Large-Scale Preparation of Ultrathin Bimetallic Nickel Iron Sulfides Branch Nanoflake Arrays for Enhanced Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. Results and Discussion
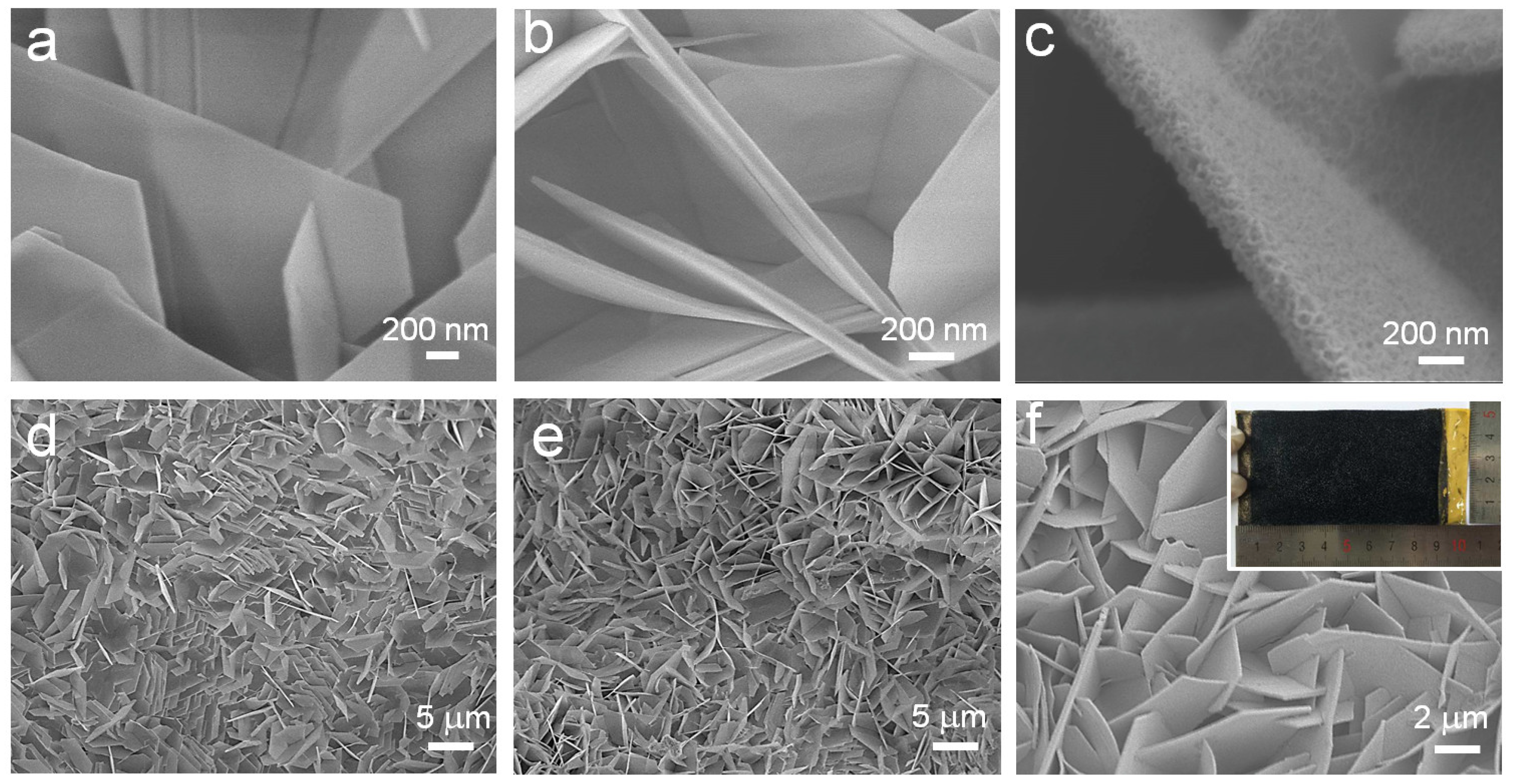
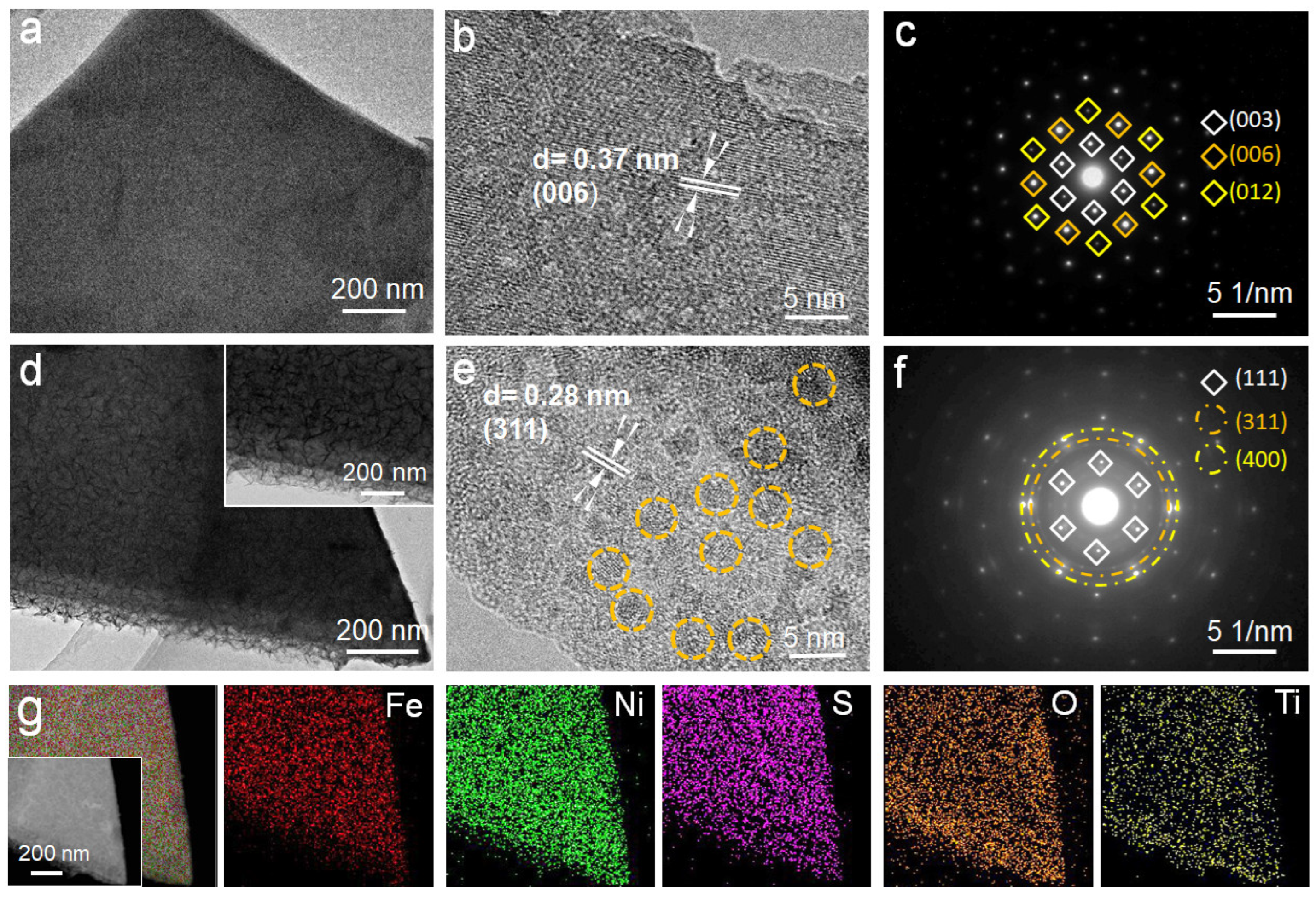
2.1. Morphology and Structure Analyses
2.2. Electrochemical Performance Analyses
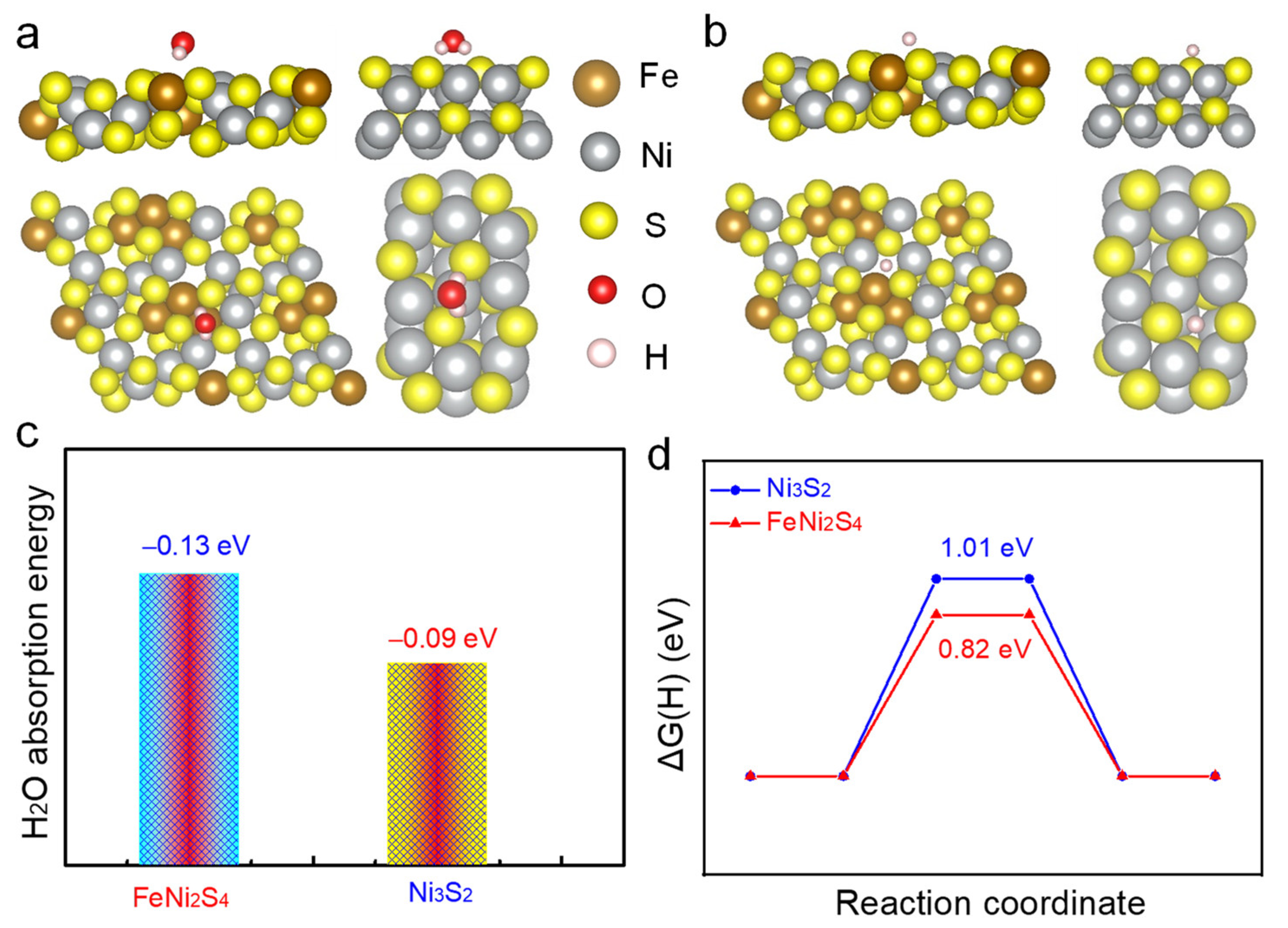
2.3. DFT Calculation
3. Materials and Methods
3.1. Synthesis of TiO2@FNS Arrays on Ni Foam
3.2. Characterization
3.3. Electrochemical Measurements
3.4. Computational Methods and Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Wang, Z.; Liu, M.; Wang, E.; Wang, B.; Xu, L.; Jiang, K.; Fan, S.; Sun, Y.; Li, J.; et al. Ultrafast self-heating synthesis of robust heterogeneous nanocarbides for high current density hydrogen evolution reaction. Nat. Commun. 2022, 13, 3338. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cheng, J.; Yang, X.; Xu, Y.; Sun, W.; Zhou, J. Facet-tunable coral-like Mo2C catalyst for electrocatalytic hydrogen evolution reaction. Chem. Eng. J. 2023, 451, 138977. [Google Scholar] [CrossRef]
- Zhao, G.; Rui, K.; Dou, S.X.; Sun, W. Heterostructures for Electrochemical Hydrogen Evolution Reaction: A Review. Adv. Funct. Mater. 2018, 28, 1803291. [Google Scholar] [CrossRef] [Green Version]
- Che, S.; Ta, N.; Yang, F.; Yang, Y.; Li, Y. I Interfacial electronic engineering of NiSe–anchored Ni–N–C composite electrocatalyst for efficient hydrogen evolution. Catalysts 2022, 12, 1525. [Google Scholar] [CrossRef]
- Zhou, Q.; Bian, Q.; Liao, L.; Yu, F.; Li, D.; Tang, D.; Zhou, H. In situ electrochemical dehydrogenation of ultrathin Co(OH)2 nanosheets for enhanced hydrogen evolution. Chin. Chem. Lett. 2023, 34, 107248. [Google Scholar] [CrossRef]
- Tong, Y.; Liu, J.; Wang, L.; Su, B.-J.; Wu, K.-H.; Juang, J.-Y.; Hou, F.; Yin, L.; Dou, S.; Liu, J.; et al. Carbon-Shielded Single-Atom Alloy Material Family for Multi-Functional Electrocatalysis. Adv. Funct. Mater. 2022, 32, 2205654. [Google Scholar] [CrossRef]
- Karmodak, N.; Andreussi, O. Catalytic Activity and Stability of Two-Dimensional Materials for the Hydrogen Evolution Reaction. ACS Energy Lett. 2020, 5, 885–891. [Google Scholar] [CrossRef]
- Zeng, F.; Mebrahtu, C.; Liao, L.; Beine, A.K.; Palkovits, R. Stability and deactivation of OER electrocatalysts: A review. J. Energy Chem. 2022, 69, 301–329. [Google Scholar] [CrossRef]
- Xu, Z.; Hao, M.; Liu, X.; Ma, J.; Wang, L.; Li, C.; Wang, W. Co(OH)2 nanoflowers decorated α-NiMoO4 nanowires as a bifunctional electrocatalyst for efficient overall water splitting. Catalysts 2022, 12, 1417; [Google Scholar] [CrossRef]
- Li, C.; Jang, H.; Kim, M.G.; Hou, L.; Liu, X.; Cho, J. Ru-incorporated oxygen-vacancy-enriched MoO2 electrocatalysts for hydrogen evolution reaction. Appl. Catal. B Environ. 2022, 307, 121204. [Google Scholar] [CrossRef]
- Ye, J.; Zang, Y.; Wang, Q.; Zhang, Y.; Sun, D.; Zhang, L.; Wang, G.; Zheng, X.; Zhu, J. Nitrogen doped FeS2 nanoparticles for efficient and stable hydrogen evolution reaction. J. Energy Chem. 2020, 56, 283–289. [Google Scholar] [CrossRef]
- Liao, L.; Zhao, Y.; Zhou, H.; Li, D.; Qi, Y.; Zhang, Y.; Sun, Y.; Zhou, Q.; Yu, F. Edge-oriented N-Doped WS2 Nanoparticles on Porous Co3N Nanosheets for Efficient Alkaline Hydrogen Evolution and Nitrogenous Nucleophile Electrooxidation. Small 2022, 18, 2203171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, Y.; He, R.; Yu, F.; Sun, J.; Wang, F.; Lan, Y.; Ren, Z.; Chen, S. One-step synthesis of self-supported porous NiSe2/Ni hybrid foam: An efficient 3D electrode for hydrogen evolution reaction. Nano Energy 2016, 20, 29–36. [Google Scholar] [CrossRef]
- Deng, S.; Yang, F.; Zhang, Q.; Zhong, Y.; Zeng, Y.; Lin, S.; Wang, X.; Lu, X.; Wang, C.-Z.; Gu, L.; et al. Phase Modulation of (1T-2H)-MoSe2/TiC-C Shell/Core Arrays via Nitrogen Doping for Highly Efficient Hydrogen Evolution Reaction. Adv. Mater. 2018, 30, 1802223. [Google Scholar] [CrossRef] [Green Version]
- Zang, Y.; Yang, B.; Li, A.; Liao, C.; Chen, G.; Liu, M.; Liu, X.; Ma, R.; Zhang, N. Tuning Interfacial Active Sites over Porous Mo2N-Supported Cobalt Sulfides for Efficient Hydrogen Evolution Reactions in Acid and Alkaline Electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 41573–41583. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Xue, S.; Zhou, S.; Qu, K.; Li, Y.; Cai, W. Pt/Mo2C heteronanosheets for superior hydrogen evolution reaction. J. Energy Chem. 2020, 47, 317–323. [Google Scholar] [CrossRef]
- Liu, W.; Geng, P.; Li, S.; Liu, W.; Fan, D.; Lu, H.; Lu, Z.; Liu, Y. Tuning electronic configuration of WP2 nanosheet arrays via nickel doping for high-efficiency hydrogen evolution reaction. J. Energy Chem. 2020, 55, 17–24. [Google Scholar] [CrossRef]
- Lin, L.; Sherrell, P.; Liu, Y.; Lei, W.; Zhang, S.; Zhang, H.; Wallace, G.G.; Chen, J. Engineered 2D Transition Metal Dichalcogenides—A Vision of Viable Hydrogen Evolution Reaction Catalysis. Adv. Energy Mater. 2020, 10, 1903870. [Google Scholar] [CrossRef]
- Morozan, A.; Johnson, H.; Roiron, C.; Genay, G.; Aldakov, D.; Ghedjatti, A.; Nguyen, C.T.; Tran, P.D.; Kinge, S.; Artero, V. Nonprecious Bimetallic Iron–Molybdenum Sulfide Electrocatalysts for the Hydrogen Evolution Reaction in Proton Exchange Membrane Electrolyzers. ACS Catal. 2020, 10, 14336–14348. [Google Scholar] [CrossRef]
- Yang, H.; Driess, M.; Menezes, P.W. Self-Supported Electrocatalysts for Practical Water Electrolysis. Adv. Energy Mater. 2021, 11, 2102074. [Google Scholar] [CrossRef]
- Sivanantham, A.; Ganesan, P.; Shanmugam, S. Hierarchical NiCo2S4Nanowire Arrays Supported on Ni Foam: An Efficient and Durable Bifunctional Electrocatalyst for Oxygen and Hydrogen Evolution Reactions. Adv. Funct. Mater. 2016, 26, 4661–4672. [Google Scholar] [CrossRef]
- Zhao, Y.; Mavrokefalos, C.K.; Zhang, P.; Erni, R.; Li, J.; Triana, C.A.; Patzke, G.R. Self-Templating Strategies for Transition Metal Sulfide Nanoboxes as Robust Bifunctional Electrocatalysts. Chem. Mater. 2020, 32, 1371–1383. [Google Scholar] [CrossRef]
- Su, H.; Song, S.; Gao, Y.; Li, N.; Fu, Y.; Ge, L.; Song, W.; Liu, J.; Ma, T. In Situ Electronic Redistribution Tuning of NiCo2S4 Nanosheets for Enhanced Electrocatalysis. Adv. Funct. Mater. 2022, 32, 2109731. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Roy, A.; Thorat, G.M.; Chung, W.-J.; Gil Seo, J. Hierarchical free-standing networks of MnCo2S4 as efficient Electrocatalyst for oxygen evolution reaction. J. Ind. Eng. Chem. 2018, 71, 452–459. [Google Scholar] [CrossRef]
- Li, L.; Dai, Y.; Xu, Q.; Zhang, B.; Zhang, F.; You, Y.; Ma, D.; Li, S.-S.; Zhang, Y.-X. Interlayer expanded nickel-iron layered double hydroxide by intercalation with sodium dodecyl sulfate for enhanced oxygen evolution reaction. J. Alloy. Compd. 2021, 882, 160752. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Srinivas, K.; Wang, M.; Liu, D.; Chen, X.; Wang, B.; Wang, S.; Chen, Y. Heterogeneous FeNi2S4/Ni3S4 nanoparticles embedded CNT networks for efficient and stable water oxidation. Journal Alloys Compounds 2022, 914, 165327. [Google Scholar] [CrossRef]
- Ren, L.; Wang, C.; Li, W.; Dong, R.; Sun, H.; Liu, N.; Geng, B. Heterostructural NiFe-LDH@Ni3S2 nanosheet arrays as an efficient electrocatalyst for overall water splitting. Electrochim. Acta 2019, 318, 42–50. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.-J.; Zhu, X.-J.; Lu, S.; Long, L.-L.; Chen, J.-J. Nanostructured metallic FeNi2S4 with reconstruction to generate FeNi-based oxide as a highly-efficient oxygen evolution electrocatalyst. Nano Energy 2020, 81, 105619. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Yuan, M.; Hao, H.; San, X.; Lv, Z.; Xu, L.; Wei, B. Operando capturing of surface self-reconstruction of Ni3S2/FeNi2S4 hybrid nanosheet array for overall water splitting. Chem. Eng. J. 2022, 427, 131944. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, H.; Zhu, Z.; Sun, J.; He, R.; Bao, J.; Chen, S.; Ren, Z. Three-Dimensional Nanoporous Iron Nitride Film as an Efficient Electrocatalyst for Water Oxidation. ACS Catal. 2017, 7, 2052–2057. [Google Scholar] [CrossRef]
- Liu, C.; Jia, D.; Hao, Q.; Zheng, X.; Li, Y.; Tang, C.; Liu, H.; Zhang, J.; Zheng, X. P-Doped Iron–Nickel Sulfide Nanosheet Arrays for Highly Efficient Overall Water Splitting. ACS Appl. Mater. Interfaces 2019, 11, 27667–27676. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Guan, P.-Y.; Li, Q.-Y.; Zhang, M.; Zang, S.-Q. MOF-Derived Flower-like MoS2@TiO2 Nanohybrids with Enhanced Activity for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2016, 8, 26794–26800. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zavabeti, A.; Wang, B.; Ren, R.; Yang, C.; Liu, Z.; Wang, Y. Nickel Phosphides Electrodeposited on TiO2 Nanotube Arrays as Electrocatalysts for Hydrogen Evolution. ACS Appl. Nano Mater. 2021, 4, 4542–4551. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, K.; Xie, D.; Zhang, Y.; Zhang, Y.; Wang, Y.; Wu, J.; Wang, X.; Fan, H.J.; Xia, X.; et al. High-Index-Faceted Ni3S2 Branch Arrays as Bifunctional Electrocatalysts for Efficient Water Splitting. Nano-Micro Lett. 2019, 11, 12. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zou, S.; Liu, X.; Lu, Y.; Dong, D. Electronic Modulation of CoP by Ce Doping as Highly Efficient Electrocatalysts for Water Splitting. ACS Sustain. Chem. Eng. 2020, 8, 10009–10016. [Google Scholar] [CrossRef]
- Tian, T.; Huang, L.; Ai, L.; Jiang, J. Surface anion-rich NiS2 hollow microspheres derived from metal–organic frameworks as a robust electrocatalyst for the hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 20985–20992. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, M.; Li, H.; Sun, G.; Ma, S. Controllable synthesis of ultrathin Co9S8 nanosheets as a highly efficient electrocatalyst for overall water splitting. Electrochim. Acta 2018, 281, 198–207. [Google Scholar] [CrossRef]
- Li, L.; Sun, C.; Shang, B.; Li, Q.; Lei, J.; Li, N.; Pan, F. Tailoring the facets of Ni3S2 as a bifunctional electrocatalyst for high-performance overall water-splitting. J. Mater. Chem. A 2019, 7, 18003–18011. [Google Scholar] [CrossRef]
- Li, N.; Liu, X.; Li, G.-D.; Wu, Y.; Gao, R.; Zou, X. Vertically grown CoS nanosheets on carbon cloth as efficient hydrogen evolution electrocatalysts. Int. J. Hydrogen Energy 2017, 42, 9914–9921. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, Y.; Gao, T.; Yao, T.; Zhang, X.; Han, J.; Wang, X.; Zhang, Z.; Xu, P.; Zhang, P.; et al. Synergistic Phase and Disorder Engineering in 1T-MoSe2 Nanosheets for Enhanced Hydrogen-Evolution Reaction. Adv. Mater. 2017, 29, 1700311. [Google Scholar] [CrossRef]
- Sun, J.; Liu, J.; Chen, H.; Han, X.; Wu, Y.; He, J.; Han, C.; Yang, G.; Shan, Y. Strongly coupled Mo2C and Ni nanoparticles with in-situ formed interfaces encapsulated by porous carbon nanofibers for efficient hydrogen evolution reaction under alkaline conditions. J. Colloid Interface Sci. 2019, 558, 100–105. [Google Scholar] [CrossRef]
- Yu, J.; Cheng, G.; Luo, W. Ternary nickel–iron sulfide microflowers as a robust electrocatalyst for bifunctional water splitting. J. Mater. Chem. A 2017, 5, 15838–15844. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blöchl, P.E.; Jepsen, O.; Andersen, O.K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B 1994, 49, 16223–16233. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, S.; Liu, C.; Zhang, Y.; Ji, Y.; Mei, B.; Yao, Z.; Lin, S. Large-Scale Preparation of Ultrathin Bimetallic Nickel Iron Sulfides Branch Nanoflake Arrays for Enhanced Hydrogen Evolution Reaction. Catalysts 2023, 13, 174. https://doi.org/10.3390/catal13010174
Deng S, Liu C, Zhang Y, Ji Y, Mei B, Yao Z, Lin S. Large-Scale Preparation of Ultrathin Bimetallic Nickel Iron Sulfides Branch Nanoflake Arrays for Enhanced Hydrogen Evolution Reaction. Catalysts. 2023; 13(1):174. https://doi.org/10.3390/catal13010174
Chicago/Turabian StyleDeng, Shengjue, Changsheng Liu, Yan Zhang, Yingxi Ji, Bingbao Mei, Zhendong Yao, and Shiwei Lin. 2023. "Large-Scale Preparation of Ultrathin Bimetallic Nickel Iron Sulfides Branch Nanoflake Arrays for Enhanced Hydrogen Evolution Reaction" Catalysts 13, no. 1: 174. https://doi.org/10.3390/catal13010174




