Synthesis of Mn-Doped ZnO Nanoparticles and Their Application in the Transesterification of Castor Oil
Abstract
:1. Introduction
2. Results and Discussion
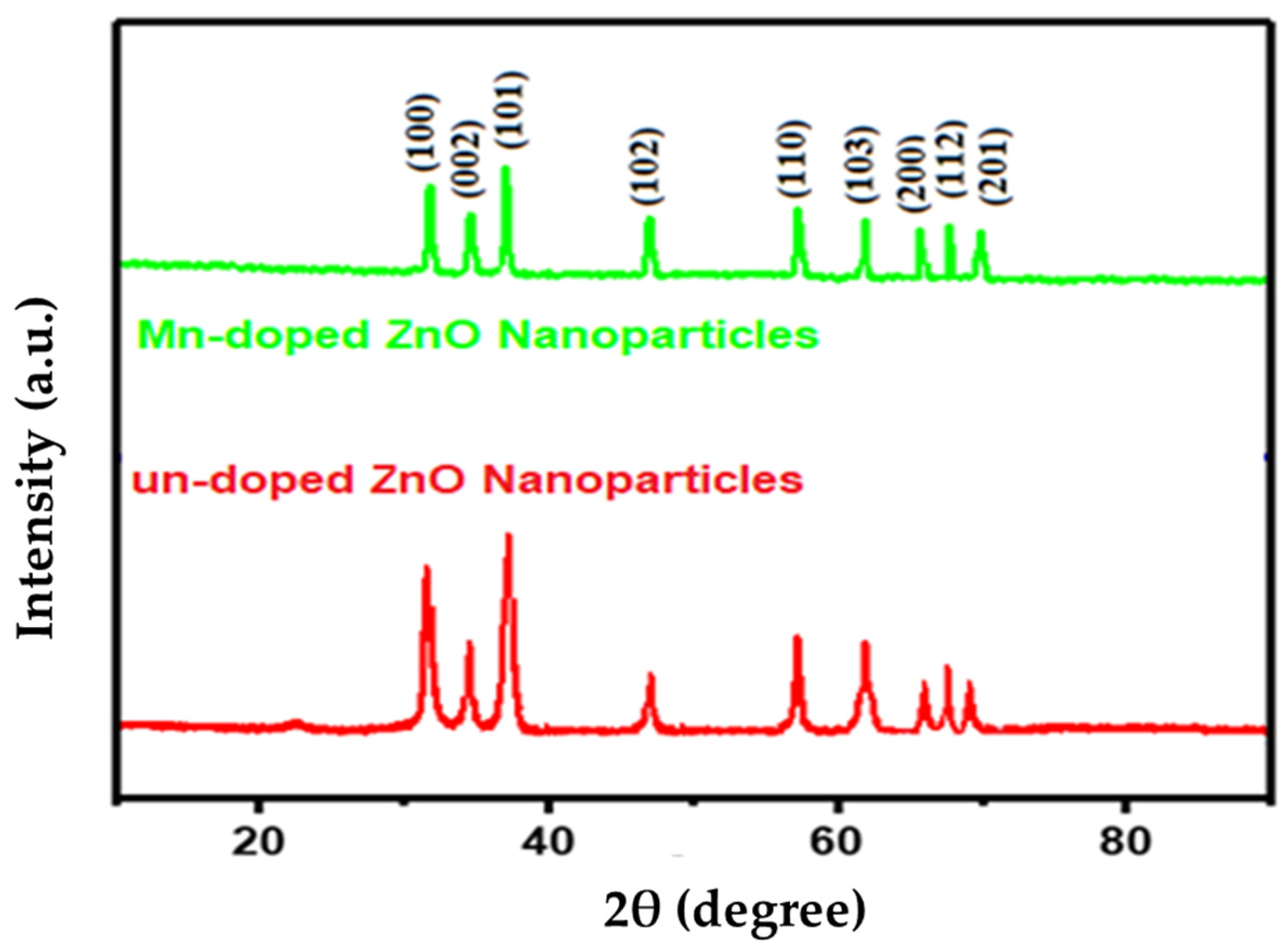
2.1. XRD Analysis
2.2. TEM and EDX Analysis
2.3. Physicochemical Analysis of Biodiesel
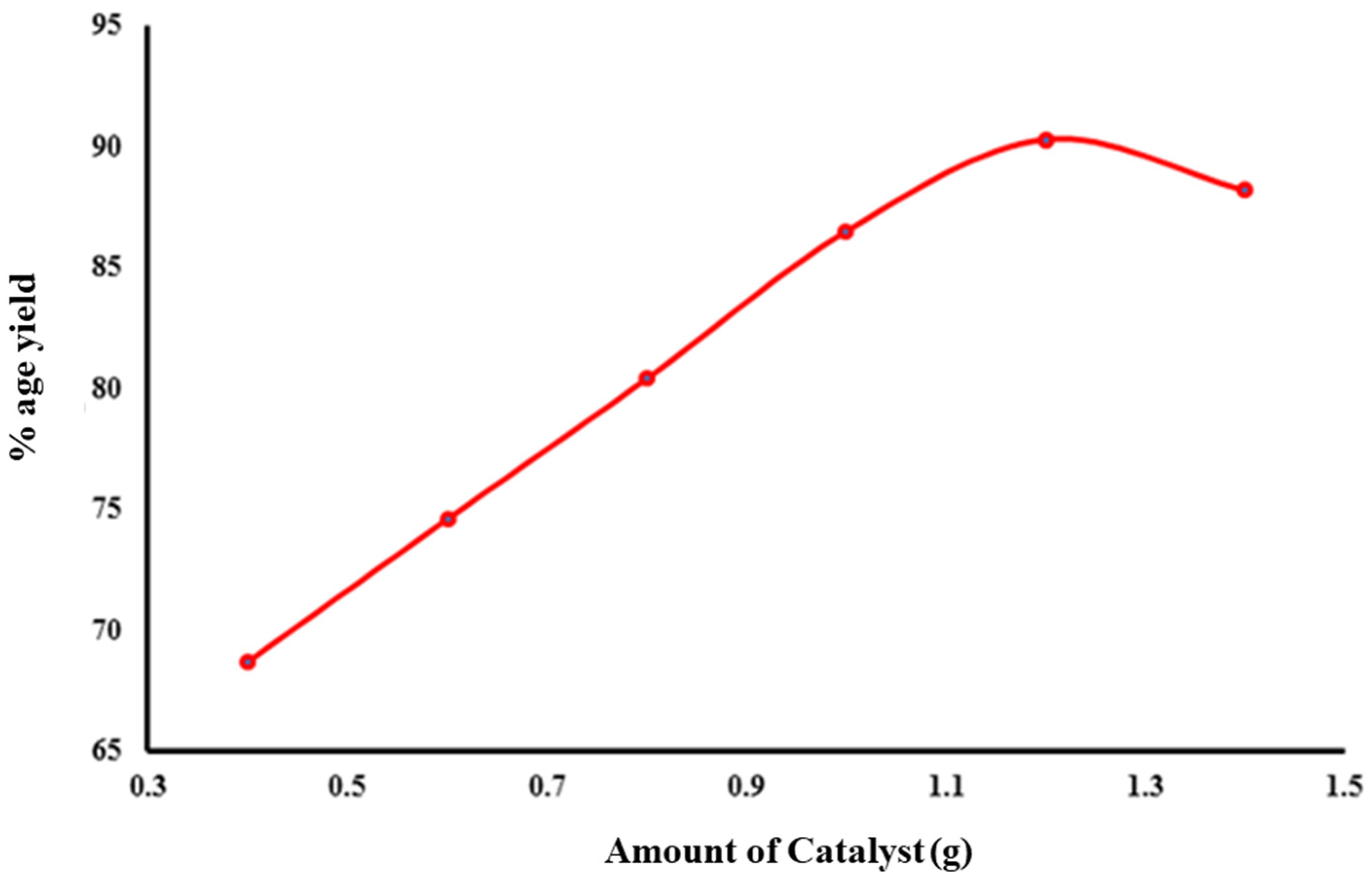
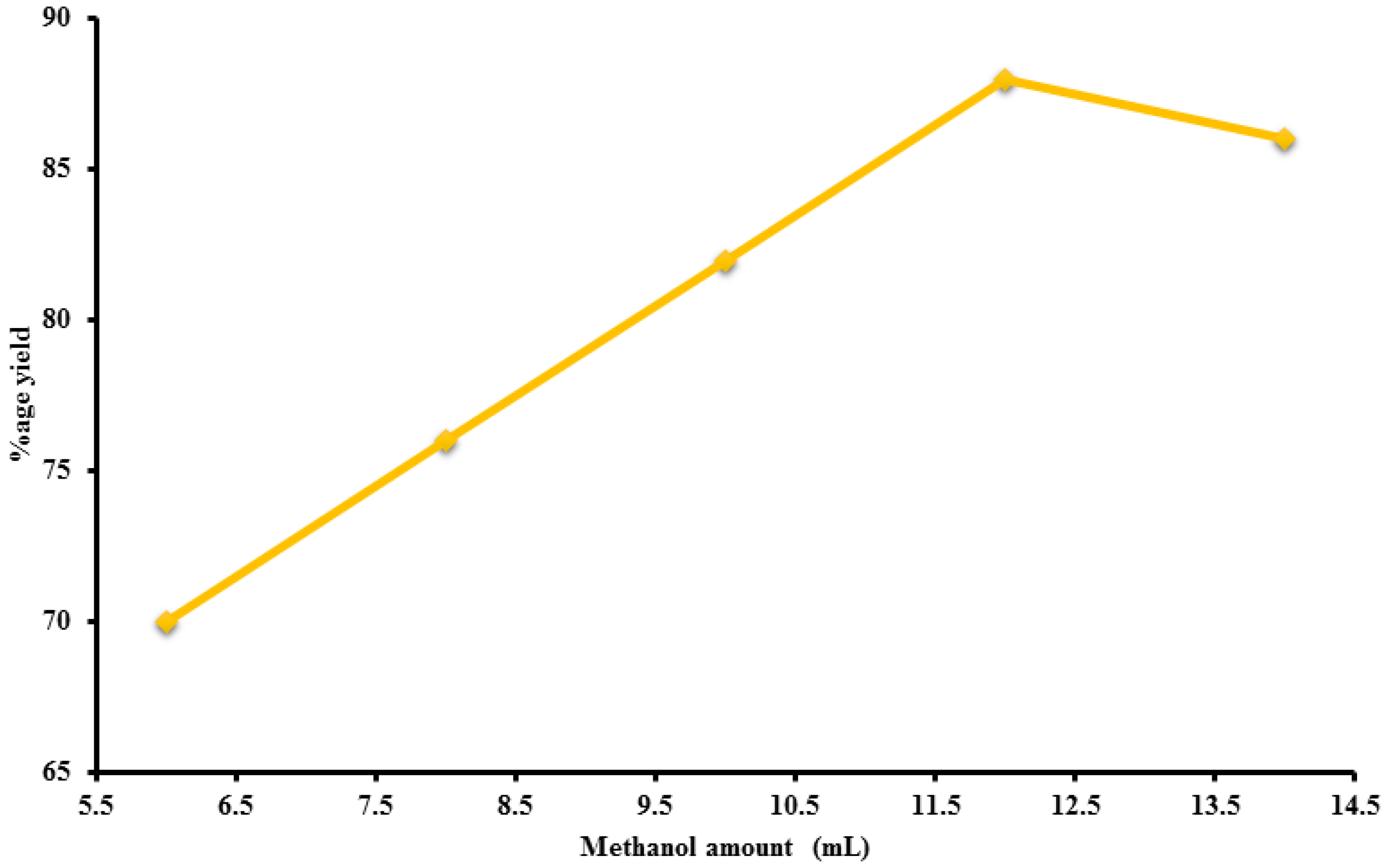
2.4. Factors Effecting Yield of Biodiesel
2.4.1. Amount of Catalyst
2.4.2. Amount of Methanol
2.4.3. Reaction Temperature
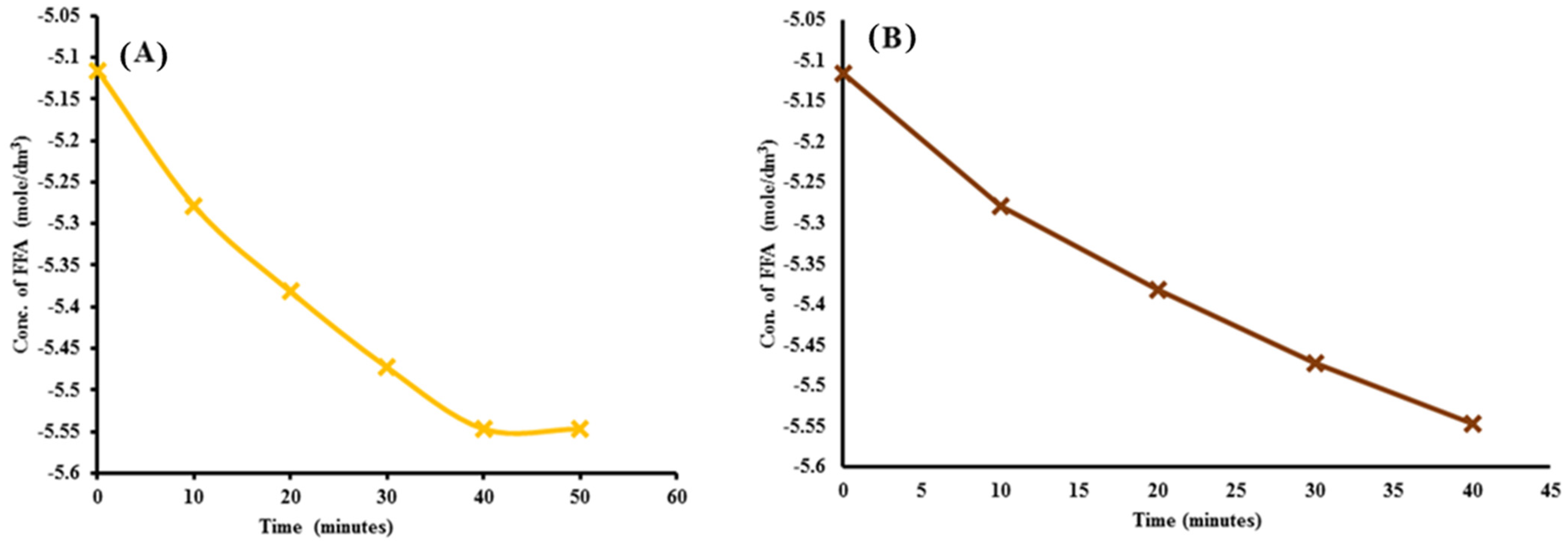
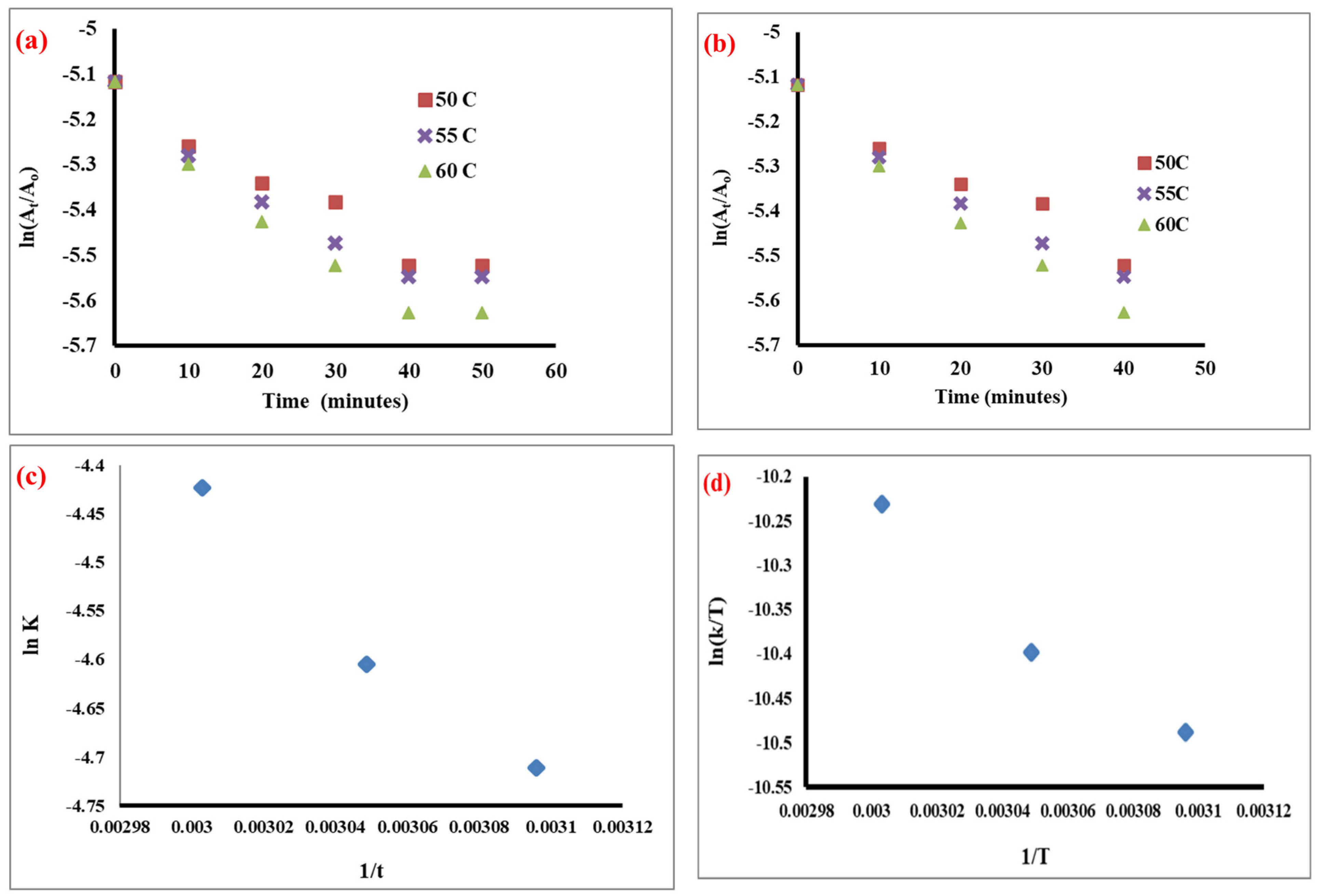
2.5. Kinetics of Transesterification Reaction
2.5.1. Pseudo-First-Order Reaction
2.5.2. Rate Constant of the Transesterification Reaction
2.6. Activation Energy
3. Materials and Methods
3.1. Required Chemicals
3.2. Preparation of Catalyst
3.3. Characterization
3.4. Transesterification Process
3.5. Physicochemical Studies of Biodiesel
3.6. Kinetic Study of Transesterification Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahmudul, H.; Hagos, F.Y.; Mamat, R.; Adam, A.A.; Ishak, W.F.W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines—A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Biogas as a renewable energy source—A review. Energy Sources Part A 2009, 31, 1280–1293. [Google Scholar] [CrossRef]
- Chaudhary, P.; Kumar, B.; Kumar, S.; Gupta, V.K. Transesterification of Castor Oil with Methanol–Kinetic Modelling. Chem. Prod. Process Model. 2015, 10, 71–80. [Google Scholar] [CrossRef]
- Karmakar, B.; Dhawane, S.H.; Halder, G. Optimization of biodiesel production from castor oil by Taguchi design. J. Environ. Chem. Eng. 2018, 6, 2684–2695. [Google Scholar] [CrossRef]
- Yan, S.; Mohan, S.; DiMaggio, C.; Kim, M.; Ng, K.S.; Salley, S.O. Long term activity of modified ZnO nanoparticles for transesterification. Fuel 2010, 89, 2844–2852. [Google Scholar] [CrossRef]
- Soliman, M.M.; Karmakar, A.; Alegria, E.C.; Ribeir, A.P.; Rúbio, G.M.; Saraiva, M.S.; da Silva, M.F.C.G.; Pombeiro, A.J. ZnO nanoparticles: An efficient catalyst for transesterification reaction of α-keto carboxylic esters. Catal. Today 2020, 348, 72–79. [Google Scholar] [CrossRef]
- Wang, A.; Quan, W.; Zhang, H.; Li, H.; Yang, S. Heterogeneous ZnO-containing catalysts for efficient biodiesel production. RSC Adv. 2021, 11, 20465–20478. [Google Scholar] [CrossRef]
- Jayakumar, M.; Karmegam, N.; Gundupalli, M.P.; Gebeyehu, K.B.; Asfaw, B.T.; Chang, S.W.; Balasubramani, R.; Awasthi, M.K. Heterogeneous base catalysts: Synthesis and application for biodiesel production—A review. Bioresour. Technol. 2021, 331, 125054. [Google Scholar] [CrossRef]
- Deshmane, V.G.; Adewuyi, Y.G. Synthesis and kinetics of biodiesel formation via calcium methoxide base catalyzed transesterification reaction in the absence and presence of ultrasound. Fuel 2013, 107, 474–482. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Sun, X.; Wu, C.; Gao, Z. Kinetics studies of biodiesel production from waste cooking oil using FeCl3-modified resin as heterogeneous catalyst. Renew. Energy 2017, 107, 522–530. [Google Scholar] [CrossRef]
- Baskar, G.; Soumiya, S. Production of biodiesel from castor oil using iron (II) doped zinc oxide nanocatalyst. Renew. Energy 2016, 98, 101–107. [Google Scholar] [CrossRef]
- Mapossa, A.B.; Dantas, J.; Costa, A.C. Transesterification reaction for biodiesel production from soybean oil using Ni0. 5Zn0. 5Fe2O4 nanomagnetic catalyst: Kinetic study. Int. J. Energy Res. 2020, 44, 6674–6684. [Google Scholar] [CrossRef]
- Kumar, S.; Deswal, V. Optimization at low temperature transesterification biodiesel production from soybean oil methanolysis via response surface methodology. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 2284–2293. [Google Scholar] [CrossRef]
- Halek, F.; Delavari, A.; Kavousi-Rahim, A. Production of biodiesel as a renewable energy source from castor oil. Clean Technol. Environ. Policy 2013, 15, 1063–1068. [Google Scholar] [CrossRef]
- Baskar, G.; Gurugulladevi, A.; Nishanthini, T.; Aiswarya, R.; Tamilarasan, K. Optimization and kinetics of biodiesel production from Mahua oil using manganese doped zinc oxide nanocatalyst. Renew. Energy 2017, 103, 641–646. [Google Scholar] [CrossRef]
- Ambat, I.; Srivastava, V.; Sillanpää, M. Recent advancement in biodiesel production methodologies using various feedstock: A review. Renew. Sustain. Energy Rev. 2018, 90, 356–369. [Google Scholar] [CrossRef]
- Donkova, B.; Dimitrov, D.T.; Kostadinov, M.; Mitkova, E.; Mehandjiev, D. Catalytic and photocatalytic activity of lightly doped catalysts M: ZnO (M = Cu, Mn). Mater. Chem. Phys. 2010, 123, 563–568. [Google Scholar] [CrossRef]
- Muruganandham, M.; Wu, J. Synthesis, characterization and catalytic activity of easily recyclable zinc oxide nanobundles. Appl. Catal. B Environ. 2008, 80, 32–41. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A review of microwave synthesis of zinc oxide nanomaterials: Reactants, process parameters and morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar]
- Belkhaoui, C.; Lefi, R.; Mzabi, N.; Smaoui, H. Synthesis, optical and electrical properties of Mn doped ZnO nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 7020–7031. [Google Scholar] [CrossRef]
- Khalid, R.; Alhazaa, A.N.; Khan, M. Synthesis, characterization and properties of Mn-doped ZnO nanoparticles. Appl. Phys. A 2018, 124, 1–8. [Google Scholar] [CrossRef]
- Baskar, G.; Selvakumari, I.A.E.; Aiswarya, R. Biodiesel production from castor oil using heterogeneous Ni doped ZnO nanocatalyst. Bioresour. Technol. 2018, 250, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Birajdar, S.D.; Alange, R.; More, S.; Murumkar, V.; Jadhav, K. Sol-gel auto combustion synthesis, structural and magnetic properties of Mn doped ZnO nanoparticles. Procedia Manuf. 2018, 20, 174–180. [Google Scholar] [CrossRef]
- Bonifácio, M.A.R.; Lira, H.D.L.; Neiva, L.S.; Kiminami, R.H.G.A.; Gama, L. Nanoparticles of ZnO doped with Mn: Structural and morphological characteristics. Mater. Res. 2017, 20, 1044–1049. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, I. Comparative study of metal (Al, Mg, Ni, Cu and Ag) doped ZnO/g-C3N4 composites: Efficient photocatalysts for the degradation of organic pollutants. Separ. Purif. Technol. 2020, 251, 117372. [Google Scholar] [CrossRef]
- Shatnawi, M.; Alsmadi, A.; Bsoul, I.; Salameh, B.; Mathai, M.; Alnawashi, G.; Alzoubi, G.M.; Al-Dweri, F.; Bawa’Aneh, M. Influence of Mn doping on the magnetic and optical properties of ZnO nanocrystalline particles. Results Phys. 2016, 6, 1064–1071. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Singh, B.K.; Pal, B.N.; Pandey, P.C. Correlation between structural, optical and magnetic properties of Mn-doped ZnO. Appl. Phys. A 2016, 122, 740. [Google Scholar] [CrossRef]
- Shanmugam, N.; Suthakaran, S.; Kannadasan, N.; Kumar, K.S. Synthesis and characterization of Te doped ZnO nanosheets for photocatalytic application. J. Heterocycl. 2015, 105, 15–20. [Google Scholar] [CrossRef]
- Ahmad, A.; Wei, Y.; Syed, F.; Tahir, K.; Rehman, A.U.; Khan, A.; Ullah, S.; Yuan, Q. The effects of bacteria-nanoparticles interface on the antibacterial activity of green synthesized silver nanoparticles. Microb. Pathogen. 2017, 102, 133–142. [Google Scholar] [CrossRef]
- Kotresh, M.; Patil, M.; Inamdar, S. Reaction temperature based synthesis of ZnO nanoparticles using co-precipitation method: Detailed structural and optical characterization. Optik 2021, 243, 167506. [Google Scholar] [CrossRef]
- Sher, M.; Khan, S.A.; Shahid, S.; Javed, M.; Qamar, M.A.; Chinnathambi, A.; Almoallim, H.S. Synthesis of novel ternary hybrid g-C3N4@ Ag-ZnO nanocomposite with Z-scheme enhanced solar light-driven methylene blue degradation and antibacterial activities. J. Environ. Chem. Eng. 2021, 9, 105366. [Google Scholar] [CrossRef]
- Qamar, M.A.; Shahid, S.; Javed, M.; Sher, M.; Iqbal, S.; Bahadur, A.; Li, D. Fabricated novel g-C3N4/Mn doped ZnO nanocomposite as highly active photocatalyst for the disinfection of pathogens and degradation of the organic pollutants from wastewater under sunlight radiations. Colloid. Surf. A Physicochem. Eng. Asp. 2021, 611, 125863. [Google Scholar] [CrossRef]
- Zhang, L.; Sheng, B.; Xin, Z.; Liu, Q.; Sun, S. Kinetics of transesterification of palm oil and dimethyl carbonate for biodiesel production at the catalysis of heterogeneous base catalyst. Bioresour. Technol. 2010, 101, 8144–8150. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yan, B.; Li, H.; Yu, S.; Liu, S.; Yu, H.; Ge, X. SO42−/ZrO2 as catalyst for upgrading of pyrolysis oil by esterification. Fuel 2018, 226, 190–194. [Google Scholar] [CrossRef]
- Galvan, D.; Orives, J.R.; Coppo, R.L.; Silva, E.T.; Angilelli, K.G.; Borsato, D. Determination of the kinetics and thermodynamics parameters of biodiesel oxidation reaction obtained from an optimized mixture of vegetable oil and animal fat. Energy Fuels 2013, 27, 6866–6871. [Google Scholar] [CrossRef]
- Othman, A.; Osman, M.; Ibrahim, E.; Ali, M.A.; Abd-Elrahim, A. Mn-doped ZnO nanocrystals synthesized by sonochemical method: Structural, photoluminescence, and magnetic properties. Mater. Sci. Eng. B 2017, 219, 1–9. [Google Scholar] [CrossRef]


| Physicochemical Characteristics | Method Used for Analysis | Standard Limits | Specification of Castor Oil | Specifications of Petroleum Diesel | Biodiesel Produced by Nano Catalysts |
|---|---|---|---|---|---|
| Density in kg/m3 | ASTM D975 | 800–990 | 919 | 854 | 900 |
| Kinematic viscosity in mm2/s at 40 °C | EN ISO 3104 | 1.9–6.0 | 50 | 3.5 | 4.7 |
| Flash point in °C | ISO 3679 | 100–170 | 213 | 89 | 122 |
| Pour point in °C | ASTM D97 | −5 to10 | 30 | −7 | 2 |
| Cetane number | EN15195 | 48–50 | 35 | 45 | 52 |
| Cloud Point in °C | ASTM D613 | −15 to 12 | 196 | −5 | 1 |
| Factor | Amount of Catalyst (g) | Methanol to Oil Ratio (mL) | Temperature (°C) | % Yield |
|---|---|---|---|---|
| Catalyst | 0.4 | 12:1 | 55 | 68.7 |
| 0.6 | 12:1 | 55 | 74.6 | |
| 0.8 | 12:1 | 55 | 80.4 | |
| 1 | 12:1 | 55 | 86.5 | |
| 1.2 | 12:1 | 55 | 90.3 | |
| 1.4 | 12:1 | 55 | 88.2 | |
| Methanol ratio | 1.2 | 6:1 | 55 | 70 |
| 1.2 | 8:1 | 55 | 76 | |
| 1.2 | 10:1 | 55 | 82 | |
| 1.2 | 12:1 | 55 | 88 | |
| 1.2 | 14:1 | 55 | 86 | |
| Temperature | 1.2 | 12:1 | 50 | 79 |
| 1.2 | 12:1 | 55 | 89 | |
| 1.2 | 12:1 | 60 | 88 | |
| 1.2 | 12:1 | 65 | 76 |
| Sr. No | Time Interval in Minutes (t) | Rate Constant (k) | Linear Regression (R2) | |||
|---|---|---|---|---|---|---|
| 1 | 0 | 0.006 | −5.116 | 55 | 0.008 | 0.925 |
| 2 | 10 | 0.0051 | −5.27851 | |||
| 3 | 20 | 0.0046 | −5.3817 | |||
| 4 | 30 | 0.0042 | −5.47267 | |||
| 5 | 40 | 0.0039 | −5.54678 | |||
| 6 | 50 | 0.0039 | −5.54678 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahid, A.; Mukhtar, Z.; Qamar, M.A.; Shahid, S.; Ali, S.K.; Shariq, M.; Alathlawi, H.J.; Hasan, M.A.; Khan, M.S.; Islam, S.; et al. Synthesis of Mn-Doped ZnO Nanoparticles and Their Application in the Transesterification of Castor Oil. Catalysts 2023, 13, 105. https://doi.org/10.3390/catal13010105
Zahid A, Mukhtar Z, Qamar MA, Shahid S, Ali SK, Shariq M, Alathlawi HJ, Hasan MA, Khan MS, Islam S, et al. Synthesis of Mn-Doped ZnO Nanoparticles and Their Application in the Transesterification of Castor Oil. Catalysts. 2023; 13(1):105. https://doi.org/10.3390/catal13010105
Chicago/Turabian StyleZahid, Afifa, Zahid Mukhtar, Muhammad Azam Qamar, Sammia Shahid, Syed Kashif Ali, Mohammad Shariq, Hussain J. Alathlawi, Mohd Abul Hasan, Mohd Shakir Khan, Saiful Islam, and et al. 2023. "Synthesis of Mn-Doped ZnO Nanoparticles and Their Application in the Transesterification of Castor Oil" Catalysts 13, no. 1: 105. https://doi.org/10.3390/catal13010105
APA StyleZahid, A., Mukhtar, Z., Qamar, M. A., Shahid, S., Ali, S. K., Shariq, M., Alathlawi, H. J., Hasan, M. A., Khan, M. S., Islam, S., Patil, B. R., Al Ansari, M. S., Nawaz, Z., & Sher, M. (2023). Synthesis of Mn-Doped ZnO Nanoparticles and Their Application in the Transesterification of Castor Oil. Catalysts, 13(1), 105. https://doi.org/10.3390/catal13010105









