Effect of Zinc on the Structure and Activity of the Cobalt Oxide Catalysts for NO Decomposition
Abstract
:1. Introduction
2. Results
2.1. Catalyst Characterisation
2.1.1. Chemical Analysis and Texture
2.1.2. Phase Composition, Raman, and Infrared Spectroscopy
2.1.3. Surface Composition
2.1.4. Scanning Electron Microscopy (SEM)
2.1.5. Reducibility
2.1.6. Basicity
2.1.7. TPD-NO
2.1.8. Work Function
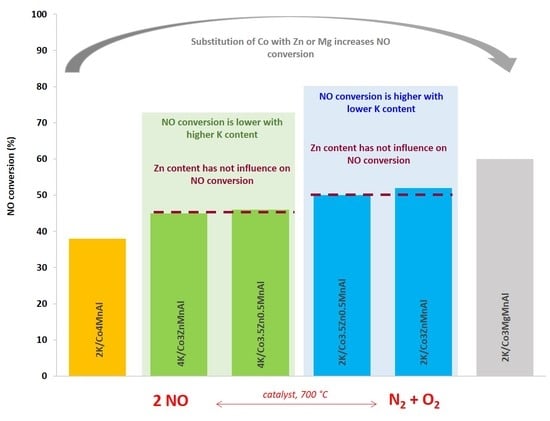
2.2. Catalytic Application—NO Decomposition
3. Discussion
- The dependence of the NO conversion on the specific surface area (Figure 11).
- The dependence of the NO conversion on the amount of adsorbed NO species in the form of loosely bound mononitrosyl species and the surface NOx− species (Figure 12).
- The dependence of the desorbed amount of NO on the amount of medium and strong basic sites (Figure 13a).
- The dependence of the NO conversion on the number of basic sites and their strength (Figure 14).
- The dependence of the NO conversion on the amount of surface lattice and chemisorbed oxygen species (Figure 15).
- The dependence of the NO conversion and TOF on the temperature of the O2 desorption during the decomposition of the intermediate KNO2 surface (Figure 16b).
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Catalyst Characterisation and Catalytic Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, P.P.; Yong, X.; Wei, M.; Li, Y.D.; Zhang, C.J. High performance catalysts BaCoO3-CeO2 prepared by the one-pot method for NO direct decomposition. ChemCatChem 2020, 12, 4297–4303. [Google Scholar] [CrossRef]
- Amirnazmi, A.; Benson, J.E.; Boudart, M. Oxygen inhibition in the decomposition of NO on metal oxides and platinum. J. Catal. 1973, 30, 55–65. [Google Scholar] [CrossRef]
- Consul, J.M.D.; Peralta, C.A.; Benvenutti, E.V.; Ruiz, J.A.C.; Pastore, H.O.; Baibich, I.M. Direct decomposition of nitric oxide on alumina-modified amorphous and mesoporous silica-supported palladium catalysts. J. Mol. Catal. A Chem. 2006, 246, 33–38. [Google Scholar] [CrossRef]
- Reddy, G.K.; Ling, C.; Peck, T.C.; Jia, H.F. Understanding the chemical state of palladium during the direct NO decomposition–influence of pretreatment environment and reaction temperature. RSC Adv. 2017, 7, 19645–19655. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.J.; Hall, W.K. Catalytic decomposition of nitric-oxide over Cu-zeolites. J. Catal. 1991, 129, 202–215. [Google Scholar] [CrossRef]
- Moden, B.; Da Costa, P.; Fonfe, B.; Lee, D.K.; Iglesia, E. Kinetics and mechanism of steady-state catalytic NO decomposition reactions on Cu-ZSM5. J. Catal. 2002, 209, 75–86. [Google Scholar] [CrossRef]
- Groothaert, M.H.; Lievens, K.; Leeman, H.; Weckhuysen, B.M.; Schoonheydt, R.A. An operando optical fiber UV-vis spectroscopic study of the catalytic decomposition of NO and N2O over Cu-ZSM-5. J. Catal. 2003, 220, 500–512. [Google Scholar] [CrossRef] [Green Version]
- Pan, K.L.; Chen, M.C.; Yu, S.J.; Yan, S.Y.; Chang, M.B. Enhancement of nitric oxide decomposition efficiency achieved with lanthanum-based perovskite-type catalyst. J. Air Waste Manag. Assoc. 2016, 66, 619–630. [Google Scholar] [CrossRef] [Green Version]
- Tofan, C.; Klvana, D.; Kirchnerova, J. Decomposition of nitric oxide over perovskite oxide catalysts: Effect of CO2, H2O and CH4. Appl. Catal. B Environ. 2002, 36, 311–323. [Google Scholar] [CrossRef]
- Masui, T.; Uejima, S.; Tsujimoto, S.; Nagai, R.; Imanaka, N. Direct NO decomposition over C-type cubic Y2O3-Pr6O11-Eu2O3 solid solutions. Catal. Today 2015, 242, 338–342. [Google Scholar] [CrossRef]
- Tsujimoto, S.; Masui, T.; Imanaka, N. Fundamental Aspects of Rare Earth Oxides Affecting Direct NO Decomposition Catalysis. Eur. J. Inorg. Chem. 2015, 242, 1524–1528. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, Z.; Wang, D.; Hong, Z.; Zhou, M.D.; Li, X.B. A review on the catalytic decomposition of NO to N2 and O2: Catalysts and processes. Catal. Sci. Technol. 2018, 8, 4563–4575. [Google Scholar] [CrossRef]
- Winter, E.R.S. The catalytic decomposition of nitric oxide by metallic oxides. J. Catal. 1971, 22, 158–170. [Google Scholar] [CrossRef]
- Park, P.W.; Kil, J.K.; Kung, H.H.; Kung, M.C. NO decomposition over sodium-promoted cobalt oxide. Catal. Today 1998, 42, 51–60. [Google Scholar] [CrossRef]
- Haneda, M.; Kintaichi, Y.; Bion, N.; Hamada, H. Alkali metal-doped cobalt oxide catalysts for NO decomposition. Appl. Catal. B Environ. 2003, 46, 473–482. [Google Scholar] [CrossRef]
- Peck, T.C.; Roberts, C.A.; Reddy, G.K. Contrasting effects of potassium addition on M3O4 (M = Co, Fe, and Mn) oxides during direct NO decomposition catalysis. Catalysts 2020, 10, 561. [Google Scholar] [CrossRef]
- Roberts, C.A.; Paidi, V.K.; Shepit, M.; Peck, T.C.; Masias, K.L.S.; van Lierop, J.; Reddy, G.K. Effect of Cu substitution on the structure and reactivity of CuxCo3−xO4 spinel catalysts for direct NOx decomposition. Catal. Today 2021, 360, 204–212. [Google Scholar] [CrossRef]
- Shelef, M.; Otto, K.; Gandhi, H. Heterogeneous decomposition of nitric oxide on supported catalysts. Atmos. Environ. 1969, 3, 107–122. [Google Scholar] [CrossRef]
- Stelmachowski, P.; Zasada, F.; Maniak, G.; Granger, P.; Inger, M.; Wilk, M.; Kotarba, A.; Sojka, Z. Optimization of multicomponent cobalt spinel catalyst for N2O abatement from nitric acid plant tail gases: Laboratory and pilot plant studies. Catal. Lett. 2009, 130, 637–641. [Google Scholar] [CrossRef]
- Inger, M.; Wilk, M.; Saramok, M.; Grzybek, G.; Grodzka, A.; Stelmachowski, P.; Makowski, W.; Kotarba, A.; Sojka, Z. Cobalt spinel catalyst for N2O abatement in the pilot plant operation-long-term activity and stability in tail gases. Ind. Eng. Chem. Res. 2014, 53, 10335–10342. [Google Scholar] [CrossRef]
- Wojcik, S.; Grzybek, G.; Stelmachowski, P.; Sojka, Z.; Kotarba, A. Bulk, surface and interface promotion of CO3O4 for the low-temperature N2O decomposition catalysis. Catalysts 2020, 10, 41. [Google Scholar] [CrossRef] [Green Version]
- Omata, K.; Takada, T.; Kasahara, S.; Yamada, M. Active site of substituted cobalt spinel oxide for selective oxidation of COH2. Appl. Catal. A Gen. 1996, 146, 255–267. [Google Scholar] [CrossRef]
- Yan, L.; Ren, T.; Wang, X.L.; Gao, Q.; Ji, D.; Suo, J.S. Excellent catalytic performance of ZnxCo1−xCo2O4 spinel catalysts for the decomposition of nitrous oxide. Catal. Commun. 2003, 4, 505–509. [Google Scholar] [CrossRef]
- Yan, L.; Ren, T.; Wang, X.L.; Ji, D.; Suo, J.S. Catalytic decomposition of N2O over MxCo1−xCo2O4 (M = Ni, Mg) spinel oxides. Appl. Catal. B Environ. 2003, 45, 85–90. [Google Scholar] [CrossRef]
- Stelmachowski, P.; Maniak, G.; Kaczmarczyk, J.; Zasada, F.; Piskorz, W.; Kotarba, A.; Sojka, Z. Mg and Al substituted cobalt spinels as catalysts for low temperature deN2O—Evidence for octahedral cobalt active sites. Appl. Catal. B Environ. 2014, 146, 105–111. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Obalová, L.; Pacultová, K.; Klegova, A.; Asiri, A.M. An investigation on the N2O decomposition activity of MnxCo1-xCo2O4 nanorods prepared by the thermal decomposition of their oxalate precursors. J. Ind. Eng. Chem. 2021, 93, 279–289. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Soliman, S.A.; Asiri, A.M. Role of rubidium promotion on the nitrous oxide decomposition activity of nanocrystalline Co3O4-CeO2 catalyst. Appl. Surf. Sci. 2019, 479, 148–157. [Google Scholar] [CrossRef]
- Shi, Z.B.; Yang, H.Y.; Gao, P.; Chen, X.Q.; Liu, H.J.; Zhong, L.S.; Wang, H.; Wei, W.; Sun, Y.H. Effect of alkali metals on the performance of CoCu/TiO2 catalysts for CO2 hydrogenation to long-chain hydrocarbons. Chin. J. Catal. 2018, 39, 1294–1302. [Google Scholar] [CrossRef]
- Mogudi, B.M.; Ncube, P.; Bingwa, N.; Mawila, N.; Mathebula, S.; Meijboom, R. Promotion effects of alkali and alkaline earth metals on catalytic activity of mesoporous Co3O4 for 4-nitrophenol reduction. Appl. Catal. B Environ. 2017, 218, 240–248. [Google Scholar] [CrossRef]
- Li, Z.J.; Zhong, L.S.; Yu, F.; An, Y.L.; Dai, Y.Y.; Yang, Y.Z.; Lin, T.J.; Li, S.G.; Wang, H.; Gao, P.; et al. Effects of sodium on the catalytic performance of CoMn catalysts for fischer-tropsch to olefin reactions. ACS Catal. 2017, 7, 3622–3631. [Google Scholar] [CrossRef]
- Maniak, G.; Stelmachowski, P.; Kotarba, A.; Sojka, Z.; Rico-Perez, V.; Bueno-Lopez, A. Rationales for the selection of the best precursor for potassium doping of cobalt spinel based deN2O catalyst. Appl. Catal. B Environ. 2013, 136, 302–307. [Google Scholar] [CrossRef]
- Pacultová, K.; Draštíková, V.; Chromčáková, Ž.; Bílková, T.; Kutláková, K.M.; Kotarba, A.; Obalová, L. On the stability of alkali metal promoters in Co mixed oxides during direct NO catalytic decomposition. Mol. Catal. 2017, 428, 33–40. [Google Scholar] [CrossRef]
- Bílková, T.; Fridrichová, D.; Pacultová, K.; Karásková, K.; Obalová, L.; Haneda, M. Reaction mechanism of NO direct decomposition over K-promoted Co-Mn-Al mixed oxides-DRIFTS, TPD and transient state studies. J. Taiwan Inst. Chem. Eng. 2021, 120, 257–266. [Google Scholar] [CrossRef]
- Grzybek, G.; Wójcik, S.; Legutko, P.; Gryboś, J.; Indyka, P.; Leszczyński, B.; Kotarba, A.; Sojka, Z. Thermal stability and repartition of potassium promoter between the support and active phase in the K-Co2.6Zn0.4O4|α-Al2O3 catalyst for N2O decomposition: Crucial role of activation temperature on catalytic performance. Appl. Catal. B Environ. 2017, 205, 597–604. [Google Scholar] [CrossRef]
- Kovanda, F.; Rojka, T.; Dobešová, J.; Machovič, V.; Bezdička, P.; Obalová, L.; Jirátová, K.; Grygar, T. Mixed oxides obtained from Co and Mn containing layered double hydroxides: Preparation, characterization, and catalytic properties. J. Solid State Chem. 2006, 179, 812–823. [Google Scholar] [CrossRef]
- An, H.; McGinn, P.J. Catalytic behavior of potassium containing compounds for diesel soot combustion. Appl. Catal. B Environ. 2006, 62, 46–56. [Google Scholar] [CrossRef]
- Pacultová, K.; Bílková, T.; Klegova, A.; Karásková, K.; Fridrichová, D.; Jirátová, K.; Kiška, T.; Balabánová, J.; Koštejn, M.; Kotarba, A.; et al. Co-Mn-Al mixed oxides promoted by K for direct NO decomposition: Effect of preparation parameters. Catalysts 2019, 9, 593. [Google Scholar] [CrossRef] [Green Version]
- Jirátová, K.; Pacultová, K.; Balabánová, J.; Karásková, K.; Klegova, A.; Bílková, T.; Jandová, V.; Koštejn, M.; Martaus, A.; Kotarba, A.; et al. Precipitated K-promoted Co-Mn-Al mixed oxides for direct no decomposition: Preparation and properties. Catalysts 2019, 9, 592. [Google Scholar] [CrossRef] [Green Version]
- Jirátová, K.; Pacultová, K.; Karásková, K.; Balabánová, J.; Koštejn, M.; Obalová, L. Direct Decomposition of NO over Co-Mn-Al Mixed Oxides: Effect of Ce and/or K Promoters. Catalysts 2020, 10, 808. [Google Scholar] [CrossRef]
- Karásková, K.; Pacultová, K.; Klegova, A.; Fridrichová, D.; Valašková, M.; Jirátová, K.; Stelmachowski, P.; Kotarba, A.; Obalová, L. Magnesium Effect in K/Co-Mg-Mn-Al Mixed Oxide Catalyst for Direct NO Decomposition. Catalysts 2020, 10, 931. [Google Scholar] [CrossRef]
- Bomfim, H.E.L.; Oliveira, A.C.; Rangel, M.d.C. Effect of zinc on the catalytic activity of hematite in ethylbenzene dehydrogenation. React. Kinet. Catal. Lett. 2003, 80, 359–364. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Paliychuk, N.; Pacia, M.; Kaspera, W.; Macyk, W.; Kotarba, A.; Bogacz, B.F.; Pedziwiatr, A.T.; Mironyuk, I.; Gargula, R.; et al. Structure-redox reactivity relationships in Co1−xZnxFe2O4: The role of stoichiometry. N. J. Chem. 2019, 43, 3038–3049. [Google Scholar] [CrossRef]
- Tiwari, P.; Verma, R.; Kane, S.N.; Tatarchuk, T.; Mazaleyrat, F. Effect of Zn addition on structural, magnetic properties and anti-structural modeling of magnesium-nickel nano ferrites. Mater. Chem. Phys. 2019, 229, 78–86. [Google Scholar] [CrossRef]
- Russo, N.; Fino, D.; Saracco, G.; Specchia, V. N2O catalytic decomposition over various spinel-type oxides. Catal. Today 2007, 119, 228–232. [Google Scholar] [CrossRef]
- Obalová, L.; Karásková, K.; Jirátová, K.; Kovanda, F. Effect of potassium in calcined Co-Mn-Al layered double hydroxide on the catalytic decomposition of N2O. Appl. Catal. B Environ. 2009, 90, 132–140. [Google Scholar] [CrossRef]
- Legutko, P.; Jakubek, T.; Kaspera, W.; Stelmachowski, P.; Sojka, Z.; Kotarba, A. Soot oxidation over K-doped manganese and iron spinels–How potassium precursor nature and doping level change the catalyst activity. Catal. Commun. 2014, 43, 34–37. [Google Scholar] [CrossRef]
- Jiratova, K.; Mikulova, J.; Klempa, J.; Grygar, T.; Bastl, Z.; Kovanda, F. Modification of Co-Mn-Al mixed oxide with potassium and its effect on deep oxidation of VOC. Appl. Catal. A Gen. 2009, 361, 106–116. [Google Scholar] [CrossRef]
- Klyushina, A.; Pacultová, K.; Karásková, K.; Jirátová, K.; Ritz, M.; Fridrichová, D.; Volodarskaja, A.; Obalová, L. Effect of preparation method on catalytic properties of Co-Mn-Al mixed oxides for N2O decomposition. J. Mol. Catal. A Chem. 2016, 425, 237–247. [Google Scholar] [CrossRef]
- Zasada, F.; Piskorz, W.; Sojka, Z. Cobalt spinel at various redox conditions: DFT+U investigations into the structure and surface thermodynamics of the (100) facet. J. Phys. Chem. C 2015, 119, 19180–19191. [Google Scholar] [CrossRef]
- Jakubek, T.; Kaspera, W.; Legutko, P.; Stelmachowski, P.; Kotarba, A. Surface versus bulk alkali promotion of cobalt-oxide catalyst in soot oxidation. Catal. Commun. 2015, 71, 37–41. [Google Scholar] [CrossRef]
- Pacultová, K.; Klegova, A.; Karásková, K.; Fridrichová, D.; Bílková, T.; Koštejn, M.; Obalová, L. Oxygen effect in NO direct decomposition over K/Co-Mg-Mn-Al mixed oxide catalyst–Temperature programmed desorption study. Mol. Catal. 2021, 510, 111695. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Obalová, L.; Karásková, K.; Wach, A.; Kustrowski, P.; Mamulová-Kutláková, K.; Michalik, S.; Jirátová, K. Alkali metals as promoters in Co-Mn-Al mixed oxide for N2O decomposition. Appl. Catal. A-Gen. 2013, 462, 227–235. [Google Scholar] [CrossRef]
- Chromčáková, Ž.; Obalová, L.; Kovanda, F.; Legut, D.; Titov, A.; Ritz, M.; Fridrichová, D.; Michalik, S.; Kuśtrowski, P.; Jirátová, K. Effect of precursor synthesis on catalytic activity of Co3O4 in N2O decomposition. Catal. Today 2015, 257, 18–25. [Google Scholar] [CrossRef]
- Karásková, K.; Pacultová, K.; Jirátová, K.; Fridrichová, D.; Koštejn, M.; Obalová, L. K-Modified Co-Mn-Al Mixed Oxide-Effect of Calcination Temperature on N2O Conversion in the Presence of H2O and NOx. Catalysts 2020, 10, 1134. [Google Scholar] [CrossRef]
- Langell, M.A.; Gevrey, F.; Nydegger, M.W. Surface composition of MnxCo1−xO solid solutions by X-ray photoelectron and Auger spectroscopies. Appl. Surf. Sci. 2000, 153, 114–127. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.C.; Shim, W.G. Catalytic combustion of VOCs over a series of manganese oxide catalysts. Appl. Catal. B Environ. 2010, 98, 180–185. [Google Scholar] [CrossRef]
- Obalová, L.; Pacultová, K.; Balabánová, J.; Jirátová, K.; Bastl, Z.; Valášková, M.; Lacný, Z.; Kovanda, F. Effect of Mn/Al ratio in Co–Mn–Al mixed oxide catalysts prepared from hydrotalcite-like precursors on catalytic decomposition of N2O. Catal. Today 2007, 119, 233–238. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoeletron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1995. [Google Scholar]
- Dupin, J.C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Nanostructured ceria-based catalysts for soot combustion: Investigations on the surface sensitivity. Appl. Catal. B Environ. 2015, 165, 742–751. [Google Scholar] [CrossRef]
- Chuang, T.J.; Brundle, C.R.; Rice, D.W. Interpretation of X-ray photoemission spectra of cobalt oxides and cobalt oxide surfaces. Surf. Sci. 1976, 59, 413–429. [Google Scholar] [CrossRef]
- Pacultová, K.; Karásková, K.; Kovanda, F.; Jirátová, K.; Šramek, J.; Kustrowski, P.; Kotarba, A.; Chromčáková, Ž.; Kočí, K.; Obalová, L. K-doped Co-Mn-Al mixed oxide catalyst for N2O abatement from nitric acid plant waste gases: Pilot plant studies. Ind. Eng. Chem. Res. 2016, 55, 7076–7084. [Google Scholar] [CrossRef]
- Kannan, S.; Swamy, C.S. Catalytic decomposition of nitrous oxide over calcined cobalt aluminum hydrotalcites. Catal. Today 1999, 53, 725–737. [Google Scholar] [CrossRef]
- Chen, H.H.; Yang, M.; Tao, S.; Chen, G.W. Oxygen vacancy enhanced catalytic activity of reduced Co3O4 towards p-nitrophenol reduction. Appl. Catal. B Environ. 2017, 209, 648–656. [Google Scholar] [CrossRef]
- Luo, Y.J.; Zheng, Y.B.; Zuo, J.C.; Feng, X.S.; Wang, X.Y.; Zhang, T.H.; Zhang, K.; Jiang, L.L. Insights into the high performance of Mn-Co oxides derived from metal organic frameworks for total toluene oxidation. J. Hazard. Mater. 2018, 349, 119–127. [Google Scholar] [CrossRef]
- Wang, Z.P.; Zhang, X.M.; Wang, L.G.; Zhang, Z.L.; Jiang, Z.; Xiao, T.C.; Umar, A.; Wang, Q. Co-Mn-Al nonstoichiometric spinel-type catalysts derived from hydrotalcites for the simultaneous removal of soot and nitrogen oxides. Sci. Adv. Mater. 2013, 5, 1449–1457. [Google Scholar] [CrossRef]
- Imanaka, N.; Masui, T. Advances in direct NOx decomposition catalysts. Appl. Catal. A Gen. 2012, 431–432, 1–8. [Google Scholar] [CrossRef]
- Haneda, M.; Hamada, H. Recent progress in catalytic NO decomposition. Comptes Rendus Chim. 2016, 19, 1254–1265. [Google Scholar] [CrossRef]
- Hong, W.-J.; Iwamoto, S.; Inoue, M. Direct NO decomposition over a Ce–Mn mixed oxide modified with alkali and alkaline earth species and CO2-TPD behavior of the catalysts. Catal. Today 2011, 164, 489–494. [Google Scholar] [CrossRef]
- Haneda, M.; Kintaichi, Y.; Hamada, H. Reaction mechanism of NO decomposition over alkali metal-doped cobalt oxide catalysts. Appl. Catal. B Environ. 2005, 55, 169–175. [Google Scholar] [CrossRef]
- Ishihara, T.; Ando, M.; Sada, K.; Takiishi, K.; Yamada, K.; Nishiguchi, H.; Takita, Y. Direct decomposition of NO into N2 and O2 over La(Ba)Mn(In)O3 perovskite oxide. J. Catal. 2003, 220, 104–114. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, D.; Li, J.; Xie, X.; Yang, X.; Wu, Y. Recycle—New possible mechanism of NO decomposition over perovskite(-like) oxides. J. Mol. Catal. A Chem. 2005, 233, 29–34. [Google Scholar] [CrossRef]
- Obalová, L.; Maniak, G.; Karásková, K.; Kovanda, F.; Kotarba, A. Electronic nature of potassium promotion effect in Co-Mn-Al mixed oxide on the catalytic decomposition of N2O. Catal. Commun. 2011, 12, 1055–1058. [Google Scholar] [CrossRef] [Green Version]
- Obalová, L.; Jirátová, K.; Kovanda, F.; Valášková, M.; Balabánová, J.; Pacultová, K. Structure–activity relationship in the N2O decomposition over Ni-(Mg)-Al and Ni-(Mg)-Mn mixed oxides prepared from hydrotalcite-like precursors. J. Mol. Catal. A Chem. 2006, 248, 210–219. [Google Scholar] [CrossRef]
















| Sample | Chemical Analysis a (wt.%) | Molar Ratio b Co:Zn(Mg):Mn:Al:K | SBETc (m2 g−1) | Vmicro · 103 (cm3 g−1) | External Surface (m2 g−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Co | Mn | Al | Zn (Mg) | K | |||||
| Co3.5Zn0.5MnAl | 43.9 | 11.1 | 5.4 | 6.9 | 0.0 | 3.5:0.5:0.9:0.9:0.0 | 57 | - | 57 |
| 2K/Co3.5Zn0.5MnAl | 46.5 | 11.5 | 5.4 | 7.1 | 1.5 | 3.5:0.5:0.9:0.9:0.2 | 45 | 2 | 39 |
| 4K/Co3.5Zn0.5MnAl | 41.8 | 11.0 | 5.3 | 6.8 | 3.0 | 3.5:0.5:1.0:1.0:0.4 | 43 | 3 | 35 |
| Co3ZnMnAl | 39.9 | 11.4 | 5.6 | 14.5 | 0.0 | 3.0:1.0:0.9:0.9:0.0 | 56 | - | 56 |
| 2K/Co3ZnMnAl | 39.6 | 11.6 | 5.8 | 14.5 | 1.4 | 3.0:1.0:0.9:1.0:0.2 | 45 | 3 | 37 |
| 4K/Co3ZnMnAl | 39.6 | 10.7 | 5.5 | 14.1 | 2.6 | 3.0:1.0:0.9:0.9:0.3 | 41 | 3 | 33 |
| 2K/Co3MgMnAl d | 42.0 | 12.5 | 6.7 | 5.3 | 1.5 | 3.0:0.9:1.0:1.0:0.2 | 47 | 3 | 39 |
| 2K/Co4MnAl e | 50.5 | 12.0 | 6.0 | 0.0 | 1.9 | 4.0:0.0:1.0:1.0:0.2 | 39 | 2 | 33 |
| Sample | Co 2p3/2 (eV) | Mn 2p3/2 (eV) | Al 2p (eV) | |||
|---|---|---|---|---|---|---|
| I. Peak (Co3+) | II. Peak (Co2+) | III. Peak (Co2+) | I. Peak (Mn3+) | II. Peak (Mn4+) | ||
| 2K/Co4MnAl | 779.8 | 781.3 | 783.4 | 641.2 | 643.1 | 72.9 |
| 2K/Co3MgMnAl * | 779.9 | 781.4 | 783.5 | 641.6 | 643.5 | 72.9 |
| 2K/Co3ZnMnAl | 780.1 | 781.6 | 783.7 | 641.2 | 643.1 | 72.9 |
| Sample | O 1s (529.5 eV) Lattice Oxygen 1 | O 1s (531.2 eV) Chemisorbed Oxygen 1 | Co2+/Co3+ Molar Ratio | Mn3+/Mn4+ Molar Ratio |
|---|---|---|---|---|
| 2K/Co4MnAl 1 | 14.4 | 4.9 | 0.7 | 2.7 |
| 2K/Co3MgMnAl * | 13.4 | 8.5 | 0.6 | 2.4 |
| 2K/Co3ZnMnAl | 13.9 | 5.6 | 0.3 | 2.8 |
| Scheme | Element | AAS (wt.%) | SEM-EDAX (wt.%) | XPS (wt.%) a | Surface-to-Bulk Weight Ratio (XPS/AAS) |
|---|---|---|---|---|---|
| 2K/Co4MnAl | Co Mn Al O K | 50.5 12.0 6.0 n.d. 1.9 | 60.8 15.0 7.8 14.3 2.2 | 28.7 15.2 10.1 37.6 7.7 | 0.6 1.3 1.7 - 4.1 |
| 2K/Co3MgMnAl | Co Mg Mn Al O K | 42.0 5.3 12.5 6.7 n.d. 1.5 | 51.7 5.0 17.0 6.8 17.0 2.6 | 20.1 5.4 12.2 16.2 39.7 5.6 | 0.5 1.0 1.0 2.4 - 3.7 |
| 2K/Co3ZnMnAl | Co Zn Mn Al O K | 39.6 14.5 11.6 5.8 n.d. 1.4 | 45.2 15.6 14.1 5.9 17.0 2.3 | 19.9 11.5 14.3 8.2 38.9 9.2 | 0.5 0.8 1.2 1.4 - 6.6 |
| 2K/Co4MnAl a | 2K/Co3MgMnAl b | 2K/Co3ZnMnAl | |
|---|---|---|---|
| Tmax from TPR-H2 (°C) c | 158; 317; 398 | 445 | 155; 389 |
| H2 consumption at 40–600 °C (mmol g−1) d | 6.1 | 5.1 | 5.4 |
| Temperature Region | I. | II. | III. | IV. | V. | III. + IV. + V. | Total (I.–V.) | |
|---|---|---|---|---|---|---|---|---|
| Tmax (°C) | 2K/Co4MnAl 2K/Co3ZnMnAl 2K/Co3MgMnAl | 124 130 95 | 226 240 133 | 329 343 253 | 450 437 420 | >650 593 603 | ||
| Peak area (a.u.) | 2K/Co4MnAl 2K/Co3ZnMnAl 2K/Co3MgMnAl | 60 40 120 | 6 49 35 | 106 35 68 | 117 133 140 | 30 74 194 | 253 242 402 | 319 331 557 |
| Experiment/Temperature Region | I. | II. | III. | IV. | V. | |
|---|---|---|---|---|---|---|
| Tmax O2 (°C) | 2K/Co4MnAl 2K/Co3ZnMnAl 2K/Co3MgMnAl | 239 265 225 | 543 572 552 | >650 >650 >650 | ||
| Tmax NO (°C) | 2K/Co4MnAl 2K/Co3ZnMnAl 2K/Co3MgMnAl | 158 161 158 | 271 259 254 | 415 405 369 | 542 568 567 | |
| O2 peak area (a.u.) | 2K/Co4MnAl 2K/Co3ZnMnAl 2K/Co3MgMnAl | 14 53 37 | 157 345 274 | 83 134 135 | ||
| NO peak area (a.u.) | 2K/Co4MnAl 2K/Co3ZnMnAl 2K/Co3MgMnAl | 63 69 161 | 234 228 419 | 56 186 281 | 270 484 762 | |
| O2/NO molar ratio | 2K/Co4MnAl 2K/Co3ZnMnAl 2K/Co3MgMnAl | 0.1 0.2 0.1 | 0.6 0.7 0.4 | |||
| Sample | WF (Fresh Sample) (eV) | WF (Used Sample) * (eV) |
|---|---|---|
| 2K/Co4MnAl | 4.53 ± 0.01 | n.d. |
| 2K/Co3MgMnAl | 4.51 ± 0.01 | 4.62 ± 0.01 |
| 2K/Co3ZnMnAl | 4.54 ± 0.01 | 4.53 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karásková, K.; Pacultová, K.; Bílková, T.; Fridrichová, D.; Koštejn, M.; Peikertová, P.; Stelmachowski, P.; Kukula, P.; Obalová, L. Effect of Zinc on the Structure and Activity of the Cobalt Oxide Catalysts for NO Decomposition. Catalysts 2023, 13, 18. https://doi.org/10.3390/catal13010018
Karásková K, Pacultová K, Bílková T, Fridrichová D, Koštejn M, Peikertová P, Stelmachowski P, Kukula P, Obalová L. Effect of Zinc on the Structure and Activity of the Cobalt Oxide Catalysts for NO Decomposition. Catalysts. 2023; 13(1):18. https://doi.org/10.3390/catal13010018
Chicago/Turabian StyleKarásková, Kateřina, Kateřina Pacultová, Tereza Bílková, Dagmar Fridrichová, Martin Koštejn, Pavlína Peikertová, Paweł Stelmachowski, Pavel Kukula, and Lucie Obalová. 2023. "Effect of Zinc on the Structure and Activity of the Cobalt Oxide Catalysts for NO Decomposition" Catalysts 13, no. 1: 18. https://doi.org/10.3390/catal13010018
APA StyleKarásková, K., Pacultová, K., Bílková, T., Fridrichová, D., Koštejn, M., Peikertová, P., Stelmachowski, P., Kukula, P., & Obalová, L. (2023). Effect of Zinc on the Structure and Activity of the Cobalt Oxide Catalysts for NO Decomposition. Catalysts, 13(1), 18. https://doi.org/10.3390/catal13010018