Hydrogen Evolution Reaction Activities of Room-Temperature Self-Grown Glycerol-Assisted Nickel Chloride Nanostructures
Abstract
:1. Introduction
2. Results
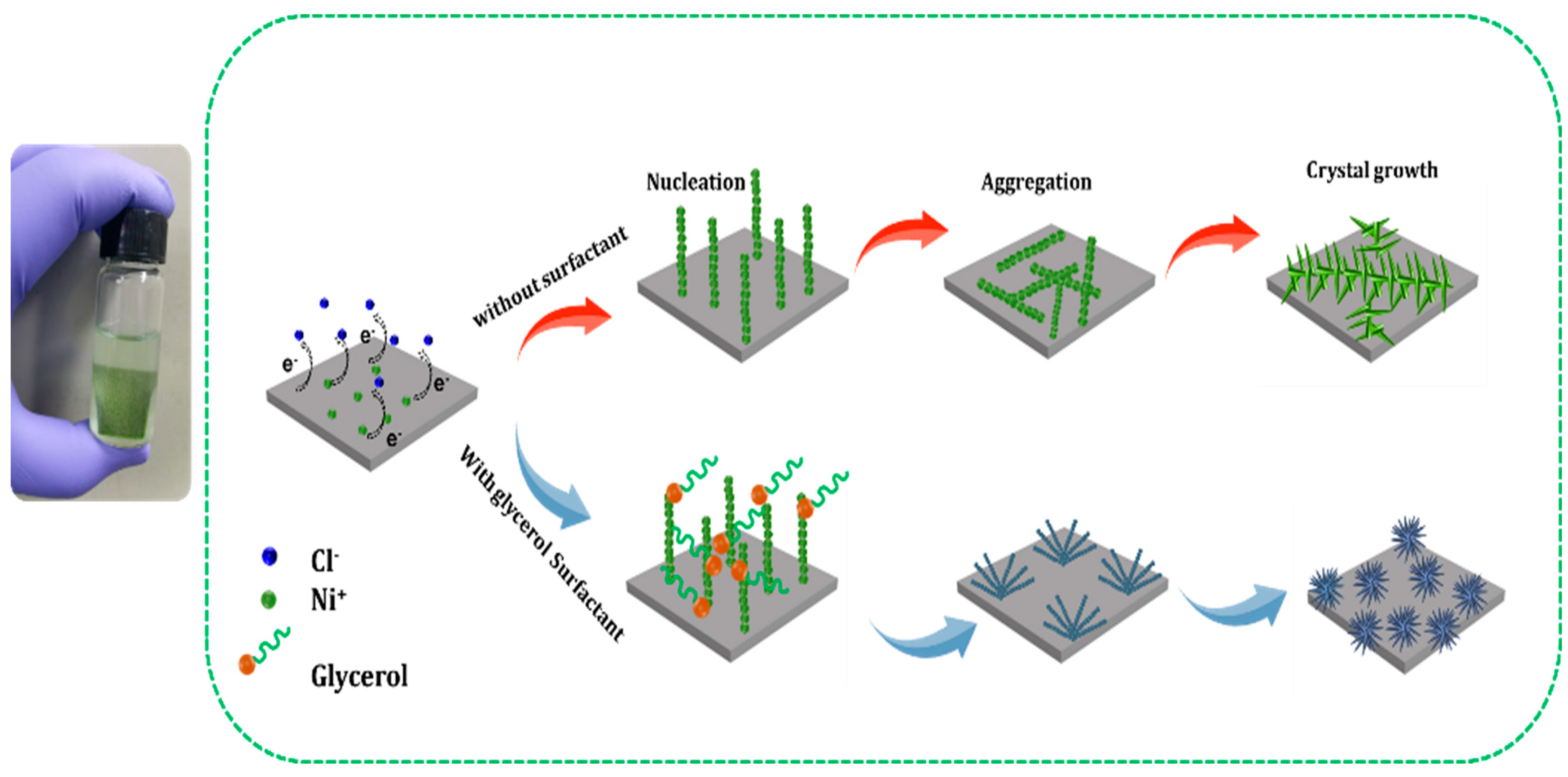
2.1. Morphology Evolution Studies
2.2. Structural Elucidation
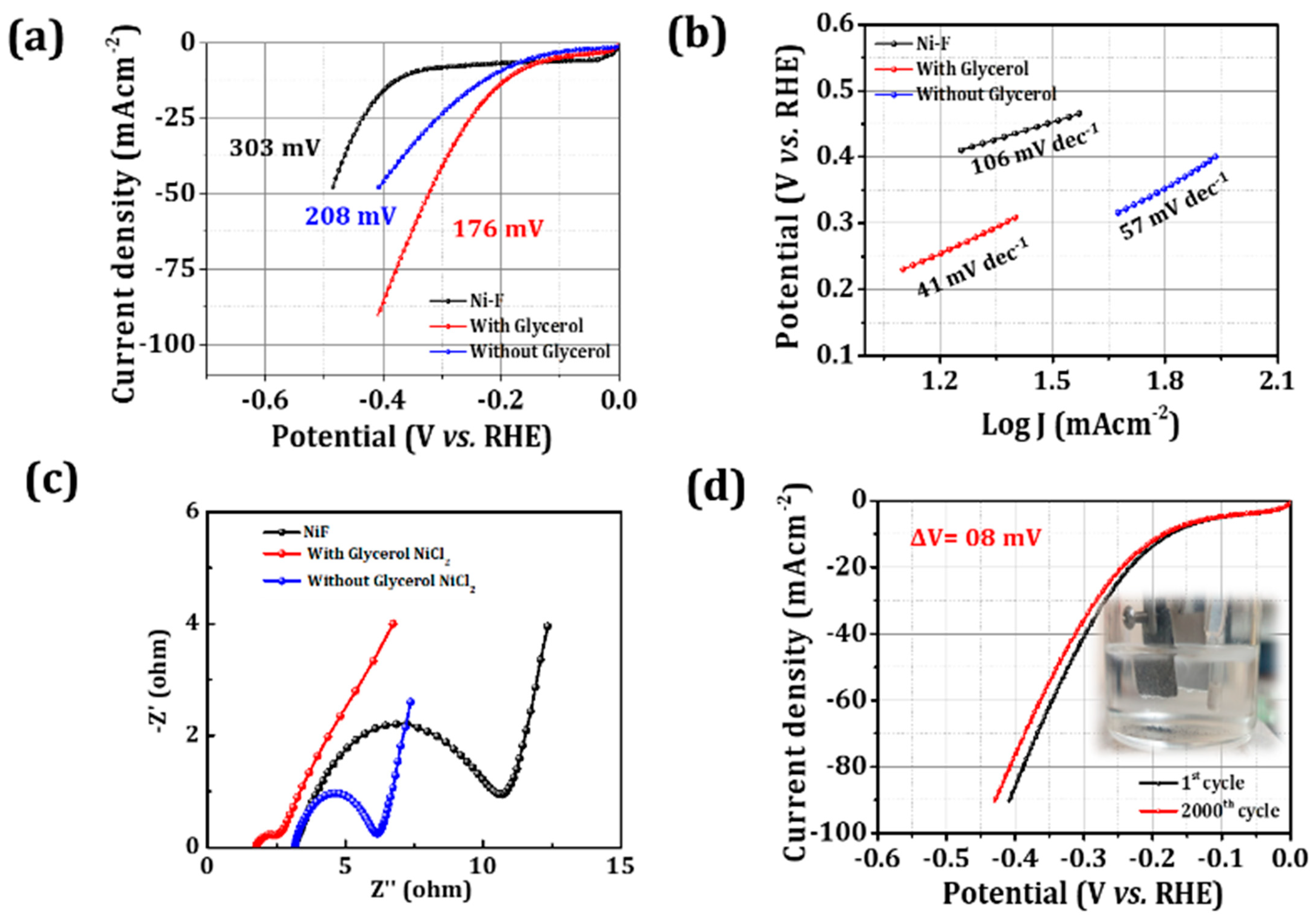
2.3. HER Activity
3. Materials and Methods
3.1. Chemicals
3.2. Characterizations
3.3. HER Confirmation
3.4. Formulae Used
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, G.; Wang, P.; Li, H.; Hu, B.; Sun, Y.; Huang, R.; Liu, L. Spin-state reconfiguration induced by alternating magnetic field for efficient oxygen evolution reaction. Nat. Commun. 2021, 12, 4827. [Google Scholar] [CrossRef]
- Anantharaj, S.; Rao Ede, S.; Sakthikumar, K.; Karthick, K.; Mishra, S.; Kundu, S. A Recent Trends and Perspectives in Electrochemical Water Splitting with an Emphasis on Sulfide, Selenide, and Phosphide Catalysts of Fe, Co, and Ni: A Review. ACS Catal. 2016, 6, 8069–8097. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, P.; Hu, B.; Shen, X.; Liu, C.; Tao, W.; Huang, P.; Liu, L. Spin-related symmetry breaking induced by half-disordered hybridization in BixEr2-xRu2O7 pyrochlores for acidic oxygen evolution. Nat. Commun. 2022, 13, 4106. [Google Scholar] [CrossRef]
- Suen, N.; Hung, S.; Quan, Q.; Zhang, N.; Xu, Y.; Chen, M. Electrocatalysis for the Oxygen Evolution Reaction: Recent Development and Future Perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
- Zhou, G.; Shan, Y.; Wang, L.; Hu, Y.; Guo, J.; Hu, F.; Shen, J.; Gu, Y.; Cui, J.; Liu, L.; et al. Photoinduced semiconductor-metal transition in ultrathin troilite FeS nanosheets to trigger efficient hydrogen evolution. Nat. Commun. 2019, 10, 399. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Shan, Y.; Zhou, G.; Long, L.; Wang, L.; Yin, K.; Guo, J.; Shen, J.; Liu, L.; Wu, X. Electric strain in dual metal Janus nanosheets induces structural phase transition for efficient hydrogen evolution. Joule 2019, 3, 2955–2967. [Google Scholar] [CrossRef]
- Chaudhari, N.; Jin, H.; Kim, B.; Lee, K. Nanostructured Materials on 3D Nickel Foam as Electrocatalysts for Water Splitting. Nanoscale 2017, 9, 12231–12247. [Google Scholar] [CrossRef]
- Giri, A.; Park, G.; Yang, H.; Pal, M.; Kwak, J.; Jeong, U. Synthesis of 2D Metal Chalcogenide Thin Films through the Process Involving Solution-Phase Deposition. Adv. Mater. 2013, 30, 1707577. [Google Scholar] [CrossRef]
- Chou, S.; Lin, J. Cathodic Deposition of Flaky Nickel Sulfide Nanostructure as an Electroactive Material for High-Performance Supercapacitors. J. Electrochem. Soc. 2013, 160, D178–D182. [Google Scholar] [CrossRef]
- Yu, S.; Yoshimura, M. Ferrite/Metal Composites Fabricated by Soft SolutionProcessing. Adv. Funct. Mater. 2002, 12, 277–285. [Google Scholar] [CrossRef]
- Yang, S.; Yao, H.; Gao, M.; Yu, S. Monodisperse Cubic Pyrite NiS2 Dodecahedrons and Microspheres Synthesized by a Solvothermal Process in a Mixed Solvent: Thermal Stability and Magnetic Properties. CrystEngComm 2009, 11, 1383–1390. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Wu, Y.; Rui, M.; Zeng, H. Two-Dimensional, Porous Nickel–Cobalt Sulfide for High-Performance Asymmetric Supercapacitors. ACS Appl. Mater. Interf. 2015, 7, 19316–19323. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Lu, Z.; Liu, J.; Lei, X.; Chang, Z.; Luo, L.; Sun, X. Metal oxide and Hydroxide Nanoarrays: Hydrothermal Synthesis and Applications as Supercapacitors and Nanocatalysts. Prog. Nat. Sci. Mater. Int. 2013, 23, 351–366. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, Y.; Kang, H.; Jin, T.; Jiao, L. Design, Synthesis, and Energy-related Applications of Metal Sulfides. Mater. Horiz. 2016, 3, 402–421. [Google Scholar] [CrossRef]
- Guo, K.; Yang, F.; Cui, S.; Chen, W.; Mi, L. Controlled synthesis of 3D Hierarchical NiSe Microspheres for High-performance Supercapacitor Design. RSC Adv. 2016, 6, 46523–46530. [Google Scholar] [CrossRef]
- Lin, H.; Liu, F.; Wang, X.; Ai, Y.; Yao, Z.; Chu, L.; Han, S.; Zhuang, X. Graphene-Coupled Flower-Like Ni3S2 for a Free-Standing 3D Aerogel with an Ultra-High Electrochemical Capacity. Electrochim. Acta 2016, 191, 705–715. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, L.; Zhao, W.; Li, X.; Huang, X.; Mai, X.; Wang, Z.; Shao, Q.; Yan, X.; Guo, Z. Highly Efficient Fe-N-C Nanoparticles Modified Porous Graphene Composites for Oxygen Reduction Reaction. J. Electrochem. Soc. 2018, 165, H510–H516. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Kumar Veerasubramani, G.; Radhakrishnan, S.; Kim, S. One pot Hydrothermal Growth of Hierarchical Nanostructured Ni3S2 on Ni foamfor Supercapacitor Application. Chem. Eng. J. Chem. 2014, 251, 116–122. [Google Scholar]
- Zhuo, M.; Zhang, P.; Chen, Y.; Li, Q. Facile Construction of Graphene-likeNi3S2 Nanosheets Through the Hydrothermally Assisted Sulfurization of NickelFoam and Their Application as Self-supported Electrodes for Supercapacitors. RSC Adv. 2015, 5, 25446–25449. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Xin, L.; Wu, M.; Lou, X. Single-crystal β-NiS Nanorod Arrays with a Hollow-structured Ni3S2 Framework for Supercapacitor Applications. J. Mater. Chem. A 2016, 4, 7700–7709. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Xiao, T.; Tana, X.; Xiang, P.; Jiang, L.; Deng, C.; Li, W.; Li, M. Controllable preparation of Nanoporous Ni3S2 films by Sulfuration of nickel foam as promising asymmetric supercapacitor electrodes. Appl. Surf. Sci. 2017, 420, 919–926. [Google Scholar] [CrossRef]
- Zhang, D.; Li, J.; Luo, J.; Xu, P.; Wei, L.; Zhou, D.; Xu, W.; Yuan, D. Ni3S2 nanowires grown on nickel foam as an efficient bifunctional electrocatalyst for water splitting with greatly practical prospects. Nanotechnology 2018, 29, 245402. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Zhu, L.; Wang, J.; Ghim, H. In Situ Chemical Etching of Tunable 3D Ni3S2 Superstructures For Bifunctional Electrocatalysts For Overall Water Splitting. J. Mater. Chem. A 2016, 4, 13916–13922. [Google Scholar] [CrossRef]
- Shinde, N.; Raut, S.; Ghule, B.; Gunturu, K.; Pak, J.; Mane, R. Recasting Ni-foam into NiF2 nanorod arrays via a hydrothermal process for hydrogen evolution reaction application. Dalton Trans. 2021, 50, 6500–6505. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yue, X.; Zhang, W.; Yu, S.; Zhang, Y.; Wang, J.; Wang, J. Nickel sulfide Microsphere Film on Ni foam as an Efficient Bifunctional Electrocatalyst for Overall Water Splitting. J. Chem. Commun. 2016, 52, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Maiaugree, W.; Tangtrakarn, A.; Lowpa, S.; Ratchapolthavisin, N.; Amornkitbamrung, V. Facile Synthesis of Bilayer Carbon/Ni3S2Nanowalls for a Counter Electrode of Dye-sensitized Solar Cell. Electrochim. Acta 2015, 174, 955–962. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Zou, J.; Zeng, X.; Zhou, L.; Huang, H. Stable Freestanding Li-ion Battery Cathodes by in Situ Conformal Coating of Conducting PolypyrroleonNiS-carbon Nanofiber Films. J. Power Sources 2016, 331, 360–365. [Google Scholar] [CrossRef]
- Salavati-niasari, M.; Davar, F.; Emadi, H. Hierarchical Nanostructured Nickel sulfide architectures through Simple Hydrothermal Method in the Presence of Thioglycoloic Acid. Chalco. Lett. 2010, 7, 647–655. [Google Scholar]
- Al-Naggar, H.; Shinde, N.M.; Kim, J.; Mane, R.S. Water splitting performance of metal and non-metal-doped transition metal oxide electrocatalysts. Coord. Chem. Rev. 2022, 474, 21486–21644. [Google Scholar]
- Feng, N.; Hu, D.; Wang, P.; Sun, X.; Li, X.; He, D. Growth of Nanostructured Nickel Sulfide Films on Ni foam as High-performance Cathodes for Lithium ion Batteries. Phys. Chem. Chem. Phys. 2013, 15, 9924–9930. [Google Scholar] [CrossRef]
- Liang, K.; Marcus, K.; Guo, L.; Li, Z.; Zhou, L.; Li, Y.; De Oliveira, S.; Orlovskaya, N.; Sohn, Y.; Yang, Y. A freestanding NiSx Porous Film as a Binder-free Electrode for Mg-ion Batteries. Chem. Commun. 2017, 53, 7608–7611. [Google Scholar] [CrossRef] [PubMed]
- Shinde, N.M.; Shinde, P.V.; Yun, J.M.; Mane, R.S.; Kim, K.H. Room-temperature chemical synthesis of 3-D dandelion-type nickel chloride (NiCl2@ NiF) supercapattery nanostructured materials. J. Colloid Interface Sci. 2020, 578, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Shi, Y.; Sun, L.; Xiao, Z.; Sun, B.; Xu, X. Controlled Synthesis of NiSNanoparticle/CdS Nanowire Heterostructures via Solution Route and Their Optical Properties. Mater. Sci. Eng. B 2013, 178, 109–116. [Google Scholar] [CrossRef]
- Cheng, Z.; Abernathy, H.; Liu, M. Raman Spectroscopy of Nickel SulfideNi3S2. J. Phys. Chem. C 2007, 111, 17997–18000. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Dehghani, H.; Behnoudnia, F. Sodium Thiosulfate-assisted Synthesis of NiS2 Nanostructure by Using Nickel (II)-Salen Precursor: Optical and Magnetic Properties. Dalton Trans. 2014, 43, 16745–16753. [Google Scholar] [CrossRef]
- Dong, J.; Cheng, Z.; Zha, S.; Liu, M. Identification of Nickel Sulfides on Ni-YSZ Cermet Exposed to H2 Fuel Containing H2S Using Raman spectroscopy. J. Power Sources 2006, 156, 461–465. [Google Scholar] [CrossRef]
- Li, H.; Chai, L.; Wang, X.; Wu, X.; Xi, G.; Liu, Y.; Qian, Y. Hydrothermal Growth and Morphology Modification of β-NiS Three-Dimensional Flowerlike Architectures. Cryst. Growth Des. 2007, 7, 1919–1920. [Google Scholar] [CrossRef]
- Lin, T.; Shuan, C.; Hung, K. High Energy Density Asymmetric supercapacitor Based on NiOOH/Ni3S2/3D Graphene and Fe3O4/Graphene Composite Electrodes. Sci. Rep. 2014, 4, 7274. [Google Scholar] [CrossRef] [Green Version]
- Jing, F.; Lv, Q.; Xiao, J.; Wang, Q.; Wang, S. Highly Active and Dual-function Self-Supported Multiphase NiS–NiS2–Ni3S2/NF Electrodes for Overall Water Splitting. J. Mater. Chem. A 2018, 6, 14207–14214. [Google Scholar] [CrossRef]
- Kang, D.; Hu, C.; Zhu, Q. Morphology controlled synthesis of hierarchical structured Fe2O3 from natural ilmenite and its high performance for dyes adsorption. App. Sur. Sci. 2018, 459, 327–335. [Google Scholar] [CrossRef]
- Deng, J.; Ren, P.; Deng, D.; Yu, L.; Yang, F.; Bao, X. Highly active and durable non-precious-metal catalysts encapsulated in carbon nanotubes for hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 1919–1923. [Google Scholar] [CrossRef]
- Suryawanshi, U.P.; Ghorpade, U.V.; Lee, D.M.; He, M.; Shin, S.W.; Kumar, P.V.; Jang, J.S.; Jung, H.R.; Suryawanshi, M.P.; Kim, J.H. Colloidal Ni2P Nanocrystals Encapsulated in Heteroatom-Doped Graphene Nanosheets: A Synergy of 0D@ 2D Heterostructure Toward Overall Water Splitting. Chem. Mater. 2020, 33, 234–245. [Google Scholar] [CrossRef]
- Babar, P.; Lokhande, A.; Karade, V.; Pawar, B.; Gang, M.G.; Pawar, S.; Kim, J.H. Bifunctional 2D electrocatalysts of transition metal hydroxide nanosheet arrays for water splitting and urea electrolysis. ACS Sustain. Chem. Eng. 2019, 7, 10035–10043. [Google Scholar] [CrossRef]
- Xiao, X.; Huang, D.; Fu, Y.; Wen, M.; Jiang, X.; Lv, X.; Li, M.; Gao, L.; Liu, S.; Wang, M.; et al. Engineering NiS/Ni2P heterostructures for efficient electrocatalytic water splitting. ACS Appl. Mater. Interfaces 2018, 10, 4689–4696. [Google Scholar] [CrossRef]
- Li, Q.; Wang, D.; Han, C.; Ma, X.; Lu, Q.; Xing, Z.; Yang, X. Construction of amorphous interface in an interwoven NiS/NiS2 structure for enhanced overall water splitting. J. Mater. Chem. A 2018, 6, 8233–8237. [Google Scholar] [CrossRef]
- Wang, T.; Guo, X.; Zhang, J.; Xiao, W.; Xi, P.; Peng, S.; Gao, D. Electronic structure modulation of NiS2 by transition metal doping for accelerating the hydrogen evolution reaction. J. Mater. Chem. A 2019, 7, 4971–4976. [Google Scholar] [CrossRef]
- Tian, T.; Huang, L.; Ai, L.; Jiang, J. Surface anion-rich NiS2 hollow microspheres derived from metal–organic frameworks as a robust electrocatalyst for the hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 20985–20992. [Google Scholar] [CrossRef]
- Wu, X.; Yang, B.; Li, Z.; Lei, L.; Zhang, X. Synthesis of supported vertical NiS2 nanosheets for hydrogen evolution reaction in acidic and alkaline solution. RSC Adv. 2015, 5, 32976–32982. [Google Scholar] [CrossRef]
- Fan, M.; Li, X.; Wei, D.; Wang, Y.; Li, M. Fabrication of Te@ NiTe2/NiS heterostructures for electrocatalytic hydrogen evolution reaction. Electrochim. Acta 2019, 328, 135075. [Google Scholar]
- Chia, X.; Sofer, Z.; Luxa, J.; Pumera, M. Unconventionally layered CoTe2 and NiTe2 as electrocatalysts for hydrogen evolution. Chem. A Eur. J. 2017, 23, 11719–11726. [Google Scholar] [CrossRef]
- De Silva, U.; Masud, J.; Zhang, N.; Hong, Y.; Liyanage, W.P.; Zaeem, M.A.; Nath, M. Nickel telluride as a bifunctional electrocatalyst for efficient water splitting in alkaline medium. J. Mater. Chem. A 2018, 6, 7608–7622. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Shaikh, S.F.; Ubaidullah, M.; Ghanem, M.A.; Mane, R.S. Self-grown one-dimensional nickel sulfo-selenide nanostructured electrocatalysts for water splitting reactions. Int. J. Hydrog. Energy 2020, 45, 15904–15914. [Google Scholar] [CrossRef]
- Lu, J.; Xiong, T.; Zhou, W.; Yang, L.; Tang, Z.; Chen, S. Metal nickel foam as an efficient and stable electrode for hydrogen evolution reaction in acidic electrolyte under reasonable overpotentials. ACS Appl. Mater. Interfaces 2016, 8, 5065–5069. [Google Scholar] [CrossRef] [PubMed]



| Catalysts | Electrolyte | ɳ (mV) | Tafel Slope (mV dec−1) | J (mA cm−2) | Ref. |
|---|---|---|---|---|---|
| Ni2P@NSG | 1.0 M KOH | 110 | 43 | 10 | [42] |
| NiFeCo | 1.0 M KOH | 108 | 64 | 10 | [43] |
| NiS/Ni2P/carbon cloth | 1.0 M KOH | 111 | 78.1 | 20 | [44] |
| NiS/NiS2 | 1.0 M KOH | 248 | 142.3 | 10 | [45] |
| Fe- NiS2 | 0.5 M H2SO4 | 198 | 42 | 10 | [46] |
| NiS2 | 1.0 M KOH | 219 | 157 | 10 | [47] |
| NiS2/graphite substrate | 0.5 M H2SO4 | 240 | 41 | 10 | [48] |
| Te@NiTe2/NiS/acetylene black | 1.0 M KOH | 101 | 118 | 10 | [49] |
| NiTe2 | 0.5 M H2SO4 | 560 | 44 | 10 | [50] |
| Ni3Te2 | 1.0 M KOH | 304 | 94.2 | 10 | [51] |
| NiSSe | 1.0 M KOH | 154 | 125 | 10 | [52] |
| NiF | 0.5 M H2SO4 | 210 | 1006.6 | 10 | [53] |
| NiF2 | 0.1 M KOH | 172 | 47 | 10 | [12] |
| NiCl2 | 1.0 M KOH | 176 | 41 | 10 | Current work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinde, N.M.; Raut, S.D.; Ghule, B.G.; Deokate, R.J.; Narwade, S.H.; Mane, R.S.; Xia, Q.; Pak, J.J.; Kim, J.-S. Hydrogen Evolution Reaction Activities of Room-Temperature Self-Grown Glycerol-Assisted Nickel Chloride Nanostructures. Catalysts 2023, 13, 177. https://doi.org/10.3390/catal13010177
Shinde NM, Raut SD, Ghule BG, Deokate RJ, Narwade SH, Mane RS, Xia Q, Pak JJ, Kim J-S. Hydrogen Evolution Reaction Activities of Room-Temperature Self-Grown Glycerol-Assisted Nickel Chloride Nanostructures. Catalysts. 2023; 13(1):177. https://doi.org/10.3390/catal13010177
Chicago/Turabian StyleShinde, Nanasaheb M., Siddheshwar D. Raut, Balaji G. Ghule, Ramesh J. Deokate, Sandesh H. Narwade, Rajaram S. Mane, Qixun Xia, James J. Pak, and Jeom-Soo Kim. 2023. "Hydrogen Evolution Reaction Activities of Room-Temperature Self-Grown Glycerol-Assisted Nickel Chloride Nanostructures" Catalysts 13, no. 1: 177. https://doi.org/10.3390/catal13010177
APA StyleShinde, N. M., Raut, S. D., Ghule, B. G., Deokate, R. J., Narwade, S. H., Mane, R. S., Xia, Q., Pak, J. J., & Kim, J.-S. (2023). Hydrogen Evolution Reaction Activities of Room-Temperature Self-Grown Glycerol-Assisted Nickel Chloride Nanostructures. Catalysts, 13(1), 177. https://doi.org/10.3390/catal13010177







