The Reaction Mechanism of the Cu(I) Catalyzed Alkylation of Heterosubstituted Alkynes
Abstract
:1. Introduction
2. Results
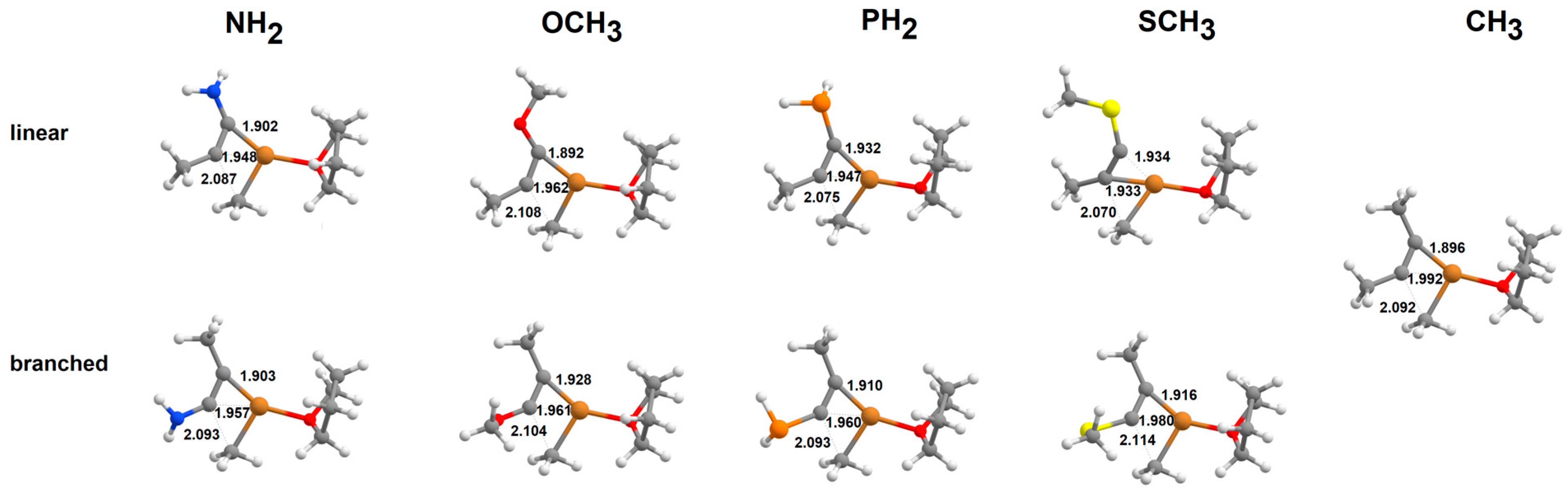
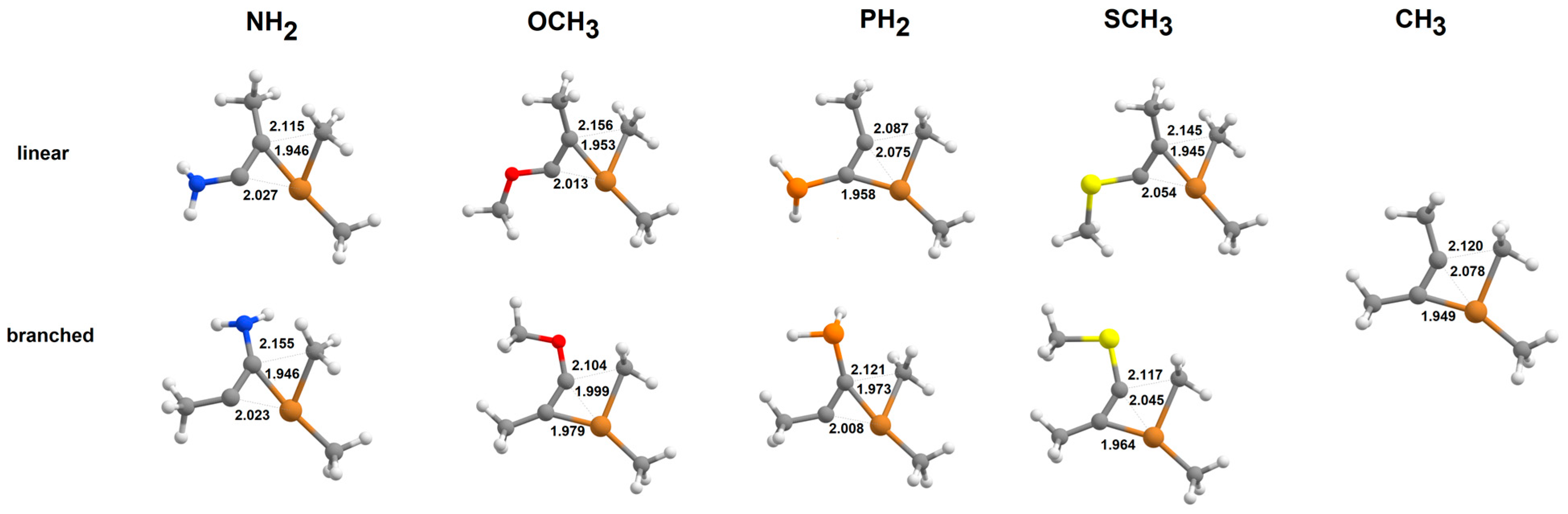
2.1. Addition of Tetrahydrofuran-Solvated Cu(CH3) to Alkynes
2.2. Addition of [Cu(CH3)2]-to Alkynes
2.3. Addition of Cu(CH3)2·Li to Alkynes
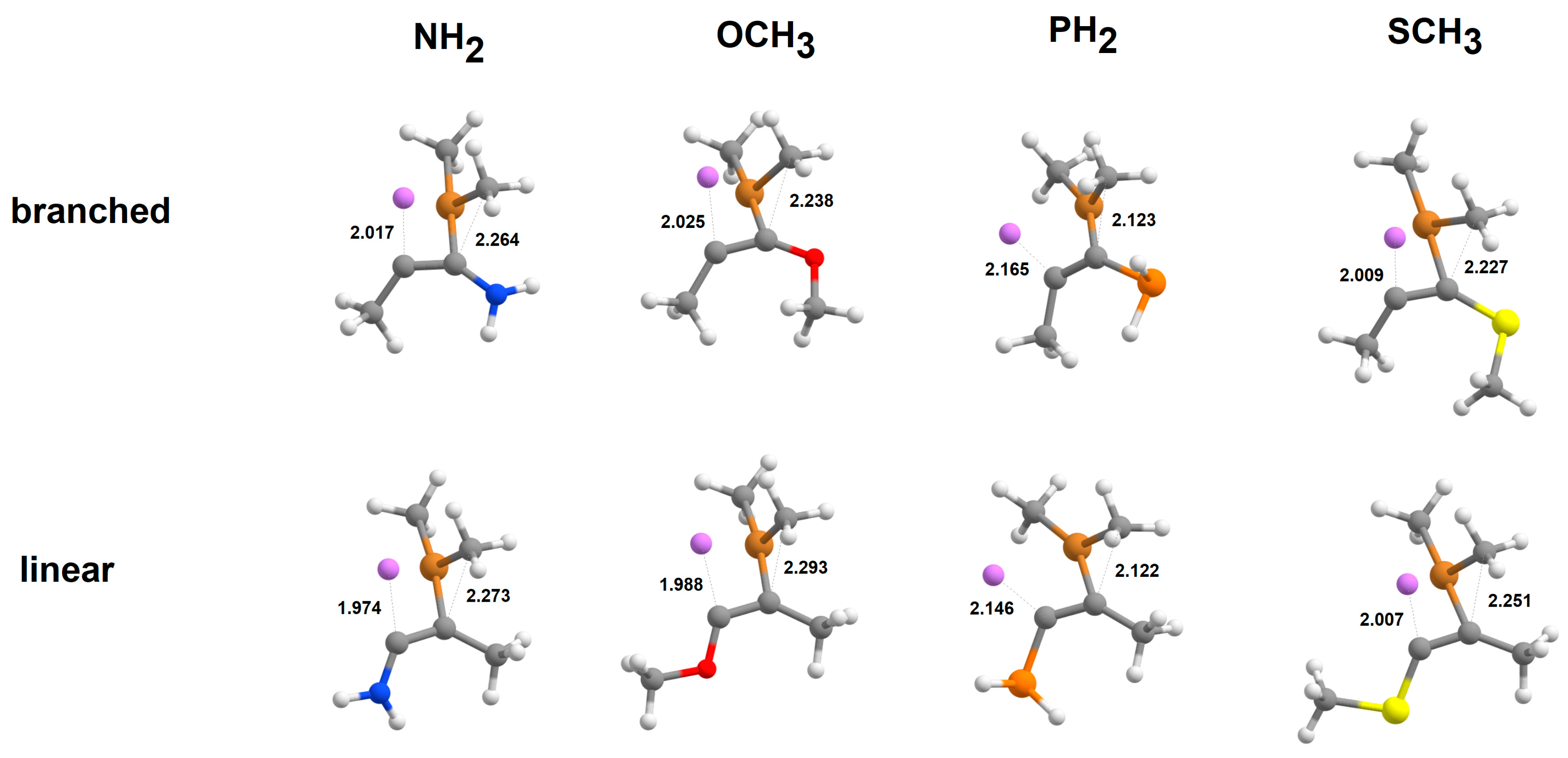
2.4. Addition of [Cu(CH3)2·Mg]+to Alkynes
2.5. Addition of Cu(CH3)2·MgBr to Heteroalkynes
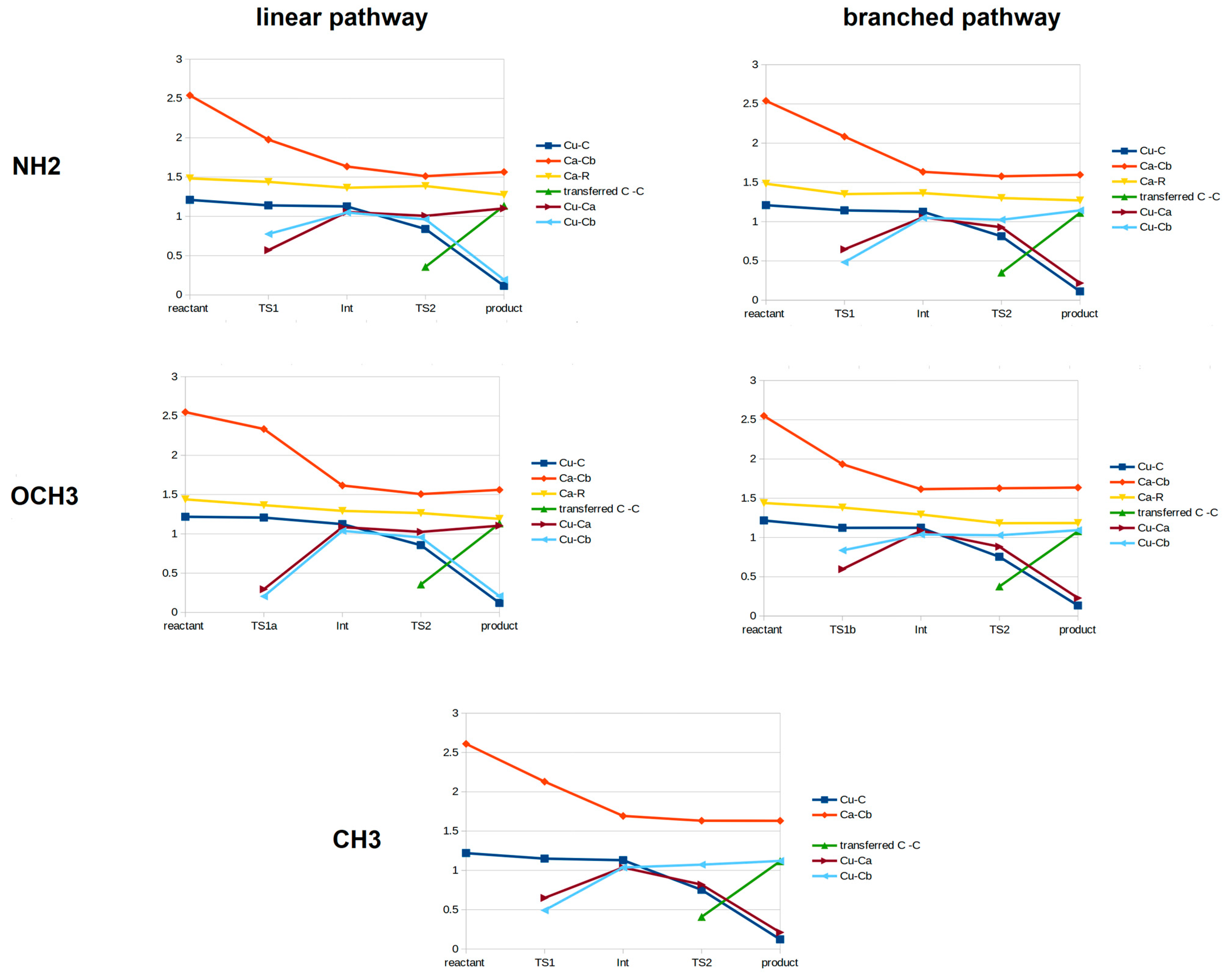
3. Discussion
4. Materials and Methods
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Chemla, F.; Ferreira, F. Carbocupration of Alkynes. In PATAI’S Chemistry of Functional Groups; John Wiley & Sons, Ltd: Chichester, UK, 2011; pp. 527–584. ISBN 9780470682531. [Google Scholar]
- Basheer, A.; Marek, I. Recent advances in carbocupration of α-heterosubstituted alkynes. Beilstein J. Org. Chem. 2010, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, D.S.; Marek, I. Copper mediated carbometalation reactions. Chem. Soc. Rev. 2016, 45, 4552–4566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varghese, J.P.; Zouev, I.; Aufauvre, L.; Knochel, P.; Marek, I. Carbocupration/Zinc Carbenoid Homologation and β-Elimination Reactions for a New Synthesis of Allenes—Application to the Enantioselective Synthesis of Chiral Allenes by Deracemization of sp3-Organometallic Derivatives. Eur. J. Org. Chem. 2002, 2002, 4151–4158. [Google Scholar] [CrossRef]
- Das, J.P.; Chechik, H.; Marek, I. A unique approach to aldol products for the creation of all-carbon quaternary stereocentres. Nat. Chem. 2009, 1, 128–132. [Google Scholar] [CrossRef]
- Marek, I.; Minko, Y.; Pasco, M.; Mejuch, T.; Gilboa, N.; Chechik, H.; Das, J.P. All-carbon quaternary stereogenic centers in acyclic systems through the creation of several C-C bonds per chemical step. J. Am. Chem. Soc. 2014, 136, 2682–2694. [Google Scholar] [CrossRef]
- Nakamura, E.; Miyachi, Y.; Koga, N.; Morokuma, K. Theoretical Studies of Heteroatom-Directed Carbometalation. Addition of MeCu, Me2Cu-, and MeLi to Substituted Acetylenes. J. Am. Chem. Soc. 1992, 114, 6686–6692. [Google Scholar] [CrossRef]
- Nakamura, E.; Mori, S.; Nakamura, M.; Morokuma, K. Theoretical studies on the addition of polymetallic lithium organocuprate clusters to acetylene. Cooperative effects of metals in a trap-and-bite reaction pathway. J. Am. Chem. Soc. 1997, 119, 4887–4899. [Google Scholar] [CrossRef]
- Granovsky, A.A. Firefly 8.0.0 2013. Available online: http://classic.chem.msu.su/gran/gamess/index.html (accessed on 1 December 2013).
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Baker, J.; Kessi, A.; Delley, B. The generation and use of delocalized internal coordinates in geometry optimization. J. Chem. Phys. 1996, 105, 192–212. [Google Scholar] [CrossRef]
- Perdew, J.; Burke, K.; Wang, Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 1996, 54, 16533–16539. [Google Scholar] [CrossRef]
- Perdew, J.P. Unified theory of exchange and correlation beyond the local density approximation. In Electronic Structure of Solids ’91, Proceedings of the 75. WE-Heraeus-Seminar and 21st Annual International Symposium on Electronic Structure of Solids, Gaussig, Germany, 11-15 March 1991; Ziesche, P., Eschrig, H., Eds.; Akademie Verlag: Berlin, Germany, 1991; Volume 17, pp. 11–20. [Google Scholar]
- Stevens, W.J.; Krauss, M.; Basch, H.; Jasien, P.G. Relativistic compact effective potentials and efficient, shared-exponent basis sets for the third-, fourth-, and fifth-row atoms. Can. J. Chem. 1992, 70, 612–630. [Google Scholar] [CrossRef]
- Bernardo, C.E.P.; Bauman, N.P.; Piecuch, P.; Silva, P.J. Evaluation of density functional methods on the geometric and energetic descriptions of species involved in Cu+-promoted catalysis. J. Mol. Model. 2013, 19, 5457–5467. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.J.; Bernardo, C.E.P. Influence of Alkyne and Azide Substituents on the Choice of the Reaction Mechanism of the Cu+-Catalyzed Addition of Azides to Iodoalkynes. J. Phys. Chem. A 2018, 122, 7497–7507. [Google Scholar] [CrossRef] [PubMed]
- Swart, M.; Güell, M.; Luis, J.M.; Solà, M. Spin-state-corrected Gaussian-type orbital basis sets. J. Phys. Chem. A 2010, 114, 7191–7197. [Google Scholar] [CrossRef] [Green Version]
- Amovilli, C.; Mennucci, B. Self-consistent-field calculation of Pauli repulsion and dispersion contributions to the solvation free energy in the polarizable continuum model. J. Phys. Chem. B 1997, 5647, 1051–1057. [Google Scholar] [CrossRef]
- Tomasi, J.; Persico, M. Molecular Interactions in Solution: An Overview of Methods Based on Continuous Distributions of the Solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
- Cossi, M.; Mennucci, B.; Pitarch, J.; Tomasi, J. Correction of cavity-induced errors in polarization charges of continuum solvation models. J. Comput. Chem. 1998, 19, 833–846. [Google Scholar] [CrossRef]
- Mennucci, B.; Tomasi, J. Continuum solvation models: A new approach to the problem of solute’s charge distribution and cavity boundaries. J. Chem. Phys. 1997, 106, 5151–5158. [Google Scholar] [CrossRef]
- Mayer, I.; Salvador, P. Overlap populations, bond orders and valences for ‘fuzzy’ atoms. Chem. Phys. Lett. 2004, 383, 368–375. [Google Scholar] [CrossRef]
- Marenich, A.V.; Jerome, S.V.; Cramer, C.J.; Truhlar, D.G. Charge model 5: An extension of Hirshfeld population analysis for the accurate description of molecular interactions in gaseous and condensed phases. J. Chem. Theory Comput. 2012, 8, 527–541. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Mendes, P.; Hoops, S.; Sahle, S.; Gauges, R.; Dada, J.; Kummer, U. Computational modeling of biochemical networks using COPASI. Methods Mol. Biol. 2009, 500, 17–59. [Google Scholar] [PubMed]

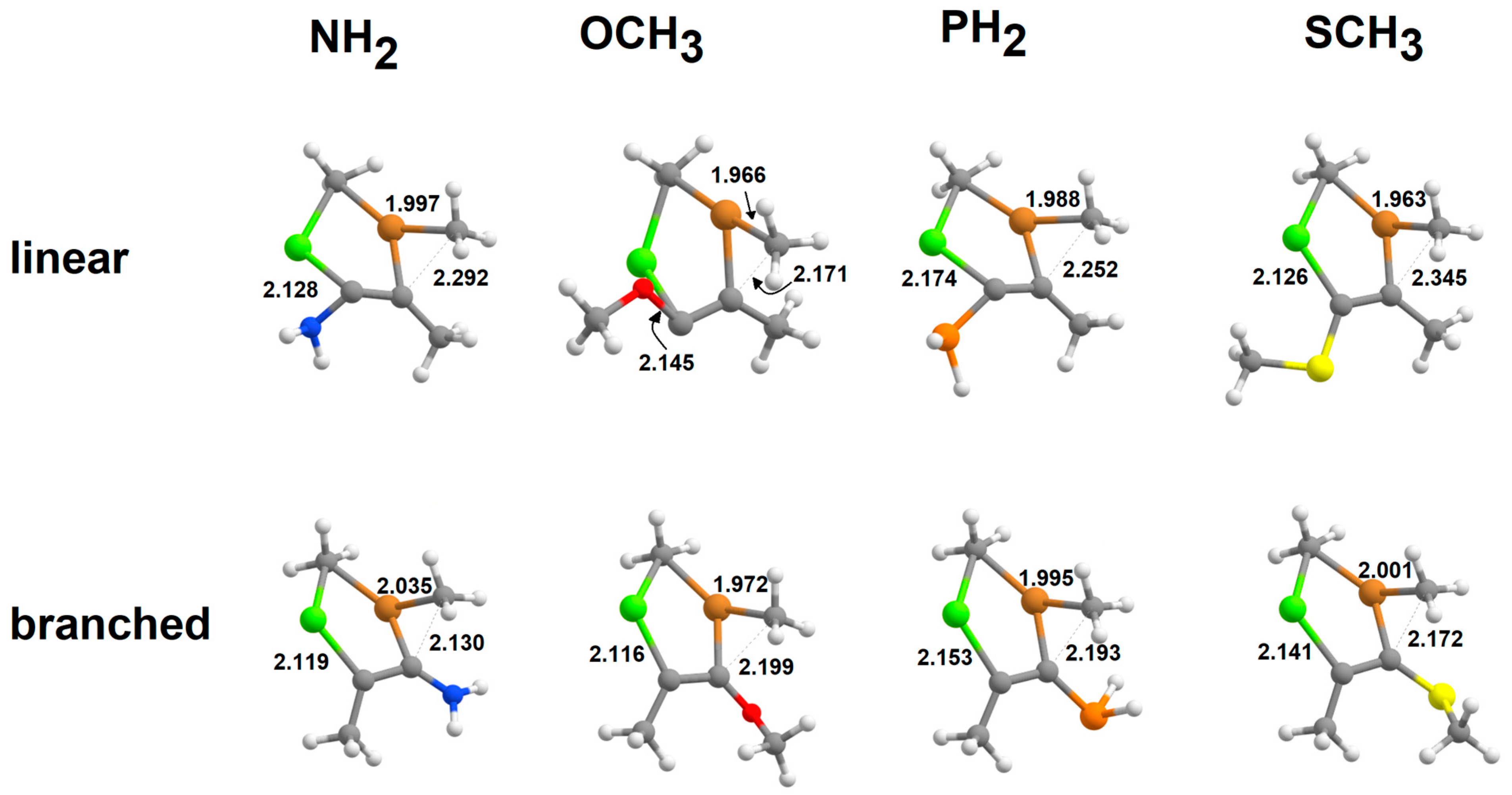
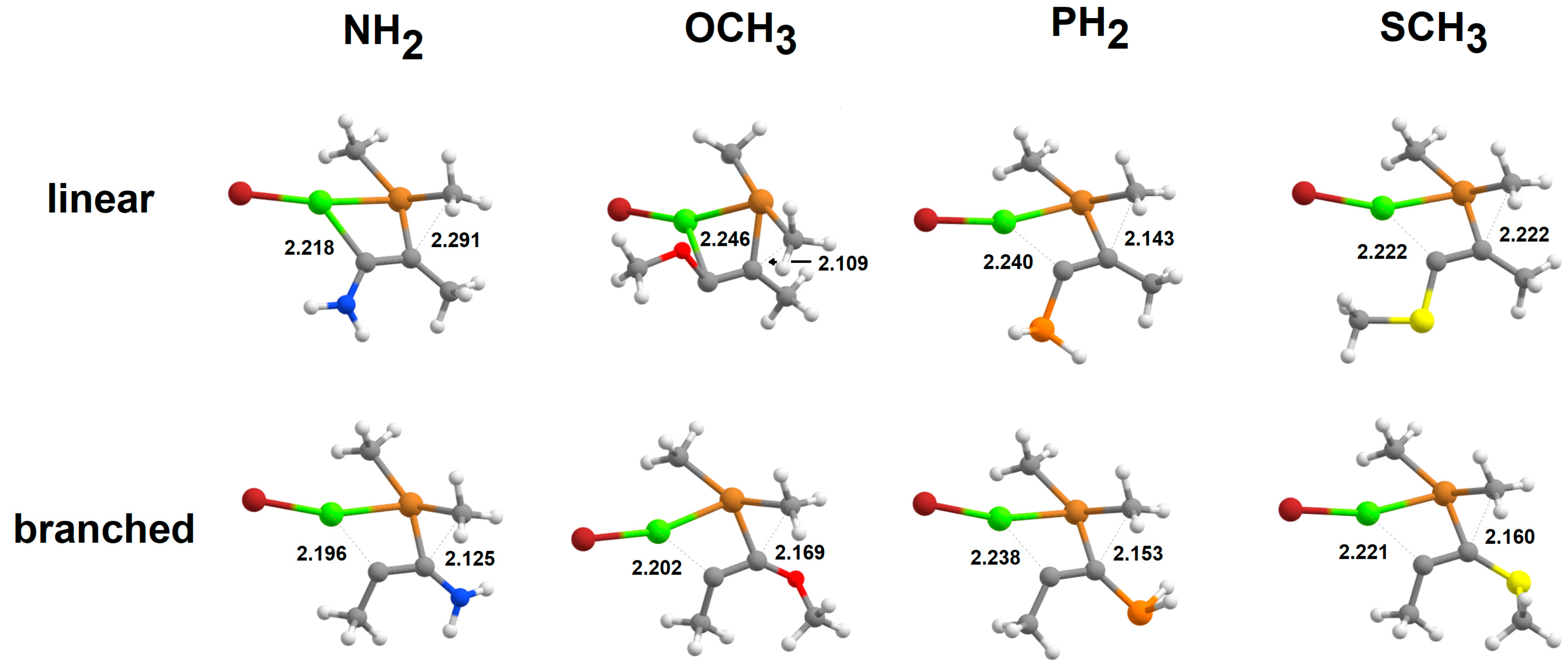
| Branched Pathway | Linear Pathway | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| R= | CH3 | NH2 | OCH3 | PH2 | SCH3 | NH2 | OCH3 | PH2 | SCH3 |
| Separated reactants | 3.6 | 13.5 | 10.2 | 6.1 | 8.8 | 13.5 | 10.2 | 6.1 | 8.8 |
| π-complex | 0.0 | 6.3 | 0.0 | 3.2 | 2.2 | 0.0 | 1.9 | 0.0 | 0.0 |
| TS | 29.5 | 33.0 | 24.6 | 30.7 | 30.8 | 31.2 | 23.4 | 26.6 | 24.7 |
| product | −26.8 | −25.5 | −33.8 | −23.6 | −28.2 | −25.4 | −36.3 | −27.0 | −31.1 |
| Branched Pathway | Linear Pathway | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| R= | CH3 | NH2 | OCH3 | PH2 | SCH3 | NH2 | OCH3 | PH2 | SCH3 |
| Separated reactants | 0.0 | 1.9 | 8.1 | 2.4 | 6.0 | 1.9 | 8.1 | 2.4 | 6.0 |
| π-complex | 2.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| TS | 28.9 | 24.7 | 25.0 | 26.8 | 31.8 | 24.0 | 23.5 | 22.5 | 20.3 |
| Product | −27.4 | −32.2 | −25.7 | −28.1 | −22.0 | −34.8 | −37.4 | −32.2 | −34.0 |
| 2-Butyne | Propyne (Linear Pathway) | Propyne (Branched Pathway) | Acetylene | |
|---|---|---|---|---|
| π-complex | −3.3 | −5.4 | −5.4 | −7.7 |
| TS | 25.4 | 20.6 | 24.1 | 16.5 |
| product | −27.9 | −33.6 | −33.6 | −39.3 |
| Activation ΔG | 28.7 | 26.0 | 29.6 | 24.2 |
| Branched Pathway | Linear Pathway | |||||||
|---|---|---|---|---|---|---|---|---|
| R= | NH2 | OCH3 | PH2 | SCH3 | NH2 | OCH3 | PH2 | SCH3 |
| TS1 | 7.7 | 13.3 | 14.3 | 13.4 | 9.5 | 11.0 | 14.3 | 13.4 |
| π-complex | −5.1 | −8.7 | −5.2 | −5.4 | −5.1 | −10.9 | −5.2 | −6.5 |
| TS2 | 18.7 | 13.7 | 21.0 | 20.9 | 16.3 | 11.9 | 13.1 | 12.8 |
| product | −36.9 | −35.4 | −27.9 | −30.5 | −37.2 | −48.7 | −31.9 | −40.5 |
| Activation ΔG | 23.8 | 22.4 | 26.2 | 26.4 | 21.3 | 22.9 | 18.3 | 19.3 |
| Branched Pathway | Linear Pathway | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| R= | CH3 | NH2 | OCH3 | PH2 | SCH3 | NH2 | OCH3 | PH2 | SCH3 |
| Pre-reactional complex | N/D | 0.0 | 1.6 | 0.0 | 1.0 | N/A | 0.0 | N/A | 0.0 |
| TS1 | N/D | 16.7 | 14.5 | 15.2 | 11.4 | N/A | 16.6 | N/A | 11.4 |
| Bidentate complex | 0.0 | 2.6 | 0.7 | −6.6 | −4.0 | 7.4 | 12.7 | −4.6 | −1.8 |
| TS2 | 8.8 | 11.8 | 3.2 | 3.4 | 4.8 | 23.8 | 11.2 | 6.6 | −3.4 |
| Product | −35.8 | −45.1 | −47.7 | −43.0 | −44.3 | −30.2 | −47.2 | −47.2 | −45.8 |
| Branched Pathway | Linear Pathway | |||||||
|---|---|---|---|---|---|---|---|---|
| R= | NH2 | OCH3 | PH2 | SCH3 | NH2 | OCH3 | PH2 | SCH3 |
| Pre-reactional complex | 9.1 | 12.4 | 18.0 | 16.9 | 13.0 | 13.5 | 13.8 | 15.1 |
| π-complex | −2.4 | −3.5 | 2.8 | 0.7 | 5.1 | 19.2 | −1.1 | 2.9 |
| TS | 11.9 | 8.3 | 19.1 | 16.9 | 10.1 | 20.3 | 13.2 | 10.3 |
| product | −41.5 | −39.4 | −24.9 | −28.7 | −32.1 | −42.5 | −26.3 | −37.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, P.J. The Reaction Mechanism of the Cu(I) Catalyzed Alkylation of Heterosubstituted Alkynes. Catalysts 2023, 13, 17. https://doi.org/10.3390/catal13010017
Silva PJ. The Reaction Mechanism of the Cu(I) Catalyzed Alkylation of Heterosubstituted Alkynes. Catalysts. 2023; 13(1):17. https://doi.org/10.3390/catal13010017
Chicago/Turabian StyleSilva, Pedro J. 2023. "The Reaction Mechanism of the Cu(I) Catalyzed Alkylation of Heterosubstituted Alkynes" Catalysts 13, no. 1: 17. https://doi.org/10.3390/catal13010017
APA StyleSilva, P. J. (2023). The Reaction Mechanism of the Cu(I) Catalyzed Alkylation of Heterosubstituted Alkynes. Catalysts, 13(1), 17. https://doi.org/10.3390/catal13010017







