Composite of α-FeOOH and Mesoporous Carbon Derived from Indian Blackberry Seeds as Low-Cost and Recyclable Photocatalyst for Degradation of Ciprofloxacin
Abstract
1. Introduction
2. Results and Discussion
2.1. X-ray Diffraction, Raman, FTIR and Surface Area Analysis
2.2. Morphological Study
2.3. UV-Vis Absorption Analysis
2.4. Catalyst Activity
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Adsorbents
3.2.1. Synthesis of α−FeOOH
3.2.2. Synthesis of Carbon
3.2.3. Synthesis of α−FeOOH/carbon composite
3.3. Characterization
3.4. Photocatalytic Studies
Ciprofloxacin Photodegradation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- de Ilurdoz, M.S.; Sadhwani, J.J.; Reboso, J.V. Antibiotic removal processes from water & wastewater for the protection of the aquatic environment-A review. J. Water Process Eng. 2022, 45, 102474. [Google Scholar]
- Babic, S.; Perisa, M.; Skoric, I. Photolytic degradation of norfloxacin, enrofloxacin and ciprofloxacin in various aqueous media. Chemosphere 2013, 91, 1635–1642. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An Overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, Q. Action and mechanism of semiconductor photocatalysis on degradation of organic pollutants in water treatment: A review. Environ. Nanotech. Monit. Manag. 2019, 12, 100255. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Niu, C.-G.; Guo, H.; Huang, D.-W.; Liang, C.; Yang, Y.-Y.; Tang, N.; Zhang, X.-G. Integrating the Z-scheme heterojunction and hot electrons injection into a plasmonic-based Zn2In2S5/W18O49 composite induced improved molecular oxygen activation for photocatalytic degradation and antibacterial performance. J. Coll. Interface Sci. 2022, 610, 953–969. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Niu, C.-G.; Huang, D.-W.; Guo, H.; Feng, H.-P.; Li, L.; Liu, H.-Y.; Fan, Q.-Q.; Qin, M.-Z. Appropriate oxygen vacancies and Mo-N bond synergistically modulate charge transfer dynamics of MoO3−x/S-CN for superior photocatalytic disinfection: Unveiling synergistic effects and disinfection mechanism. J. Hazard. Mater. 2023, 445, 130481. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Feng, H.-P.; Niu, C.-G.; Huang, D.-W.; Guo, H.; Liang, C.; Liu, H.-Y.; Chen, S.; Tang, N.; Li, L. Constructing a plasma-based Schottky heterojunction for near-infrared-driven photothermal synergistic water disinfection: Synergetic effects and antibacterial mechanisms. Chem. Eng. J. 2021, 426, 131902. [Google Scholar] [CrossRef]
- Medhi, R.; Marquez, M.D.; Lee, T.R. Visible-light-active doped metal oxide nanoparticles: Review of their synthesis, properties, and applications. ACS Appl. Nano Mater. 2020, 3, 6156–6185. [Google Scholar] [CrossRef]
- Wolski, L.; Grzelak, K.; Mu´nko, M.; Frankowski, M.; Grzyb, T.; Nowaczyk, G. Insight into photocatalytic degradation of ciprofloxacin over CeO2/ZnO nanocomposites: Unravelling the synergy between the metal oxides and analysis of reaction pathways. Appl. Surface Sci. 2021, 563, 150338. [Google Scholar] [CrossRef]
- Wen, X.-J.; Niu, C.-G.; Zhang, L.; Liang, C.; Guo, H.; Zeng, G.-M. Photocatalytic degradation of ciprofloxacin by a novel Z-scheme CeO2–Ag/AgBr photocatalyst: Influencing factors, possible degradation pathways, and mechanism insight. J. Catal. 2018, 358, 141–154. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, H.; Wang, C.; Mao, X.; Wang, Y.; Yang, Y.; Liu, G. Visible light induced photocatalytic degradation of rhodamine B on one-dimensional iron oxide particles. J. Phys. Chem. C 2010, 114, 17051–17061. [Google Scholar] [CrossRef]
- Ruales-Lonfat, C.; Barona, J.F.; Sienkiewicz, A.; Bensimon, M.; V´elez-Colmenares, J.; Ben´ıtez, N.; Pulgar´ın, C. Iron oxides semiconductors are efficients for solar water disinfection: A comparison with photo-Fenton processes at neutral pH. Appl. Catal. B 2015, 166–167, 497–508. [Google Scholar] [CrossRef]
- Pervez, M.N.; Ma, S.; Huang, S.; Naddeo, V.; Zhao, Y. Photo-Fenton Degradation of Ciprofloxacin by Novel Graphene Quantum Dots/α-FeOOH Nanocomposites for the production of safe drinking water from surface water. Water 2022, 14, 2260. [Google Scholar] [CrossRef]
- Dutta, D.P. Composites of Sb2O4 and biomass-derived mesoporous disordered carbon as versatile anodes for sodium-ion batteries. ChemSelect 2020, 5, 1846. [Google Scholar]
- Araga, R.; Soni, S.; Sharma, C.S. Fluoride adsorption from aqueous solution using activated carbon obtained from KOH-treated jamun (Syzygium cumini) seed. J. Environ. Chem. Eng. 2017, 5, 5608–5616. [Google Scholar] [CrossRef]
- Raj, K.A.; Panda, M.R.; Dutta, D.P.; Mitra, S. Bio-derived mesoporous disordered carbon: An excellent anode in sodium-ion battery and full-cell lab prototype. Carbon 2019, 143, 402–412. [Google Scholar] [CrossRef]
- Abrashev, M.V.; Ivanov, V.G.; Stefanov, B.S.; Todorov, N.D.; Rosell, J.; Skumryev, V. Raman spectroscopy of alpha-FeOOH (goethite) near antiferromagnetic to paramagnetic phase transition. J. Appl. Phys. 2020, 127, 205108. [Google Scholar] [CrossRef]
- Vernekar, D.; Jagadeesan, D. Tunable acid–base bifunctional catalytic activity of FeOOH in an orthogonal tandem reaction. Catal. Sci. Technol. 2015, 5, 4029. [Google Scholar] [CrossRef]
- Sharma, S.; Kaur, M.; Sharma, C.; Choudhary, A.; Paul, S. Biomass-Derived Activated Carbon-Supported Copper Catalyst: An Efficient Heterogeneous Magnetic Catalyst for Base-Free Chan–Lam Coupling and Oxidations. ACS Omega 2021, 6, 19529–19545. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Fang, D.; Yu, Y.; Xu, Z.; Liang, J.; Zhou, L. Enhanced catalytic performance of β-FeOOH by coupling with single-walled carbon nanotubes in a visible-light-Fenton-like process. Sci. Eng. Comp. Mater. 2018, 25, 9–15. [Google Scholar] [CrossRef]
- Huang, H.W.; Li, X.W.; Wang, J.J.; Dong, F.; Chu, P.K.; Zhang, T.R.; Zhang, Y.H. Anionic group self-doping as a promising strategy: Band-gap engineering and multi-functional applications of high-performance CO32−-doped Bi2O2CO3. ACS Catal. 2015, 5, 4094–4103. [Google Scholar] [CrossRef]
- He, Z.Q.; Wang, D.; Fang, H.Y.; Chen, J.M.; Song, S. Highly efficient and stable Ag/AgIO3particles for photocatalytic reduction of CO2 under visible light. Nanoscale 2014, 6, 10540. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.P.; Ballal, A.; Chopade, S.; Kumar, A. A study on the effect of transition metal (Ti4+, Mn2+, Cu2+ and Zn2+)-doping on visible light photocatalytic activity of Bi2MoO6 nanorods. J. Photochem. Photobiol. A Chem. 2017, 346, 105–112. [Google Scholar] [CrossRef]
- Zhang, T.; Lv, C.; Wang, X.; Wang, S.; Xie, Y.; Yu, M.; Sun, C.; Pan, K. Surface active-site engineering in NiCoSe2/nitrogen-doped carbon dodecahedrons for efficient triiodide reduction in photovoltaics. Appl. Surf. Sci. 2023, 610, 155483. [Google Scholar] [CrossRef]
- Song, T.; Zhang, Z.; Zhao, B.; Wang, L.; Kan, W.; Sun, L.; Wang, X. Boosting catalytic performance of hierarchical Co/Co0.85Se microspheres via Mott-Schottky effect toward triiodide reduction and alkaline hydrogen evolution. J. Alloys Compds. 2022, 918, 165608. [Google Scholar] [CrossRef]
- Liu, Z.; Xin, W.; Xie, W.; Wang, X.; Li, N.; Yuan, Z.; Li, Y.; Wang, J. Integration of hierarchical tin Sulfide@Sulfur-Doped carbon porous composites with enhanced performance for triiodide reduction. Dyes Pigm. 2022, 204, 110458. [Google Scholar] [CrossRef]
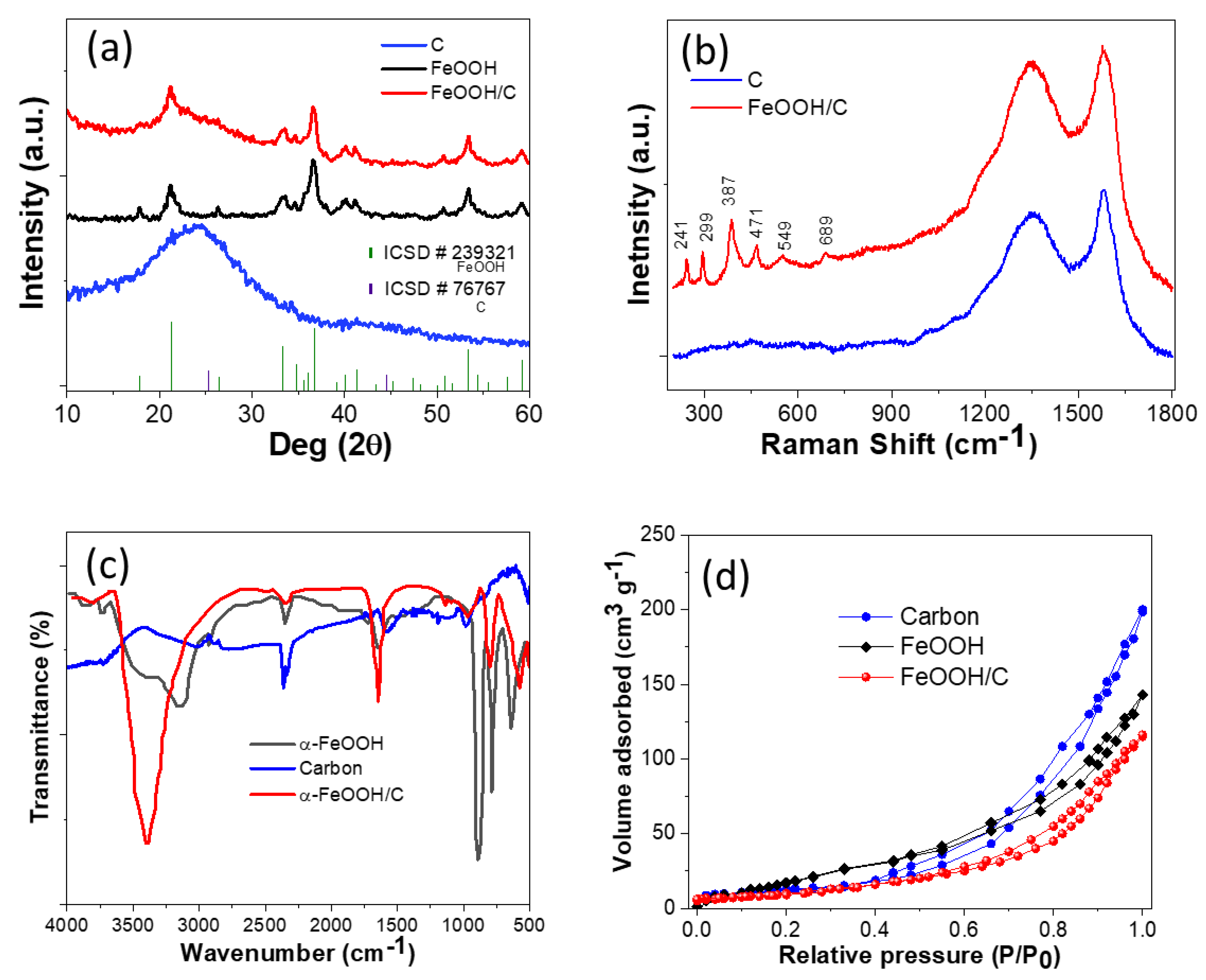
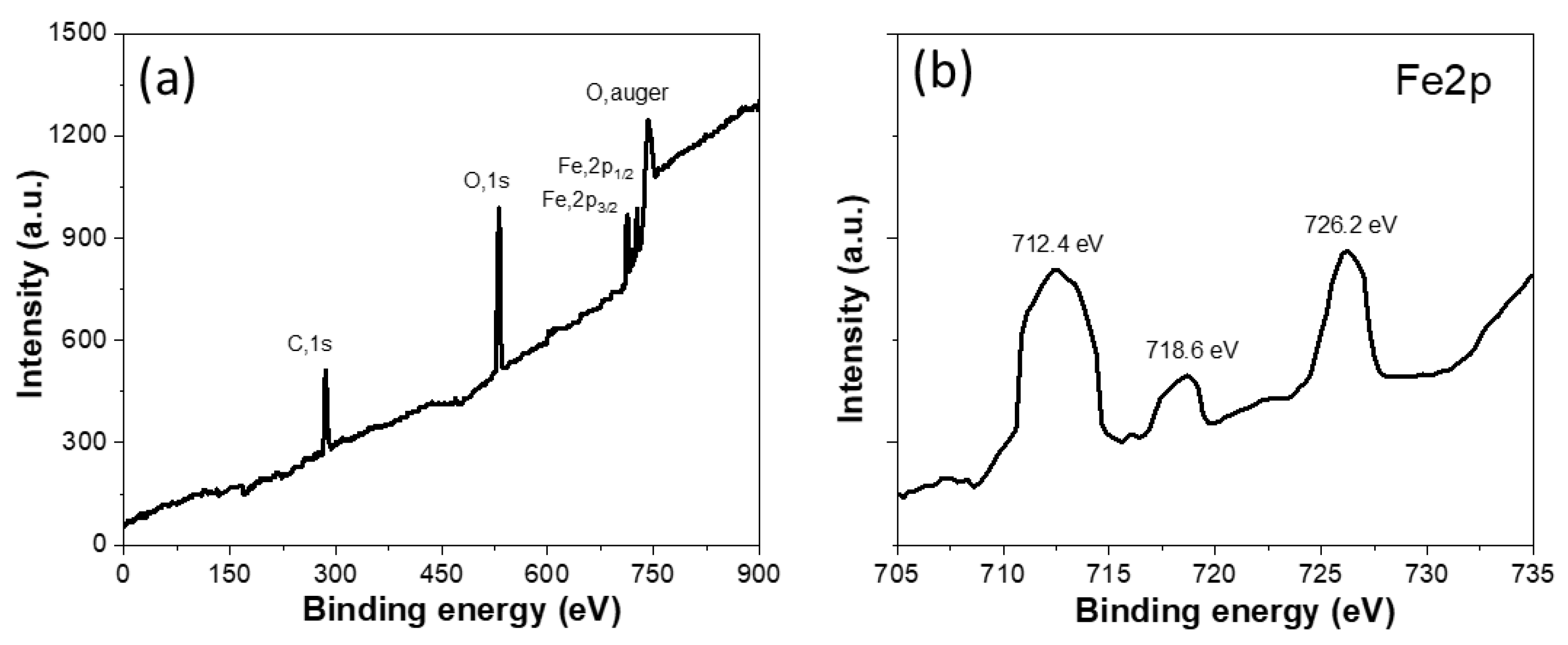


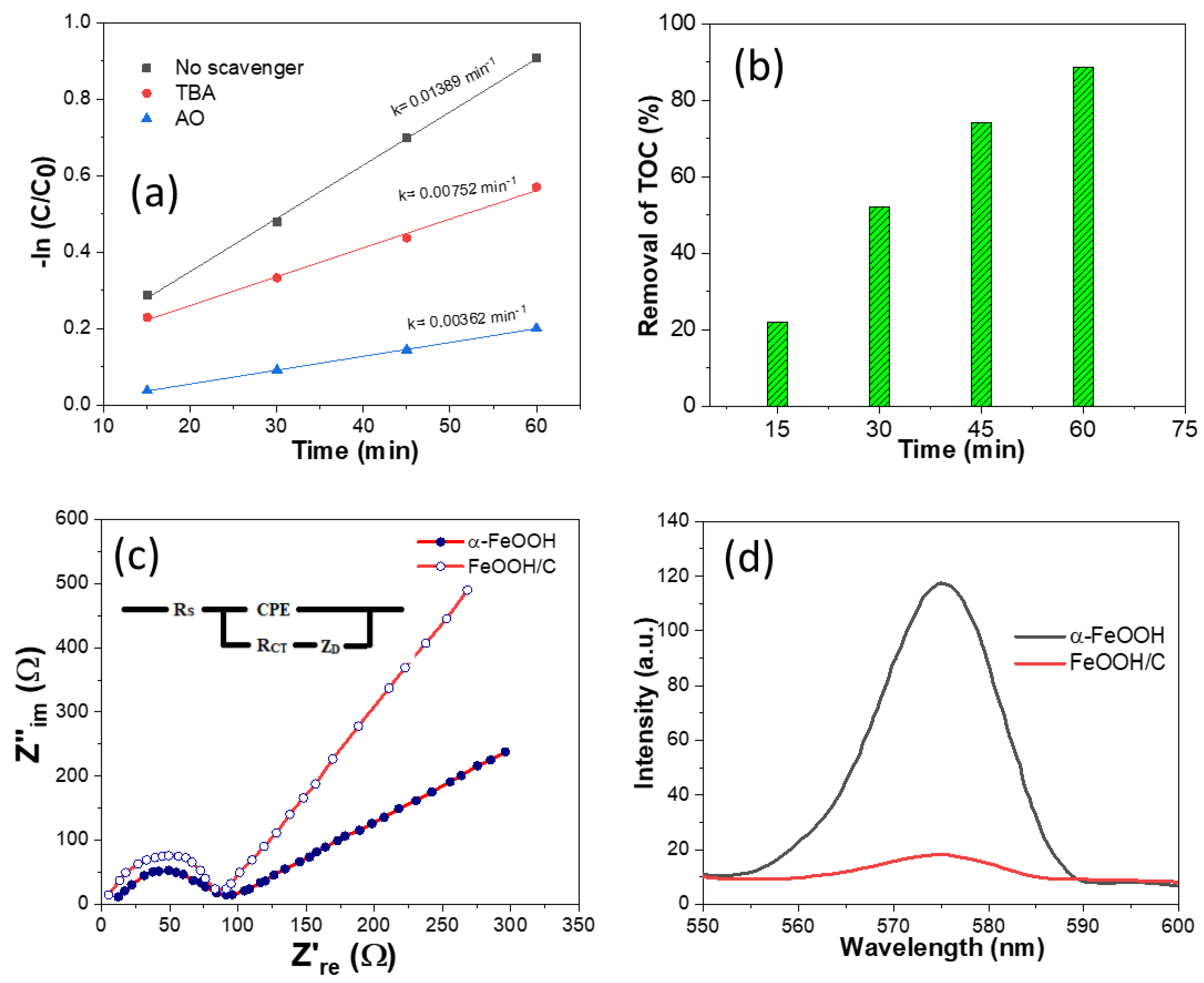
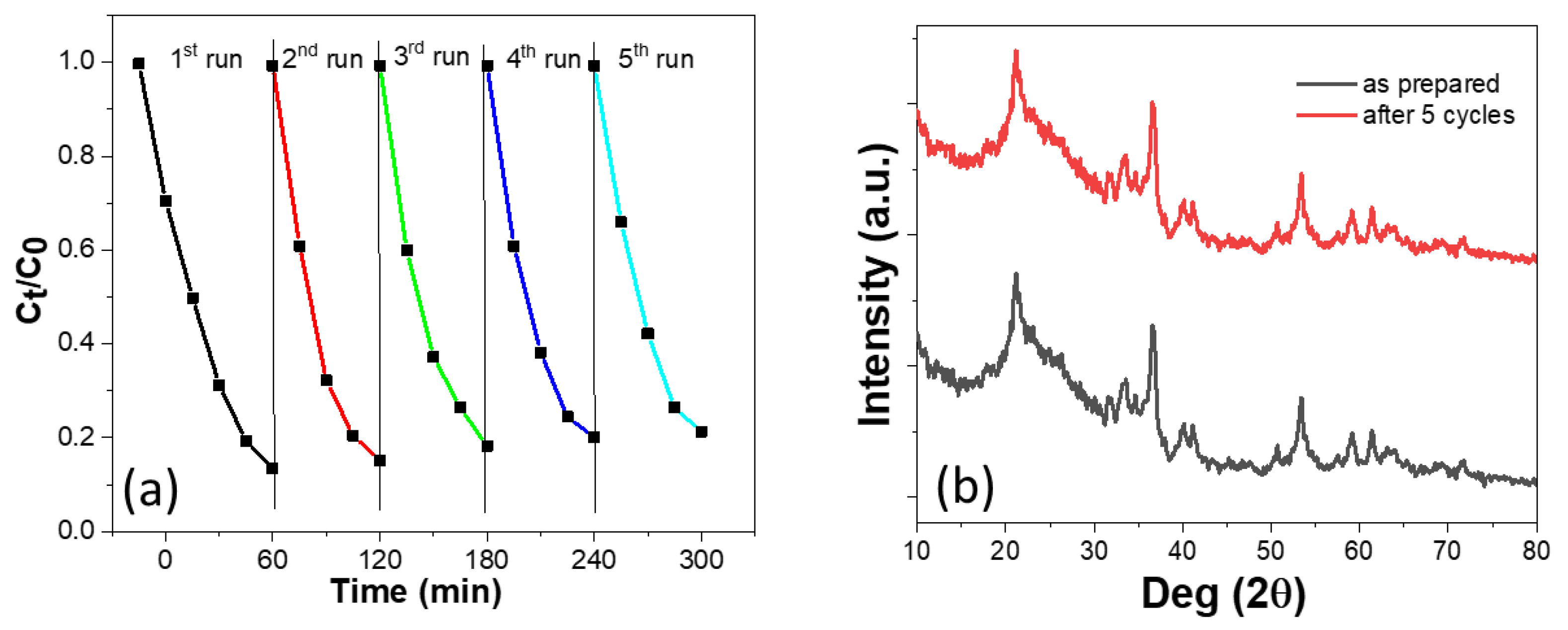
| Samples | Specific Surface Area (m2 g−1) | Mean Pore Diameter (nm) | Total Pore Volume (cm3 g−1) |
|---|---|---|---|
| C | 64.35 | 8.06 | 0.14 |
| α−FeOOH | 38.75 | 3.02 | 0.08 |
| α−FeOOH/C | 52.25 | 6.17 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, D.P.; Abraham, S. Composite of α-FeOOH and Mesoporous Carbon Derived from Indian Blackberry Seeds as Low-Cost and Recyclable Photocatalyst for Degradation of Ciprofloxacin. Catalysts 2023, 13, 191. https://doi.org/10.3390/catal13010191
Dutta DP, Abraham S. Composite of α-FeOOH and Mesoporous Carbon Derived from Indian Blackberry Seeds as Low-Cost and Recyclable Photocatalyst for Degradation of Ciprofloxacin. Catalysts. 2023; 13(1):191. https://doi.org/10.3390/catal13010191
Chicago/Turabian StyleDutta, Dimple P., and Sebin Abraham. 2023. "Composite of α-FeOOH and Mesoporous Carbon Derived from Indian Blackberry Seeds as Low-Cost and Recyclable Photocatalyst for Degradation of Ciprofloxacin" Catalysts 13, no. 1: 191. https://doi.org/10.3390/catal13010191
APA StyleDutta, D. P., & Abraham, S. (2023). Composite of α-FeOOH and Mesoporous Carbon Derived from Indian Blackberry Seeds as Low-Cost and Recyclable Photocatalyst for Degradation of Ciprofloxacin. Catalysts, 13(1), 191. https://doi.org/10.3390/catal13010191





