Ni-Free SOFC Anode Material with Thermal and Redox Stabilities for the Direct Utilization of Ethanol
Abstract
:1. Introduction
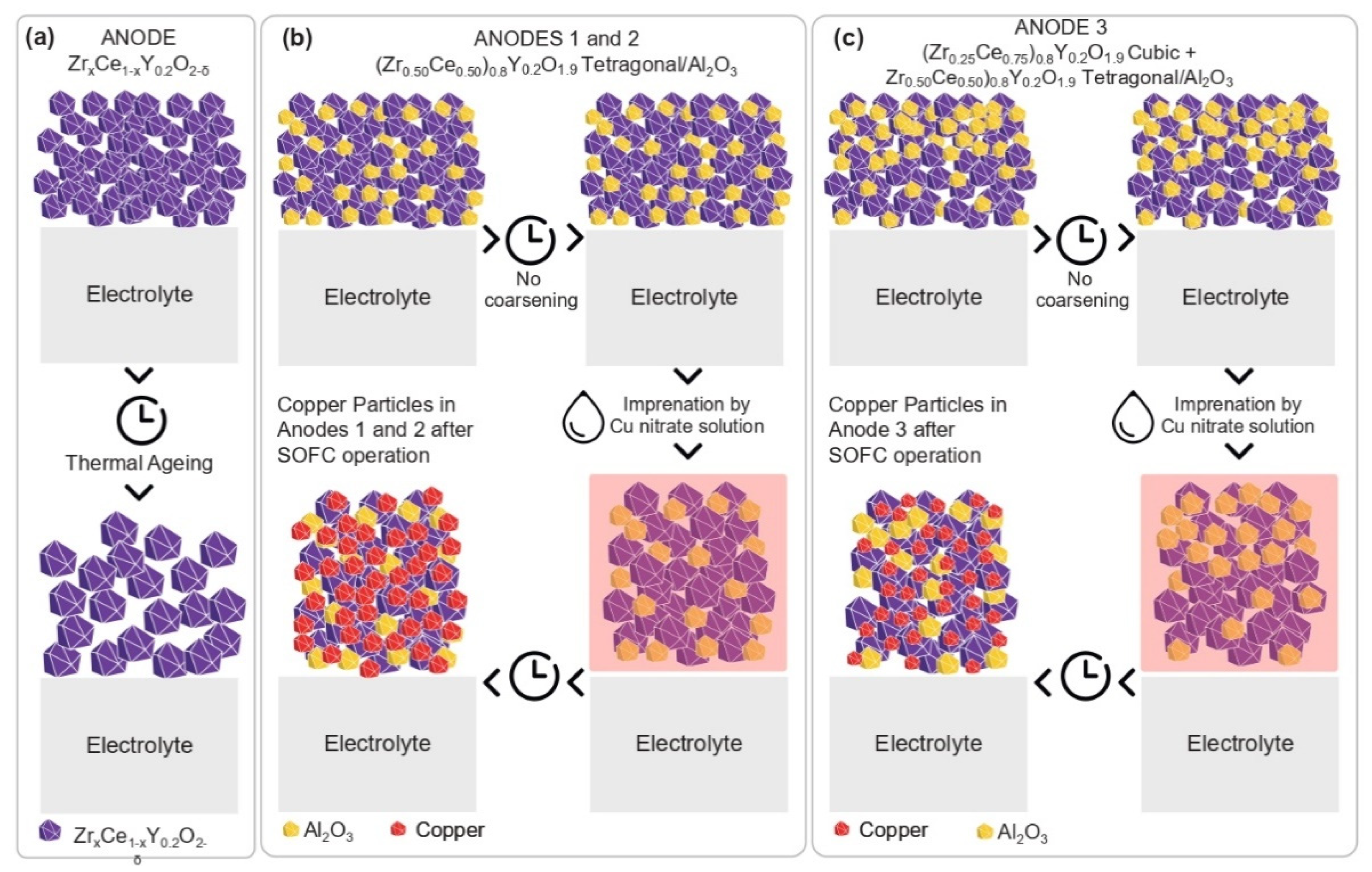
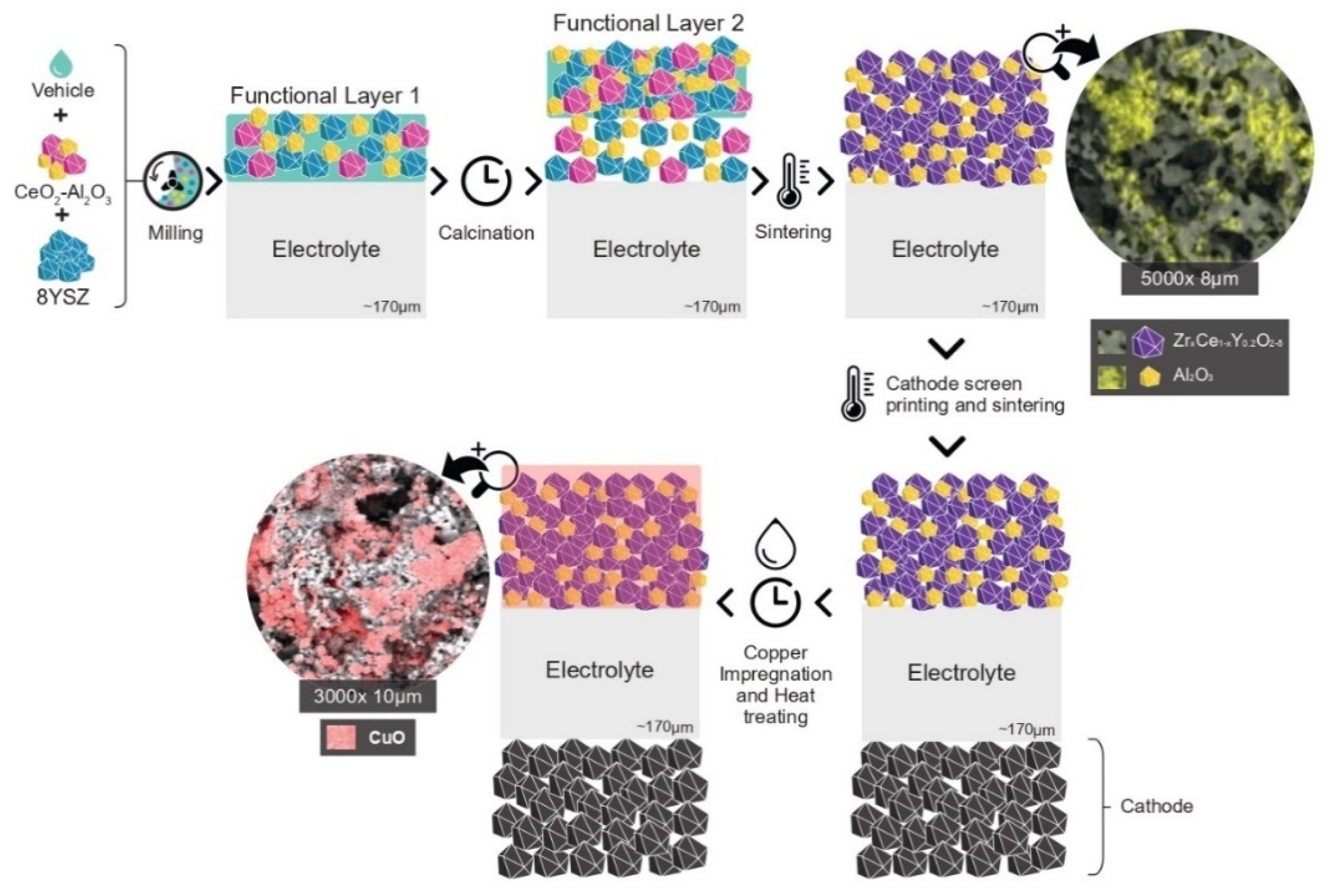
- A new methodology was created for the synthesis of the electrocatalyst and preparation of the anodic electrode. A simple ceramic processing of the precursor materials CeO2-Al2O3 and 8YSZ, and sintering of the functional layers, favored the reaction between Ceria and 8YSZ in order to produce an anode composed of electrochemical active solid solutions such as (Zr0.25Ce0.75)0.8Y0.2O1.9, cubic, and (Zr0.50Ce0.50)0.8Y0.2O1.9, tetragonal, in addition to the Al2O3 phase. These solid solutions were homogeneously distributed in the anode’s microstructure, which exhibited an excellent OSC, while the presence of dispersed alumina improved the thermal, structural and mechanical stabilities;
- The electrochemical activity of the solid solutions was directly related to their compositions and structure and favored a greater formation of defects in the surface and bulk sites, allowing the oxygen mobility to be improved. In this way, when operating in an SOFC-reducing atmosphere, the Ce4+ reacted with electrons, which were produced when an oxygen vacancy was formed, and it was reduced to Ce3+;
- The crystallization of the Al2O3 phase in separate grains prevented its reaction with CeO2, avoiding the formation of the undesired perovskite CeAlO3 phase, since it induced a loss in the OSC. In the anode’s microstructure, the Al2O3 phase acted against thermal degradation, avoiding the coarsening of the solid solution and increasing the thermal, redox and mechanical stabilities of the solid solution;
- The presence of alumina in the anode’s microstructure prevented the deactivation of the OSC properties, favoring the redox process of Ceria in the solid solution at high temperatures.
2. Results and Discussion
2.1. Anodes’ Structural Analysis
2.2. Morphology and Microstructure Analysis before Copper Impregnation
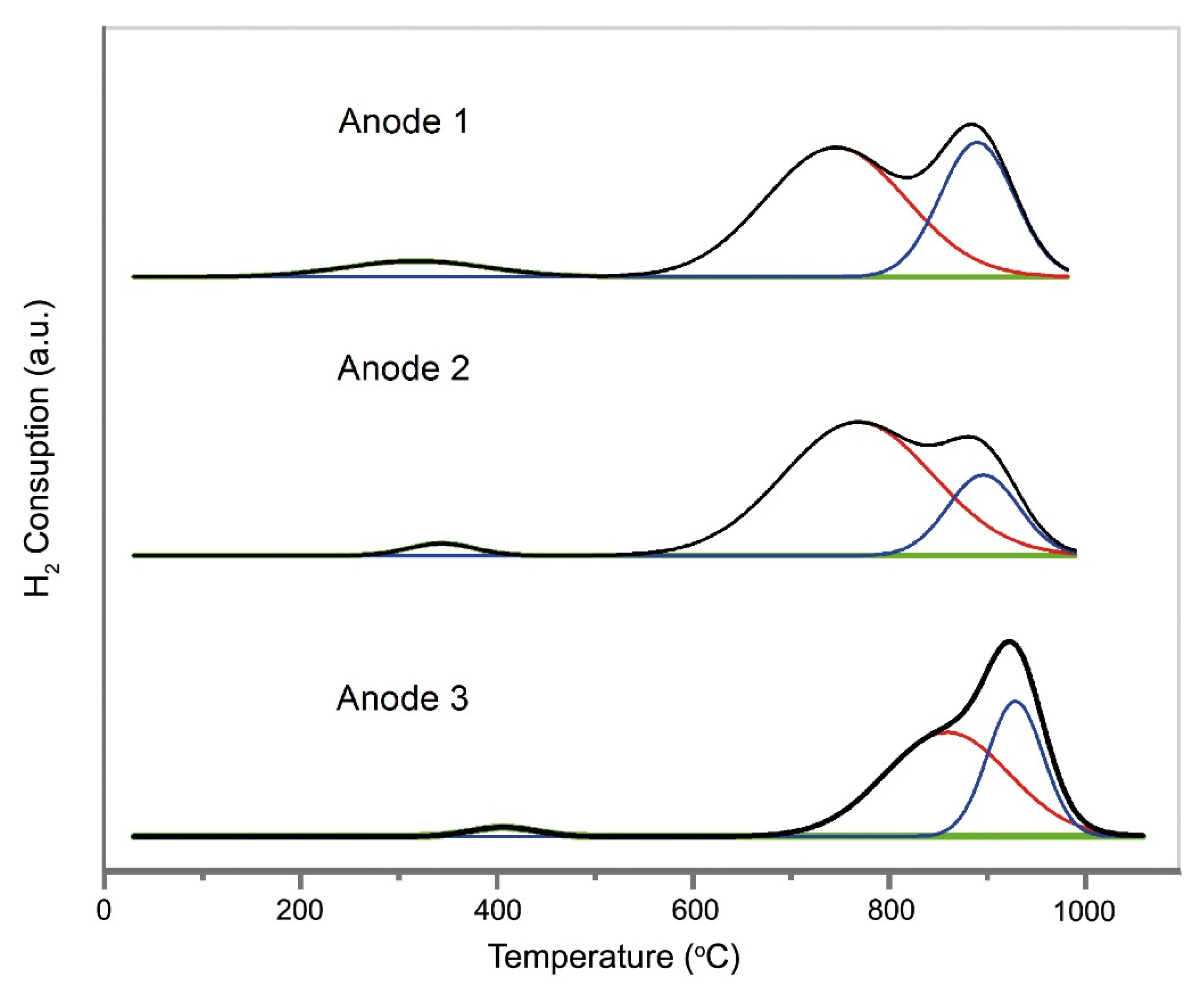
2.3. Reducibility and Oxygen Storage Capacity, OSC, Properties
2.4. Morphology and Microstructure Analysis after Copper Impregnation
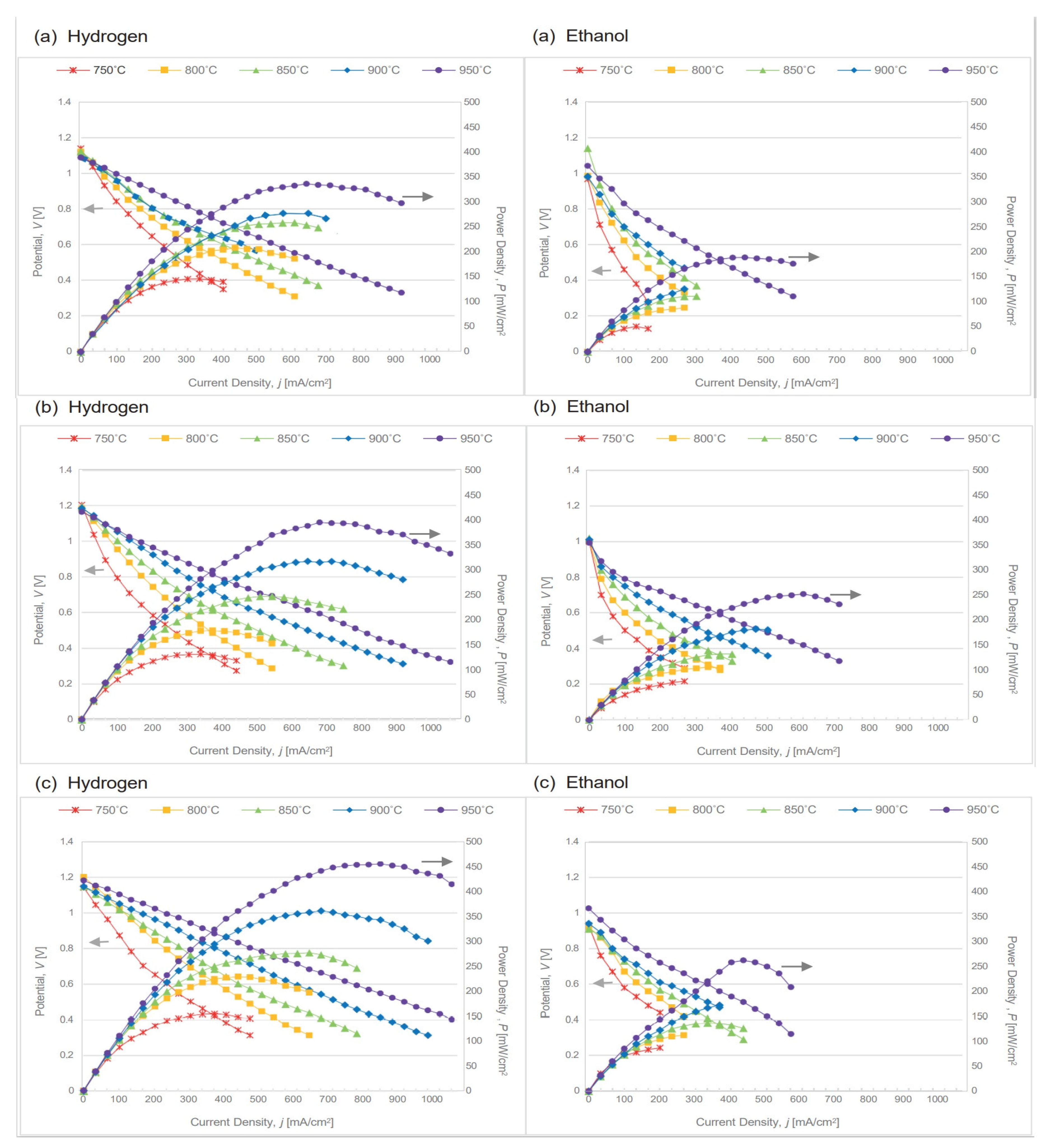
2.5. SOFC’s Electrochemical Performance
3. Materials and Methods
3.1. Anode Concept
3.2. Cells Fabrication
3.3. Characterization Techniques and Performance Test
4. Conclusions
- I.
- The activities of the solid solutions were directly related to their compositions and structure in order to favor a greater formation of defects in the surface and bulk sites, allowing for the oxygen ion mobility to be improved;
- II.
- The presence of a cubic phase, rich in Cerium, favored the best performance since it had more Cerium available to be used for the redox process;
- III.
- The crystallization of the Al2O3 phase in separate grains prevented its reaction with CeO2, avoiding the formation of the undesired perovskite CeAlO3 phase. In the anode’s microstructure, the Al2O3 phase acted against thermal degradation, avoiding the coarsening of the solid solution and increasing the thermal and redox stability of the solid solution;
- IV.
- The presence of alumina prevented a strong deactivation of the OSC properties even after sintering at 1500 °C, favoring the redox process of Ceria in a solid solution at high temperatures.
5. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Liu, Y.; Chen, Z.; Li, M.; Rao, M.; Wang, X.; Feng, P.; Zhou, F.; Ling, Y. Enhanced conversion efficiency and coking resistance of solid oxide fuel cells with vertical-microchannel anode fueled in CO2 assisted low-concentration coal-bed methane. Sep. Purif. Technol. 2022, 288, 120665. [Google Scholar] [CrossRef]
- Gupta, G.K.; Dean, A.M.; Ahn, K.; Gorte, R.J. Comparison of conversion and deposit formation of ethanol and butane under SOFC conditions. J. Power Source 2006, 158, 497–503. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Qin, D.; Gu, Y.; Yu, H.; Tao, S.; Qian, B.; Chao, Y. Improving electrochemical performance of (Cu, Sm) CeO2 anode with anchored Cu nanoparticles for direct utilization of natural gas in solid oxide fuel cells. J. Eur. Ceram. Soc. 2022, 42, 3254–3263. [Google Scholar] [CrossRef]
- Escudero, M.J.; Valero, C.; Cauqui, M.; Goma, D.; Yeste, M.P. Ni-Ce-ZrO2 system as anode material for direct internal reforming biogas solid oxide fuel cells. Fuel 2022, 322, 124247. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Y.; Li, G.; Hu, C. CeZrOx Promoted Water-Gas Shift Reaction under Steam–Methane Reforming Conditions on Ni-HTASO5. J. Catal. 2020, 10, 1110. [Google Scholar] [CrossRef]
- Vacharapong, P.; Arayawate, S.; Katanyutanon, S.; Toochinda, P.; Lawtrakul, L.; Charojrochkul, S. Enhancement of Ni Catalyst Using CeO2–Al2O3 Support Prepared with Magnetic Inducement for ESR. J. Catal. 2020, 10, 1357. [Google Scholar] [CrossRef]
- Murray, E.P.; Tsai, T.; Barnett, S.A. A direct-methane fuel cell with a ceria-based anode. Nature 1999, 400, 649–651. [Google Scholar] [CrossRef]
- Park, S.; Vohs, J.M.; Gorte, R.J. Direct oxidation of hydrocarbons in a solid-oxide fuel cell. Nature 2000, 404, 265–267. [Google Scholar] [CrossRef]
- Gross, M.D.; Vohs, J.M.; Gorte, R.J. Recent progress in solid anodes for direct utilization of hydrocarbons. J. Mater. Chem. 2007, 17, 3071–3077. [Google Scholar] [CrossRef] [Green Version]
- Gorte, R.J.; Park, S.; Vohs, J.M.; Wang, C. Anodes for direct oxidation of dry hydrocarbons in a solid oxide fuel cell. Adv. Mater. 2000, 12, 1465. [Google Scholar] [CrossRef]
- Park, S.; Gorte, R.J.; Vohs, J.M. Tape casting solid oxide fuel cells for the direct oxidation of hydrocarbons. J. Electrochem. Soc. 2001, 148, A443–A447. [Google Scholar] [CrossRef]
- Gorte, R.J.; Vohs, J.M. Novel SOFC anode for the direct electrochemical oxidation of hydrocarbons. J. Catal. 2003, 216, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Ahn, K.; He, H.; Vohs, J.M.; Gorte, R.J. Enhanced thermal stability of SOFC anodes made with CeO2-ZrO2 solutions. Electrochem. Solid-State Lett. 2005, 8, A414–A417. [Google Scholar] [CrossRef]
- He, H.; Vohs, J.M.; Gorte, R.J. Effect of synthesis conditions on the performance of Cu-CeO2-YSZ anode in SOFCs. J. Electrochem. Soc. 2003, 150, A1470–A1475. [Google Scholar] [CrossRef]
- Perrichon, V.; Laachir, A.; Abouarnadasse, S.; Touret, O.; Blanchard, G. Thermal stability of a high surface area ceria under reducing atmosphere. Appl. Catal. A Gen. 1995, 129, 69–82. [Google Scholar] [CrossRef]
- Hori, C.E.; Permana, H.; Simon Ng, K.Y.; Brenner, A.; More, K.; Rahmoeller, K.M.; Belton, D. Thermal stability of oxygen storage properties in a mixed CeO2-ZrO2 system. Appl. Catal. B Environ. 1998, 16, 105–117. [Google Scholar] [CrossRef]
- Ping, L.; Chen, X.; Yongdan, L.; Schwank, W.J. A review on oxygen storage capacity of CeO2-based materials: Influence factors, measurement techniques, and applications in reactions related to catalytic automotive emissions control. Catal. Today 2019, 327, 90–115. [Google Scholar] [CrossRef]
- Zimicz, M.G.; Núnez, P.; Ruiz-Morales, J.C.; Lamas, D.G.; Larrondo, S.A. Electro-catalytic performance of 60%NiO/Ce0.9Zr0.1O2 cermet as anode of intermediate temperature solid oxide fuel cells. J. Power Source 2013, 238, 87–94. [Google Scholar] [CrossRef]
- Cimenti, M.; Hill, J.M. Direct utilization of ethanol on ceria-based anodes for solid oxide fuel cells. Asia-Pac. J. Chem. Eng. 2009, 4, 45–54. [Google Scholar] [CrossRef]
- Song, S.; Han, M.; Zhang, J.; Fan, H. NiCu-Zr0.1Ce0.9O2-δ anode materials for intermediate temperature solid oxide fuel cells using hydrocarbon fuels. J. Power Source 2013, 233, 62–68. [Google Scholar] [CrossRef]
- Song, S.; Fuentes, R.O.; Baker, R.T. Nanoparticulate ceria–zirconia anode materials for intermediate temperature solid oxide fuel cells using hydrocarbon fuels. J. Mater. Chem. 2010, 20, 9760–9769. [Google Scholar] [CrossRef]
- Monte, R.D.; Kaspar, J. Heterogeneous environmental catalysis–a gentle art: CeO2–ZrO2 mixed oxides as a case history. Catal. Today 2005, 100, 27–35. [Google Scholar] [CrossRef]
- Aneggi, E.; Boaro, M.; de Leitenburg, C.; Dolcetti, G.; Trovarelli, A. Trovarelli, Insights into the redox properties of ceria-based oxides and their implications in catalysis. J. Alloys Compd. 2006, 408–412, 1096–1102. [Google Scholar] [CrossRef]
- Shah, P.M.; Burnett, J.W.H.; Morgan, D.J.; Davies, T.E.; Taylor, S.H. Ceria–Zirconia Mixed Metal Oxides Prepared via Mechanochemical Grinding of Carbonates for the Total Oxidation of Propane and Naphthalene. J. Catal. 2019, 9, 475. [Google Scholar] [CrossRef] [Green Version]
- Boaro, M.; Pappacena, A.; Abate, C.; Ferluga, M.; Llorca, J.; Trovarelli, A. Effect of redox treatments on Ce0.50Zr0.50O2 based solid oxide fuel cell anodes. J. Power Source 2014, 270, 79–91. [Google Scholar] [CrossRef]
- Venâncio, S.A.; de Miranda, P.E.V. Synthesis of CeAlO3/CeO2–Al2O3 for use as a solid oxide fuel cell functional anode material. Ceram. Int. 2011, 37, 3139–3152. [Google Scholar] [CrossRef]
- Yokokawa, H.; Sakai, N.; Horita, T.; Yamaji, K.; Xiong, Y.; Otake, T.; Yugami, H.; Kawada, T.; Mizusaki, J. Phase diagram calculations of ZrO2-based ceramics with an emphasis on the reduction/oxidation equilibria of cerium ions in the ZrO2-YO1.5-CeO2-CeO1.5 system ZrO2-YO1.5-CeO2-CeO1.5 system. J. Phase Equilibria 2001, 22, 331–338. [Google Scholar] [CrossRef]
- Kaspar, J.; Fornasiero, P.; Balducci, G.; Di Monte, R.; Hickey, N.; Sergo, V. Effect of ZrO2 content textural and structural and structural properties of e CO2-ZrO2 solid solutions made by citrate complexation route. Inorg. Chim. Acta 2003, 349, 217–226. [Google Scholar] [CrossRef]
- Pavlova, S.; Smirnova, M.; Bobin, A.; Cherepanova, S.; Kaichev, V.; Ishchenko, A.; Selivanova, A.; Rogov, V.; Rogerand, A.-C.; Sadykov, V. Structural, Textural, and Catalytic Properties of Ni-CexZr1−xO2 Catalysts for Methane Dry Reforming Prepared by Continuous Synthesis in Supercritical Isopropanol. Energies 2020, 13, 3728. [Google Scholar] [CrossRef]
- Vidal, H.; Bernal, S.; Kašpar, J.; Pijolat, M.; Perrichon, V.; Blanco, G.; Pintado, J.; Baker, R.; Colon, G.; Fally, F. Influence of high temperature treatments under net oxidizing and reducing conditions on the oxygen storage and buffering properties of a Ce0.68Zr0.32O2 mixed oxide. Catal. Today 1999, 54, 93–100. [Google Scholar] [CrossRef]
- Benjaram, M.R.; Ataullah, K. Raman and X-ray photoelectron spectroscopy study of CeO2-ZrO2 and V2O5/CeO2-ZrO2 catalysts. Langmuir 2003, 19, 3025–3030. [Google Scholar] [CrossRef]
- Kurapova, O.Y.; Glukharev, A.G.; Glumov, O.V.; Konakov, V.G. The effect of the sintering parameters on the structure and oxygen ion conductivity of Y2O3–ZrO2–CeO2 ceramics. Open Ceram. 2021, 5, 100086. [Google Scholar] [CrossRef]
- Eufinger, J.-P.; Daniels, M.; Schmale, K.; Berendts, S.; Ulbrich, G.; Lerch, M.; Wiemhöfer, H.-D.; Janek, J. The model case of an oxygen storage catalyst–non-stoichiometry, point defects and electrical conductivity of single crystalline CeO2-ZrO2-Y2O3 solid solution. Phys. Chem. 2014, 16, 25583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fornasiero, P.; Dimonte, R.; Rao, G.; Kaspar, J.; Meriani, S.; Trovarelli, A.; Graziani, M. Rh-Loaded CeO2-ZrO2 Solid Solutions as Highlt Efficient Oxygen Exchangers: Dependence of the Reduction Behavior and the Oxygen Storage Capacity on the Structural-Properties. J. Catal. 1995, 151, 168–177. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, C.-H.; Raitano, J.M.; Hanson, J.C.; Caliebe, W.A.; Khalid, S.; Chan, S.-W. Phase stability in ceria-zirconia binary oxide nanoparticles: The effect of the Ce3+ concentration and the redox environment. J. Appl. Phys. 2006, 99, 084313. [Google Scholar] [CrossRef]
- Kakuta, N.; Ikawa, S.; Eguchi, T.; Murakami, K. Oxidation behavior of reduced (CeO2)1-x-(ZrO2) x=0, 0.2, 0.5) catalysts. J. Alloys Compd. 2006, 408–412, 1078–1083. [Google Scholar] [CrossRef]
- Kaspar, J.; Fornasiero, P.; Hickey, N. Automotive catalytic converters: Current status and some perspectives. Catal. Today 2003, 77, 419–449. [Google Scholar] [CrossRef]
- Kozlov, A.I.; Kim, D.H.; Yezerets, A.; Andersen, P.; Kung, H.H.; Kung, M.C. Effect of Preparation Method and Redox Treatment on the Reducibility and Structure of Supported Ceria-Zirconia Mixed Oxide. J. Catal. 2002, 209, 417–429. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Zhan, W.; Guo, Y.; Guo, Y.; Lu, G. Preparation of high oxygen storage capacity and thermally stable ceria-zirconia solid solution. Catal. Sci. Technol. 2016, 6, 897–907. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lu, G.; Guo, Y.; Jiang, L.; Guo, Y.; Li, C. Effect of additives on the structure characteristics, thermal stability, reducibility and catalytic activity of CeO2-ZrO2 solid solution for methane combustion. J. Mater. Sci. 2009, 44, 1294–1301. [Google Scholar] [CrossRef]
- Wei, Z.; Li, H.; Zhang, X.; Yan, S.; Lv, Z.; Chen, Y.; Gong, M. Preparation and property investigation of CeO2–ZrO2–Al2O3 oxygen-storage compounds. J. Alloy. Compd. 2008, 455, 322–326. [Google Scholar] [CrossRef]
- Balducci, G.; Kašpar, J.; Fornasiero, P.; Graziani, M.; Islam, M.S.; Gale, J.D. Computer simulation studies of bulk reduction and oxygen migration in CeO2-ZrO2 solid solutions. J. Phys. Chem. B 1997, 101, 1750–1753. [Google Scholar] [CrossRef]
- Shawn, R.; Salvador, S.; Alexis, M.; Chris, B. Recent advances in gasoline three-way catalyst formulations: A review. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2019, 234, 937–949. [Google Scholar] [CrossRef]
- Venâncio, S.A.; de Miranda, P.E.V. Solid oxide fuel cell anode for the direct utilization of ethanol as fuel. Scr. Mater. 2011, 65, 1065–1068. [Google Scholar] [CrossRef]
- Li, W.; Guan, B.; Liu, M.; Wei, B.; Zhu, X.; Wang, Z.; Lü, Z. On the limiting factor of impregnation methods for developing Cu/CeO2 anodes for solid oxide fuel cells. J. Solid State Electr. 2018, 22, 1735–1743. [Google Scholar] [CrossRef]
- Tiwari, P.K.; Yue, X.; Irvine, J.; Basu, S. La and Ca-Doped A-Site Deficient Strontium Titanates Anode for Electrolyte Supported Direct Methane Solid Oxide Fuel Cell. J. Electrochem. Soc. 2017, 164, 1030–1036. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Liu, Z.; Pang, L.; Han, Y.; Yao, S.; Wang, X. Preparation of Y2O3–ZrO2–CeO2 solid solution by co-precipitation and its electrical property. Phys. B Phys. Condens. Matter 2021, 612, 412972. [Google Scholar] [CrossRef]
- Venâncio, S.A.; de Miranda, P.E.V. Direct utilization of carbonaceous fuels in multifunctional SOFC anodes for the electrosynthesis of chemicals or the generation of electricity. Int. J. Hydrogen Energy 2017, 42, 13927–13938. [Google Scholar] [CrossRef]
- Cimenti, M.; Hill, J.M. Direct Utilization of Liquid Fuels in SOFC for Portable Applications: Challenges for the Selection of Alternative Anodes. Energy 2009, 2, 377–410. [Google Scholar] [CrossRef] [Green Version]
- Rotzoll, G. High temperature pyrolysis of ethanol. J. Anal. Appl. Pyrol. 1985, 9, 43–52. [Google Scholar] [CrossRef]
- Cimenti, M.; Hill, J.M. Thermodynamic analysis of solid oxide fuel cells operated with methanol and ethanol under direct utilization, steam reforming, dry reforming or partial oxidation conditions. J. Power Source 2009, 186, 377–384. [Google Scholar] [CrossRef]
- Ayesha, I.; Muhammad, F.A. Influence of CdS dopant on oxygen vacancies and Ce3+ formation in CeO2-ZnO nanocomposites: Structural, optical and catalytic properties. J. Mater. Sci. Mater. Electron. 2017, 28, 2788–2794. [Google Scholar] [CrossRef]
- Subramani, V.; Basile, A.; Veziroglu, T.N. Compendium of hydrogen energy. In Hydrogen Production and Purification; Subramani, V., Basile, A., Veziroglu, T.N., Eds.; Woodhead Publishing Series: Sawston, UK, 2015; pp. 10–11. [Google Scholar]
- Miranda, P.E.V.; Venâncio, S.A.; de Miranda, H.V. Method for the Direct Oxidation and/or Internal Reforming of Ethanol, Solid Oxide Fuel Cell for Direct Oxidation and/or Internal Reforming of Ethanol, Catalyst and Multifunctional Electrocatalytic Anode for Direct Oxidation and/or Internal Reforming of Ethanol. US Patent 9,431,663 B2, 30 August 2016. [Google Scholar]
- Sobukawa, H. Development of ceria-zirconia solid solutions and future trends. RD Rev. Toyota CRDL 2002, 37, 1–5. [Google Scholar]
- Suzuki, T.; Morikawa, A.; Suda, A.; Sobukawa, H.; Sugiura, M.; Kanazawa, T.; Suzuki, J.; Takada, T. Alumina-Ceria-Zirconia Composite Oxide for Three-Way Catalyst, Special Issue Oxygen Storage Materials for Automotive-Ceria-Zirconia solid solution. RD Rev. Toyota CRDL 2002, 37, 28–33. [Google Scholar]


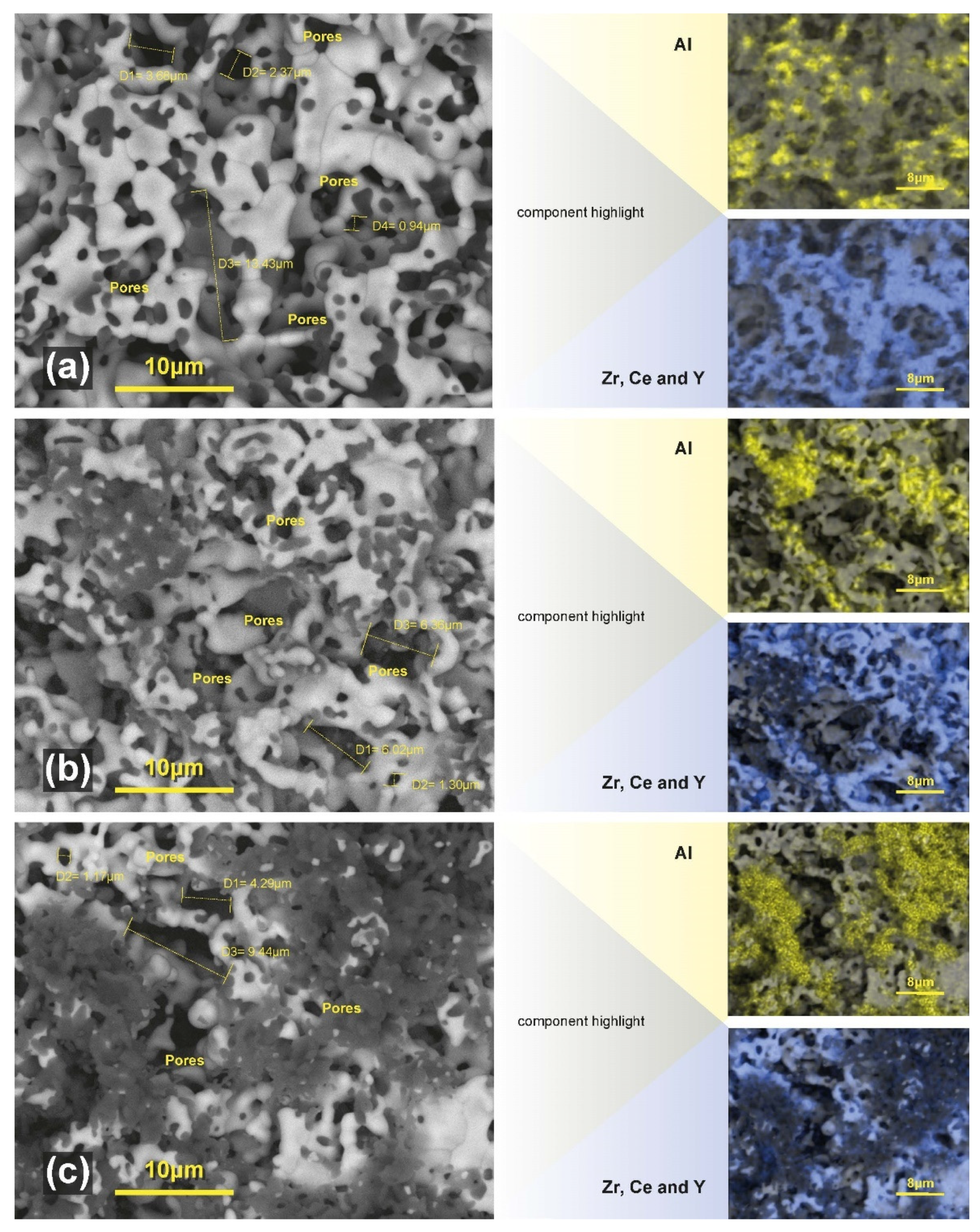

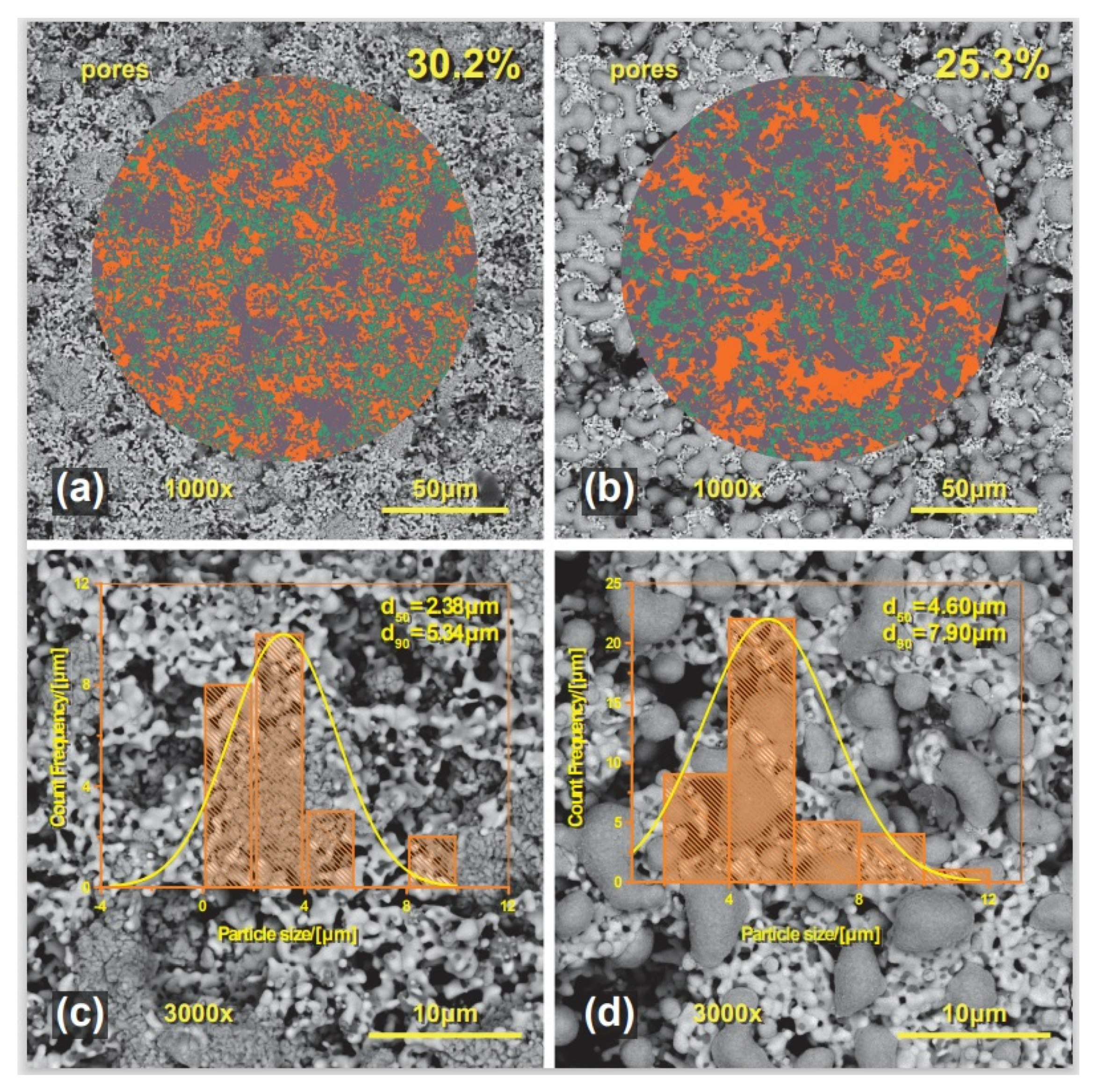


| Sample | Phases | Space Group | Lattice Parameters (nm) | Crystallite Size (nm) | Phases Fraction (Mass. %) | Interatomic Distances (nm) | Refinement Coefficients |
|---|---|---|---|---|---|---|---|
| ANODE 1 | (Zr0.50Ce0.50)0.8Y0.2O1.9 (Tetragonal) | P 42/n m c | a = b = 0.369(9) c = 0.522(7) | 82.08 ± 0.06 | 83.0 ± 1 | Zr-O: 0.2337 | Rp = 23.5 Rwp = 18.2 χ2 = 1.55 |
| Al2O3 (Hexagonal) | R-3 c | a = b = 0.475(2) c = 1.297(2) | 124.32 ± 0.09 | 17.0 ± 1 | Al-O: 0.1963 | ||
| ANODE 2 | (Zr0.50Ce0.50)0.8Y0.2O1.9 (Tetragonal) | P 42/n m c | a = b = 0.368(5) c = 0.520(3) | 89.33 ± 0.04 | 82.0 ± 1 | Zr-O: 0.2326 | Rp = 23.1 Rwp = 18.5 χ2 = 1.62 |
| Al2O3 (Hexagonal) | R-3 c | a = b = 0.475(1) c = 1.297(0) | 122.87 ± 0.09 | 18.0 ± 1 | Al-O: 0.1979 | ||
| ANODE 3 | (Zr0.25Ce0.75)0.8Y0.2O1.9 (Cubic) | F m-3 m | a = b = c = 0.529(7) | 25.636 ± 0.04 | 49.3 ± 1.73 | Zr-O: 0.2294 | Rp = 25.9 Rwp = 21.3 χ2 = 1.49 |
| (Zr0.50Ce0.50)0.8Y0.2O1.9 (Tetragonal) | P 42/n m c | a = b = 0.372(3) c = 0.528(2) | 17.1 ± 0.1 | 29.7 ± 1.55 | Zr-O: 0.2223 | ||
| Al2O3 (Hexagonal) | R-3 c | a = b = 0.474(9) c = 1.297(0) | 106.88 ± 0.07 | 21.1 ± 2 | Al-O: 0.1987 |
| Present Study | Literature [41] | ||||
|---|---|---|---|---|---|
| Sample | TPR | OSC * | Sample | OSC ** | |
| Peak Temperature (T(°C)) | H2 Consumption (µmolg−1 Anode Materials) | O2 Uptake (µmolg−1 Anode Materials) | O2 Uptake (µmolg−1) | ||
| ANODE 1 | 200–980 | 446.4 | 446.4 | Ce0.5Zr0.5O2 | 318.5 |
| ANODE 2 | 280–980 | 446.4 | 424.1 | Ce0.5Zr0.5O2/Al2O3 (90/10) a | 351.5 |
| ANODE 3 | 340–1000 | 375 | 415.2 | Ce0.5Zr0.5O2/Al2O3 (75/25) b | 365.4 |
| Anode | Functional Layer 1 | Functional Layer 2 | ||||
|---|---|---|---|---|---|---|
| 8YSZ (wt.%) | CeO2-Al2O3 (wt.%) | Cornstarch (wt.%) | 8YSZ (wt.%) | CeO2-Al2O3 (wt.%) | Cornstarch (wt.%) | |
| ANODE 1 | 50 | 50 | 20 | 50 | 50 | 20 |
| ANODE 2 | 70 | 30 | 20 | 50 | 50 | 20 |
| ANODE 3 | 70 | 30 | 20 | 0 | 100 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venâncio, S.A.; de Miranda, P.E.V. Ni-Free SOFC Anode Material with Thermal and Redox Stabilities for the Direct Utilization of Ethanol. Catalysts 2023, 13, 134. https://doi.org/10.3390/catal13010134
Venâncio SA, de Miranda PEV. Ni-Free SOFC Anode Material with Thermal and Redox Stabilities for the Direct Utilization of Ethanol. Catalysts. 2023; 13(1):134. https://doi.org/10.3390/catal13010134
Chicago/Turabian StyleVenâncio, Selma Aparecida, and Paulo Emilio Valadão de Miranda. 2023. "Ni-Free SOFC Anode Material with Thermal and Redox Stabilities for the Direct Utilization of Ethanol" Catalysts 13, no. 1: 134. https://doi.org/10.3390/catal13010134





