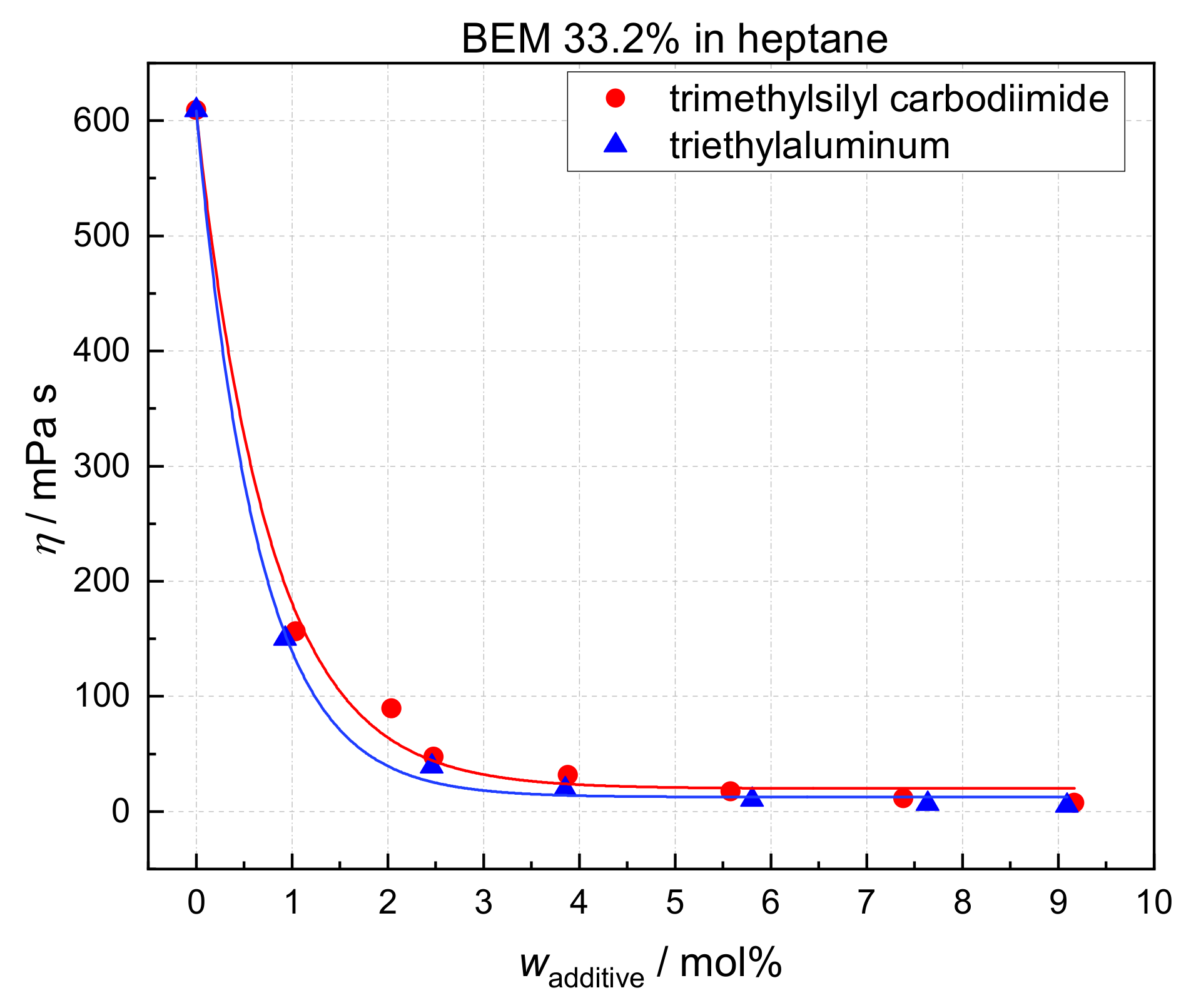
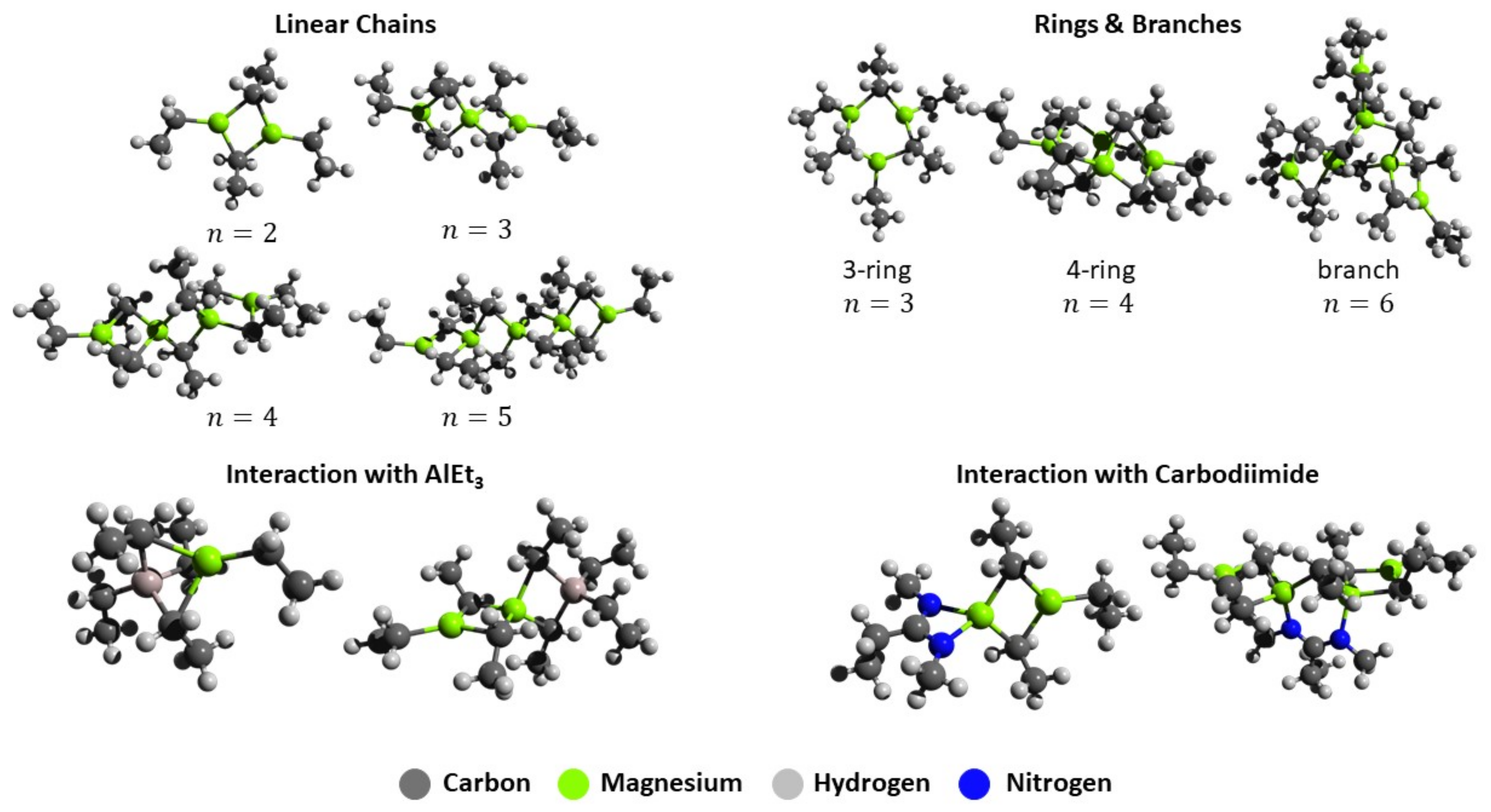
| Entry | Alkyl | wMg (wt%) | wAlkyl (wt%) | wAl (ppm) | Solvent | η (mPa·s) |
|---|---|---|---|---|---|---|
| 1 | BEM | 4.39 | 20.0 | 901 | Heptane 1 | 61.8 |
| 2 | BEM | 4.32 | 19.6 | 900 | toluene | 127.8 |
| 3 | BOMAG | 2.97 | 20.4 | 780 | Heptane 1 | 40.2 |
| 4 | BOMAG | 2.82 | 19.3 | 600 | toluene | 68.0 |
| 5 | di-octyl magnesium | 1.93 | 19.9 | 560 | Heptane 1 | 15.0 |
| 6 | n-butyl-sec-butyl magnesium | 5.56 | 31.7 | 17 | n-hexane | 2.4 |
| Entry | Alkyl | Additive | Solvent | wAdditive (mol%) | wAlkyl (wt%) | wMg (wt%) | wAl (ppm) | η (mPa·s) |
|---|---|---|---|---|---|---|---|---|
| 1 | BEM | - | toluene | - | 19.64 | 4.32 | 900 | 127.8 |
| 2 | BEM | triethylaluminum | toluene | 2.67 | 19.64 | 4.32 | 900 | 14.0 |
| 3 | BEM | trimethylsilyl carbodiimide | toluene | 2.44 | 19.64 | 4.32 | 900 | 18.3 |
| 4 | BOMAG | - | toluene | - | 19.31 | 2.82 | 600 | 68.0 |
| 5 | BOMAG | triethylaluminum | toluene | 2.72 | 19.31 | 2.82 | 600 | 9.5 |
| 6 | BOMAG | trimethylsilyl carbodiimide | toluene | 2.75 | 19.31 | 2.82 | 600 | 11.8 |
| 7 | BEM | - | heptane 1 | - | 19.95 | 4.39 | 901 | 61.8 |
| 8 | BEM | triethylaluminum | heptane 1 | 2.51 | 18.95 | 4.17 | 856 | 7.23 |
| 9 | BEM | trimethylsilyl carbodiimide | heptane 1 | 2.48 | 20.01 | 4.40 | 903 | 9.68 |
| 10 | BOMAG | - | heptane 1 | - | 20.41 | 2.97 | 780 | 40.20 |
| 11 | BOMAG | triethylaluminum | heptane 1 | 2.53 | 20.02 | 2.91 | 738 | 6.33 |
| 12 | BOMAG | trimethylsilyl carbodiimide | heptane 1 | 2.52 | 19.91 | 2.91 | 734 | 7.62 |
| Methyl | Ethyl | Butyl | n-Butyl sec-Butyl | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | n | ΔE/n | ΔG/n | ΔE/n | ΔG/n | ΔE/n | ΔG/n | ΔE/n | ΔG/n |
| Linear Chains | |||||||||
| 1 | 2 | −8.7 | −2.5 | −9.7 | −3.4 | −10.6 | −2.4 | −7.7 | −0.6 |
| 2 | 3 | −13.2 | −5.5 | −14.7 | −5.2 | −15.8 | −5.3 | - | - |
| 3 | 4 | −15.2 | −5.9 | −17.1 | −6.3 | −18.5 | −6.5 | - | - |
| 4 | 5 | −16.2 | −5.9 | −17.6 | −5.4 | −19.8 | −7.0 | −15.5 | −2.6 |
| Rings and Branches | |||||||||
| 5 | 3 | −7.6 | 1.0 | −9.9 | 0.1 | −10.8 | 0.3 | −11.0 | −0.3 |
| 6 | 4 | −15.2 | −5.9 | −16.3 | −5.4 | −17.9 | −6.0 | −16.6 | −4.5 |
| 7 | 5 | −15.4 | −4.9 | −17.5 | −5.2 | −19.9 | −6.1 | −15.4 | −1.2 |
| Methyl | Ethyl | Butyl | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4-Ring | 6-Ring | 4-Ring | 6-Ring | 4-Ring | 6-Ring | ||||||||
| Entry | Carbodiimide | ΔE | ΔG | ΔE | ΔG | ΔE | ΔG | ΔE | ΔG | ΔE | ΔG | ΔE | ΔG |
| 1 | Me | −76.5 | −50.9 | −118.7 | −68.3 | −85.0 | −54.7 | −138.8 | −76.2 | −83.8 | −56.1 | −132.5 | −73.7 |
| 2 | Et | −75.8 | −51.1 | −116.9 | −64.2 | −85.7 | −127.3 | - | - | −83.6 | −53.9 | −132.7 | −73.9 |
| 3 | Si(Me)3 | −61.0 | −35.1 | −101.2 | −48.1 | −71.9 | −39.7 | −123.2 | −59.0 | −70.1 | −40.3 | −116.3 | −56.7 |
| 4 | Cyclohexyl | −72.0 | −45.1 | −119.7 | −67.1 | −81.1 | −48.6 | −140.2 | −75.1 | −79.4 | −48.8 | - | - |
| Entry | ΔE | ΔG | ΔE | ΔG | |
|---|---|---|---|---|---|
| R | (MgR2) (AlEt3) | (MgR2)2 (AlMe3) | |||
| 1 | Methyl | −21.5 | −8.9 | −16.7 | 9.5 |
| 2 | Ethyl | −25.1 | −10.4 | −48.4 | −18.9 |
| 3 | Butyl | −27.3 | −11.4 | −53.0 | −20.9 |
| Entry | Additive | wAdditive (wt%) | wTi (wt%) | Rp (kgPE·gCat−1·h−1) | Rp,Ti (kgPE·gTi−1·h−1) |
|---|---|---|---|---|---|
| 1 | no additive | 0 | 7.4 | 25.4 ± 0.982 | 343.5 ± 13.26 |
| 2 | trimethylsilyl carbodiimide | 2.57 | 10.83 | 33.1 ± 0.006 | 305.1 ± 0.057 |
| 3 | tert-butyl carbodiimide | 2.63 | 7.62 | 26.5 ± 0.460 | 347.3 ± 6.034 |
| 4 | trimethylsilyl isothiocyanate | 2.48 | 11.06 | 24.6 ± 3.317 | 222.9 ± 29.99 |
| 5 | tert-butyl isothiocyanate | 2.78 | 7.85 | 24.3 ± 1.639 | 309.8 ± 20.89 |
| Entry | Additive | Mn (g·mol−1) | Mw (g·mol−1) | Ð |
|---|---|---|---|---|
| 1 | no additive | 35,900 ± 4680 | 234,333 ± 27,107 | 6.5 ± 0.13 |
| 2 | trimethylsilyl carbodiimide | 37,200 ± 282 | 227,150 ± 899 | 6.1 ± 0.12 |
| 3 | tert-butyl carbodiimide | 35,500 ± 5939 | 214,150 ± 316 | 6.0 ± 0.13 |
| 4 | trimethylsilyl isothiocyanate | 39,533 ± 7182 | 241,433 ± 44,831 | 6.1 ± 0.03 |
| 5 | tert-butyl isothiocyanate | 49,600 ± 0 | 281,950 ± 24,745 | 5.7 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarz, J.F.; Holtrichter-Rößmann, T.; Liedtke, C.G.; Diddens, D.; Paulik, C. Modified Magnesium Alkyls for Ziegler–Natta Catalysts. Catalysts 2022, 12, 973. https://doi.org/10.3390/catal12090973
Schwarz JF, Holtrichter-Rößmann T, Liedtke CG, Diddens D, Paulik C. Modified Magnesium Alkyls for Ziegler–Natta Catalysts. Catalysts. 2022; 12(9):973. https://doi.org/10.3390/catal12090973
Chicago/Turabian StyleSchwarz, Julia Felicitas, Thorsten Holtrichter-Rößmann, Claus Günter Liedtke, Diddo Diddens, and Christian Paulik. 2022. "Modified Magnesium Alkyls for Ziegler–Natta Catalysts" Catalysts 12, no. 9: 973. https://doi.org/10.3390/catal12090973
APA StyleSchwarz, J. F., Holtrichter-Rößmann, T., Liedtke, C. G., Diddens, D., & Paulik, C. (2022). Modified Magnesium Alkyls for Ziegler–Natta Catalysts. Catalysts, 12(9), 973. https://doi.org/10.3390/catal12090973








