Fabrication and Characterization of Dextranase Nano-Entrapped Enzymes in Polymeric Particles Using a Novel Ultrasonication–Microwave Approach
Abstract
:1. Introduction
2. Results and Discussions
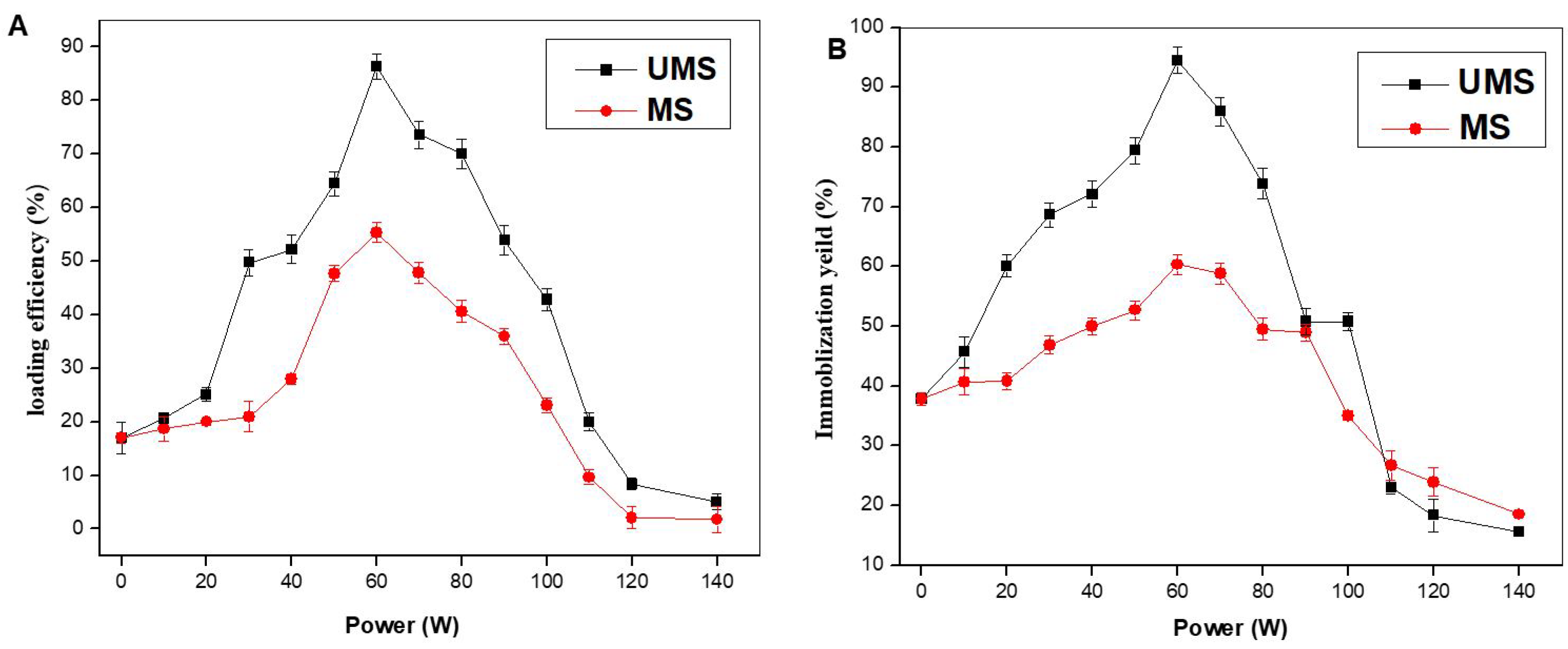
2.1. Effluence of UMS Treatment on the LE and IY of the Immobilized Enzyme
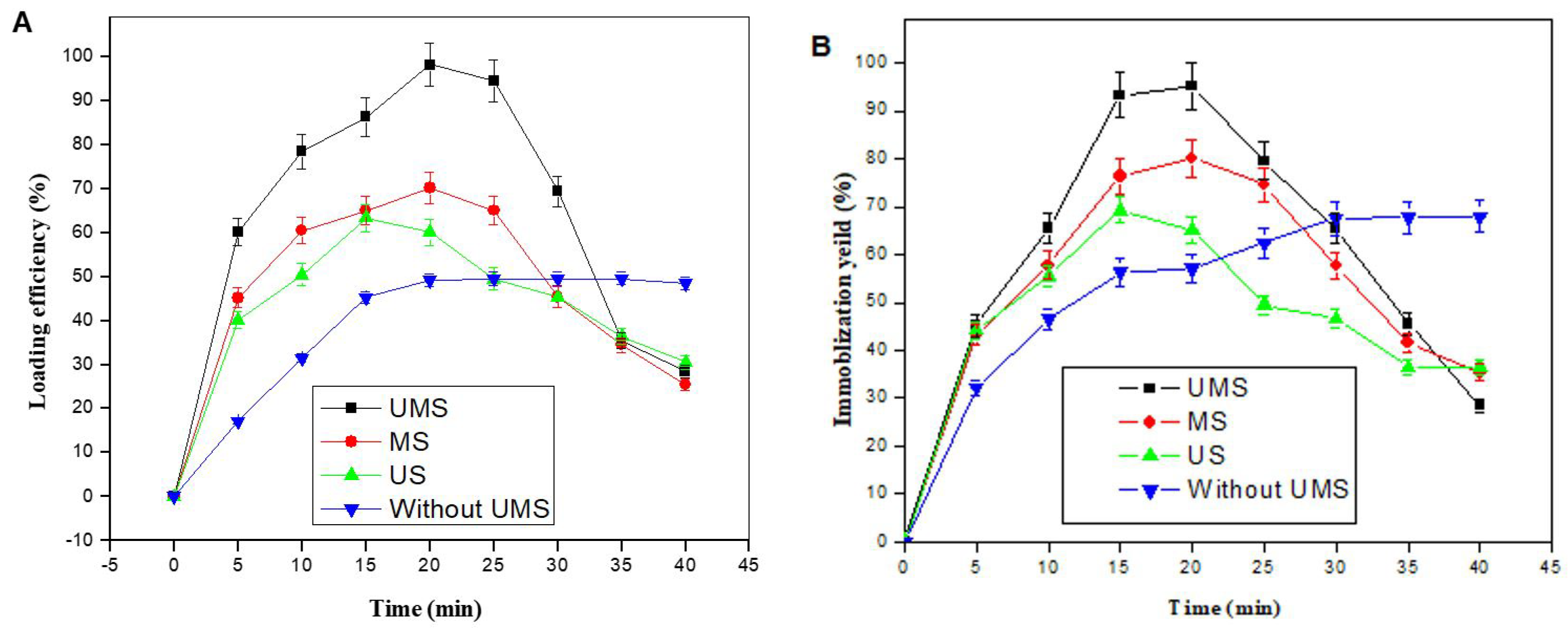
2.2. Effects of UMS Time on the LE and IY of the IE
2.3. Characterization of the Immobilized Enzyme
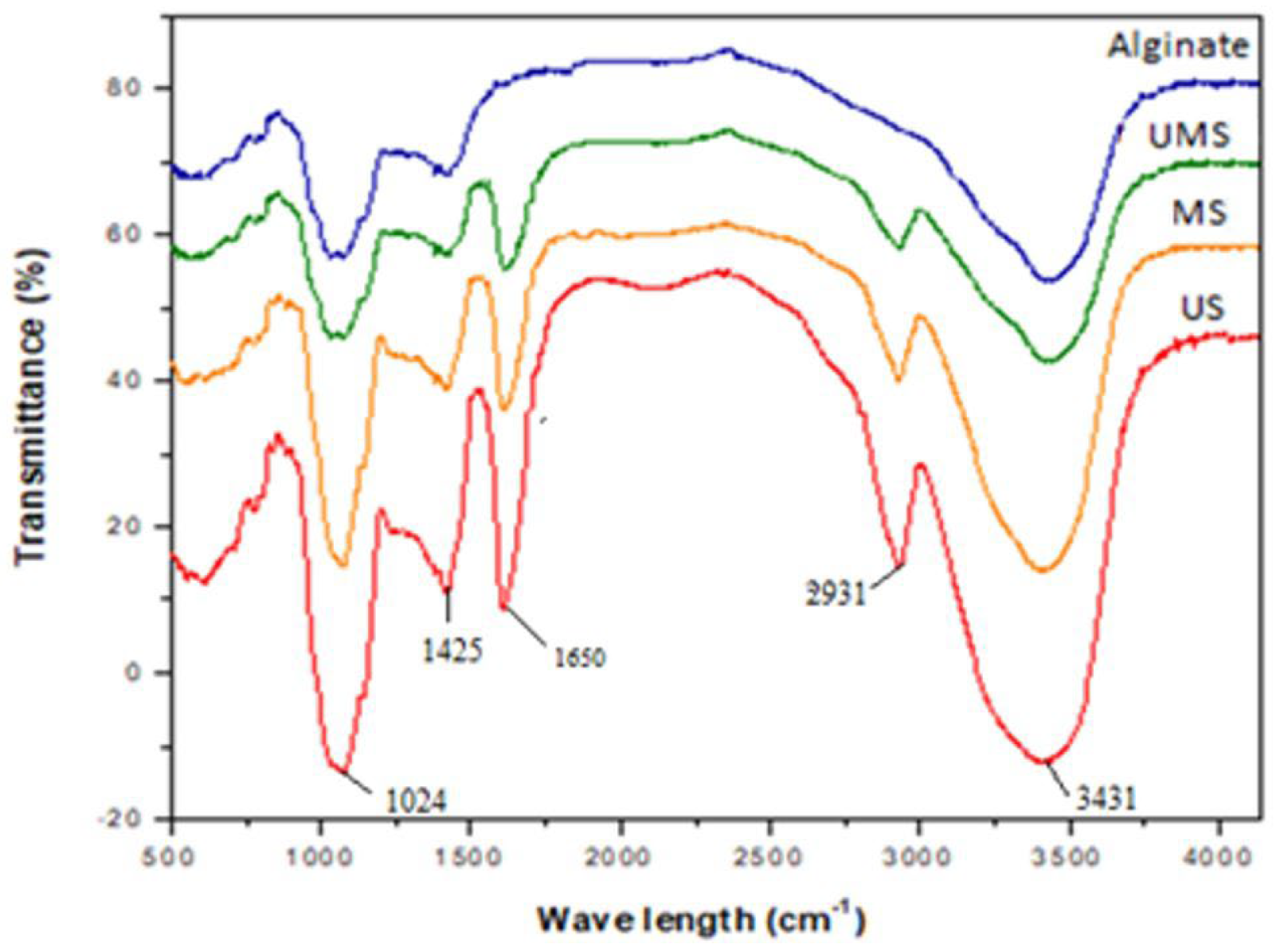
2.3.1. Fourier Transform Infrared Spectra
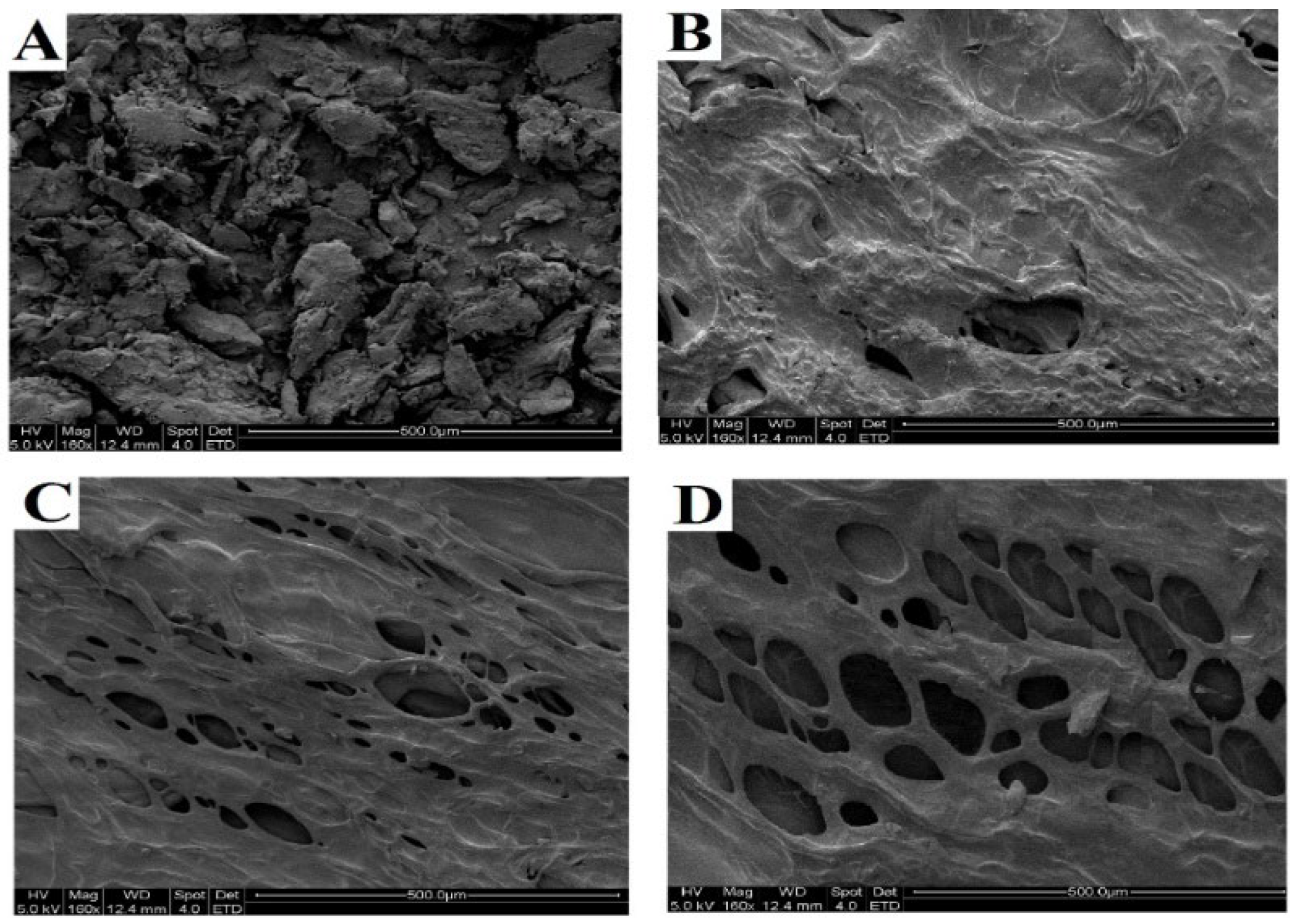
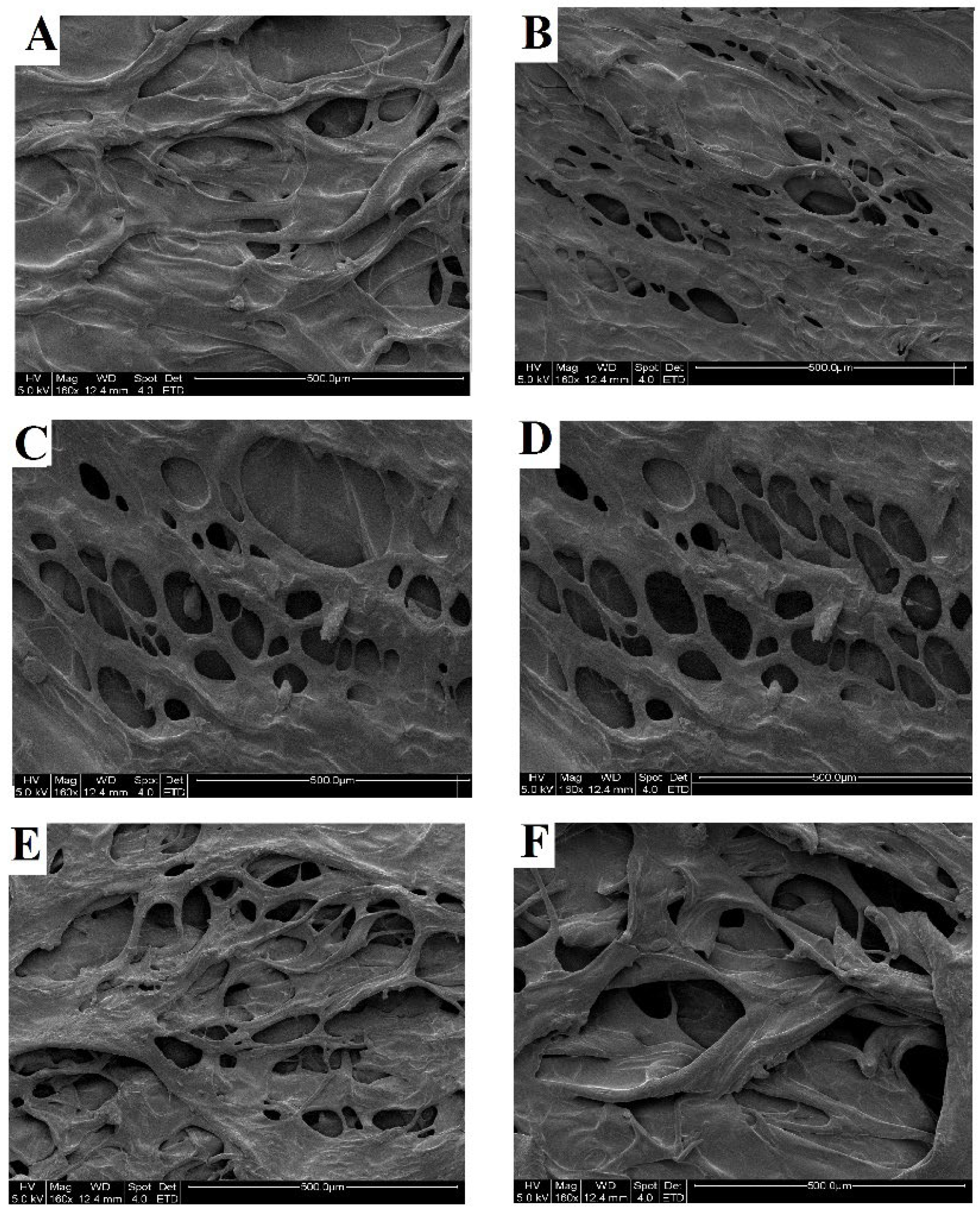
2.3.2. Scanning Electron Microscopy (SEM) Analysis
2.4. Study of the IE Kinetics
2.5. Thermodynamic Studies of IEs
2.6. Reusability
3. Materials and Methods
3.1. Materials
3.2. Ultrasonication–Microwave Shock Apparatus
3.3. Measurement of Enzyme Activities
3.4. Chromatographic Analysis and Conditions
3.5. Preparation of the IE with Ultrasonication Treatment
3.6. Preparation of the IE with MS Treatment
3.7. Preparation of the IE with Ultrasonication–Microwave Shock Treatment
3.8. Preparation of the IE without Ultrasonication–Microwave Shock Treatment
3.9. Characterization of the IE
3.9.1. Fourier Transform Infrared Spectroscopy (FT-IR)
3.9.2. Scanning Electron Microscopy (SEM) Analysis
3.10. Kinetic and Thermodynamic Studies of the IE
3.11. Protein Concentration
3.12. Recycled Hydrolysis of Dextran Catalyzed with IEs
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, J.; Zhang, Y.; Simpson, B. Food enzymes immobilization: Novel carriers, techniques and applications. Curr. Opin. Food Sci. 2022, 43, 27–35. [Google Scholar] [CrossRef]
- Wilson, L.; Palomo, J.M.; Fernández-Lorente, G.; Illanes, A.; Guisán, J.M.; Fernández-Lafuente, R. Improvement of the functional properties of a thermostable lipase from alcaligenes sp. via strong adsorption on hydrophobic supports. Enzym. Microb. Technol. 2006, 38, 975–980. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, S.; Wei, L.; Liao, Y.; Li, D.; Wu, G.; Wang, W. Efficient removal of dextran in sugar juice by immobilized α-dextranase from Chaetomium gracile. Int. J. Food Eng. 2022, 18, 751–759. [Google Scholar] [CrossRef]
- Sorndech, W. Isomaltooligosaccharides as Prebiotics and Their Health Benefits. Probiotics, Prebiotics and Synbiotics: Technological Advancements Towards Safety and Industrial Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 361–377. [Google Scholar]
- Wan, N.; Kou, P.; Pang, H.-Y.; Chang, Y.-H.; Cao, L.; Liu, C.; Zhao, C.-J.; Gu, C.-B.; Fu, Y.-J. Enzyme pretreatment combined with ultrasonic-microwave-assisted surfactant for simultaneous extraction of essential oil and flavonoids from Baeckea frutescens. Ind. Crops Prod. 2021, 174, 114173. [Google Scholar] [CrossRef]
- Hu, B.; Wang, H.; He, L.; Li, Y.; Li, C.; Zhang, Z.; Liu, Y.; Zhou, K.; Zhang, Q.; Liu, A.; et al. A method for extracting oil from cherry seed by ultrasonic-microwave assisted aqueous enzymatic process and evaluation of its quality. J. Chromatogr. A 2019, 1587, 50–60. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, T.; Tian, X.; Gai, D. Effect of microwave shock on the structure of glucoamylase. Process Biochem. 2012, 47, 2323–2328. [Google Scholar] [CrossRef]
- Meridor, D.; Gedanken, A. Preparation of enzyme nanoparticles and studying the catalytic activity of the Immobilized nanoparticles on polyethylene films. Ultrason. Sonochem. 2013, 20, 425–431. [Google Scholar] [CrossRef]
- Bashari, M.; Jin, Z.; Wang, J.; Zhan, X. A novel technique to improve the biodegradation efficiency of dextranase enzyme using the synergistic effects of ultrasound combined with microwave shock. Innov. Food Sci. Emerg. Technol. 2016, 35, 125–132. [Google Scholar] [CrossRef]
- Bashari, M.; Wang, P.; Eibaid, A.; Tian, Y.; Xu, X.; Jin, Z. Ultrasound-assisted dextranase entrapment onto Ca-alginate gel beads. Ultrason. Sonochem. 2013, 20, 1008–1016. [Google Scholar] [CrossRef]
- Bashari, M.; Abbas, S.; Xu, X.; Jin, Z. Combined of ultrasound irradiation with high hydrostatic pressure (US/HHP) as a new method to improve immobilization of dextranase onto alginate gel. Ultrason. Sonochem. 2014, 21, 1325–1334. [Google Scholar] [CrossRef]
- Ul, S.Q.; Aman, A.; Syed, N.; Bano, S.; Azhar, A. Characterization of dextransucrase immobilized on Ca-alginate beads from Leuconostoc mesenteroides PCSIR-4. Ital. J. Biochem. 2007, 56, 158–162. [Google Scholar]
- Basto, C.; Silva, C.J.; Gubitzb, G.; Cavaco-Paulo, A. Stability and decolourization ability of Trametesvillosa laccase in liquid ultrasonic fields. Ultrason. Sonochem. 2007, 14, 355–362. [Google Scholar] [CrossRef]
- Chen, R.; Wei, Q.; Wei, X.; Liu, Y.; Zhang, X.; Chen, X.; Yin, X.; Xie, T. Stable and efficient immobilization of bi-enzymatic NADPH cofactor recycling system by consecutive microwave irradiation. PLoS ONE 2020, 15, e0242564. [Google Scholar]
- Ariffin, M.F.K.; Idris, A. Fe2O3/Chitosan coated superparamagnetic nanoparticles supporting lipase enzyme from Candida Antarctica for microwave assisted biodiesel production. Renew. Energy 2022, 185, 1362–1375. [Google Scholar] [CrossRef]
- Abdel-Naby, M.A.; Ismail, A.M.; Abdel-Fattah, A.M.; Abdel-Fattah, A.F. Preparation and some properties of immobilized Penicillium funiculosum 258 dextranase. Proc. Biochem. 1999, 34, 391–398. [Google Scholar] [CrossRef]
- Yadav, J.; Paragas, E.; Korzekwa, K.; Nagar, S. Time-dependent enzyme inactivation: Numerical analyses of in vitro data and prediction of drug-drug interactions. Pharmacol. Ther. 2020, 206, 107449. [Google Scholar] [CrossRef]
- Nogueira, B.M.; Carretoni, C.; Cruz, R.; Freitas, S.; Melo, P.A., Jr.; Costa-Félix, R.; Pinto, J.C.; Nele, M. Microwave activation of enzymatic catalysts for biodiesel production. J. Mol. Catal. B Enzym. 2010, 67, 117–121. [Google Scholar] [CrossRef]
- Pereira, R.; Tojeira, A.; Vaz, D.C.; Mendes, A.; Bártolo, P. Preparation and characterization of films based on alginate and aloe vera. Int. J. Polym. Anal. 2011, 16, 449–464. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, H.; Wang, X.; Zu, H.; Wang, C.; Dong, D.; Lyu, M.; Wang, S. Immobilization of Dextranase on Nano-Hydroxyapatite as a Recyclable Catalyst. Materials 2021, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, L.; Fang, Y.; Zhang, X.; Lyu, M.; Wang, S. The adsorption of dextranase onto Mg/Fe-layered double hydroxide: Insight into the immobilization. Nanomaterials 2018, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Verma, A.; Satlewal, A. Application of nanoparticle-immobilized thermostable beta-glucosidase for improving thesugarcane juice properties. Innov. Food Sci. Emerg. Technol. 2016, 33, 472–482. [Google Scholar] [CrossRef]
- Almeida, L.C.; Barbosa, A.S.; Fricks, A.T.; Freitas, L.S.; Lima, Á.S.; Soares, C.M. Use of conventional or non-conventional treatments of biochar for lipase immobilization. Process Biochem. 2017, 61, 124–129. [Google Scholar] [CrossRef]
- Zahirinejad, S.; Hemmati, R.; Homaei, A.; Dinari, A.; Hosseinkhani, S.; Mohammadi, S.; Vianello, F. Nano-organic supports for enzyme immobilization: Scopes and perspectives. Colloids Surf. B Biointerfaces 2021, 204, 111774. [Google Scholar] [CrossRef]
- Sharma, J.; Singh, A.; Kumar, R.; Mittal, A. Partial purification of an alkaline protease from a new strain of Aspergillus Oryzae AWT 20 and its enhanced stabilization in entrapped Ca-alginate beads. Internet J. Microbiol. 2006, 2, 1–4. [Google Scholar]
- Quiroga, E.; Illanes, C.O.; Ochoa, N.A.; Barberis, S. Performance improvement of araujiain, a cystein phytoprotease, by immobilization within Ca-alginate beads. Process Biochem. 2011, 46, 1029–1034. [Google Scholar] [CrossRef]
- Wang, Y.; Hasebe, Y. Acridine orange-induced signal enhancement effect of tyrosinase-immobilized carbon-felt-based flow biosensor for highly sensitive detection of monophenolic compounds. Anal. Bioanal. Chem. 2011, 399, 1151–1162. [Google Scholar] [CrossRef]
- Krajewska, B.; Leszko, M.; Zaborska, W. Urease immobilized on chitosan membrane: Preparation and properties. J. Chem. Technol. Biotechnol. 1990, 48, 337–350. [Google Scholar] [CrossRef]
- Antony, N.; Balachandran, S.; Mohanan, P.V. Immobilization of diastase α-amylase on nano zinc oxide. Food Chem. 2016, 211, 624–630. [Google Scholar] [CrossRef]
- Eggleston, G.; Monge, A. Optimization of sugarcane factory application of commercial dextranases. Proc. Biochem. 2005, 40, 1881–1894. [Google Scholar] [CrossRef]
- Brown, C.F.; Inkerman, P.A. Specific method for quantitative measurement of the total dextran content of raw sugar. J. Agric. Food Chem. 1992, 40, 227–233. [Google Scholar] [CrossRef]
- Won, K.; Kim, S.; Kim, K.J.; Park, H.W.; Moon, S.J. Optimization of lipase entrapment in Ca-alginate gel beads. Proc. Biochem. 2005, 40, 2149–2154. [Google Scholar] [CrossRef]
- Ma, H.; Huang, L.; Jia, J.; He, R.; Luo, L.; Zhu, W. Effect of energy-gathered ultrasound on alcalase. Ultrason. Sonochem. 2011, 18, 419–424. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of micro-gram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
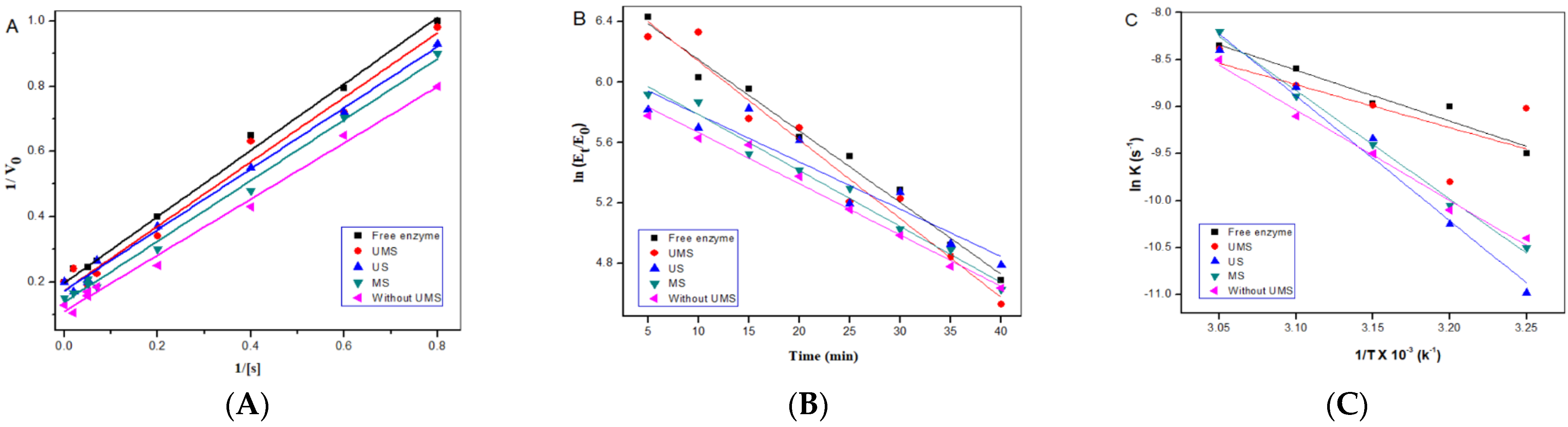


| VMax (μΜ min−1) | KM (μΜ) | Kcat (min−1) | Kcat/KM (μΜ−1 min−1) | |
|---|---|---|---|---|
| Free enzyme | 1.910 ± 0.17 | 0.189 ± 0.23 | 194.22 | 1027.61 |
| UMS | 1.235 ± 0.02 | 0.184 ± 0.17 | 182.30 | 990.76 |
| US | 1.188 ± 0.21 | 0.151 ± 0.21 | 143.73 | 951.85 |
| MS | 1.103 ± 0.11 | 0.164 ± 0.13 | 161.27 | 983.35 |
| Without UMS | 0.991 ± 0.30 | 0.134 ± 0.05 | 131.48 | 981.19 |
| K × 10−3 (min−1) | t½ (min) | R2 | |
|---|---|---|---|
| Free enzyme | 0.70 ± 0.33 | 16.25 ± 1.57 | 0.989 |
| UMS | 0.75 ± 0.17 | 15.41 ± 1.15 | 0.982 |
| US | 0.82 ± 0.12 | 12.01 ± 1.13 | 0.987 |
| MS | 0.79 ± 0.09 | 14.60 ± 1.45 | 0.977 |
| Without UMS | 1.00 ± 0.08 | 11.60 ± 1.37 | 0.990 |
| ln (A) | Ea (kJ mol−1) | ΔH (kJ mol−1) | ΔS (kJ mol−1 K−1) | ΔG (kJ mol−1) | |
|---|---|---|---|---|---|
| Free enzyme | 25.53 | 69.41 ± 3.52 | 66.72 ± 1.39 | −0.0891 ± 0.0082 | 60.08 ± 0.98 |
| UMS | 24.10 | 60.11 ± 1.98 | 59.13 ± 0.98 | −0.1068 ± 0.0023 | 109.32 ± 0.28 |
| US | 21.23 | 53.87 ± 3.01 | 49.99 ± 1.23 | −0.1502 ± 0. 0045 | 99.21 ± 1.02 |
| MS | 21.95 | 55.23 ± 1.79 | 50.38 ± 1.01 | −0.1489 ± 0.0019 | 98.97 ± 1.54 |
| Without UMS | 22.11 | 58.32 ± 13.34 | 56.73 ± 1.12 | −0.1229 ± 0.0033 | 97.08 ± 1.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashari, M.; Ahmed, H.; Mustafa, A.B.; Riaz, A.; Wang, J.; Saddick, S.Y.; Omar, A.S.; Afifi, M.; Al-Farga, A.; AlJumaiah, L.Z.; et al. Fabrication and Characterization of Dextranase Nano-Entrapped Enzymes in Polymeric Particles Using a Novel Ultrasonication–Microwave Approach. Catalysts 2023, 13, 125. https://doi.org/10.3390/catal13010125
Bashari M, Ahmed H, Mustafa AB, Riaz A, Wang J, Saddick SY, Omar AS, Afifi M, Al-Farga A, AlJumaiah LZ, et al. Fabrication and Characterization of Dextranase Nano-Entrapped Enzymes in Polymeric Particles Using a Novel Ultrasonication–Microwave Approach. Catalysts. 2023; 13(1):125. https://doi.org/10.3390/catal13010125
Chicago/Turabian StyleBashari, Mohanad, Hani Ahmed, Ayman Balla Mustafa, Asad Riaz, Jinpeng Wang, Salina Yahya Saddick, Abdulkader Shaikh Omar, Mohamed Afifi, Ammar Al-Farga, Lulwah Zeyad AlJumaiah, and et al. 2023. "Fabrication and Characterization of Dextranase Nano-Entrapped Enzymes in Polymeric Particles Using a Novel Ultrasonication–Microwave Approach" Catalysts 13, no. 1: 125. https://doi.org/10.3390/catal13010125
APA StyleBashari, M., Ahmed, H., Mustafa, A. B., Riaz, A., Wang, J., Saddick, S. Y., Omar, A. S., Afifi, M., Al-Farga, A., AlJumaiah, L. Z., Abourehab, M. A. S., Belal, A., & Zaky, M. Y. (2023). Fabrication and Characterization of Dextranase Nano-Entrapped Enzymes in Polymeric Particles Using a Novel Ultrasonication–Microwave Approach. Catalysts, 13(1), 125. https://doi.org/10.3390/catal13010125







